Abstract
Erectile dysfunction, prostatic hyperplasia and lower urinary tract symptoms hare important pathogenetic links. Endothelial dysfunction and hormonal alterations represent the main aspects. The present article examines the anatomical, physiological, and pathophysiological characteristics of this association, finalizing the text to an interpretation of the clinical management of these patients based on these functional considerations.
Introduction
Low urinary tract symptoms (LUTS)/benign prostatic hyperplasia (BPH) and erectile dysfunction (ED), both severely affecting the aging male, are often associated [Citation1] and share similar pathophysiological mechanisms. Emerging studies have shown that common therapeutic approaches may be effective in improving both LUTS and DE in patients with BPH [Citation2,Citation3]. To clarify the mechanisms that underlie this association and to better understand the rational of such therapeutic approaches, anatomy, physiology, pathophysiology and the clinical approach of these two conditions are deeply discussed in this review.
Anatomy and physiology of erectile dysfunction
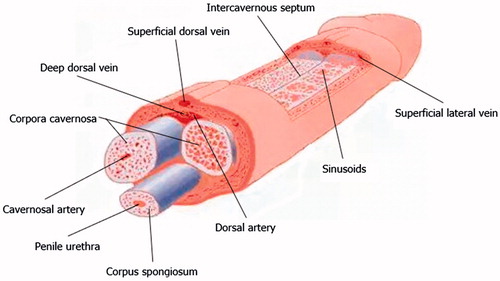
The central erectile structures are bilateral corpora cavernosa, seen as dorsolaterally placed low-reflectivity bodies on ultrasound, surrounded by the thick fibrous tunica albuginea. The corpora cavernosa are formed by multiple sinusoids composed of endothelium and smooth muscle. These sinusoids are capable of substantial volume expansion. The ventrally located corpus spongiosum is enclosed by a thinner layer of tunica albuginea and surrounds the penile urethra. The corpus spongiosum is anatomically independent of the cavernosa. The three corpora are enclosed by the more superficial Buck’s fascia.
The penile arterial supply displays slight variation in its anatomy. The penis is usually supplied by branches of the internal pudendal artery, which continue as the penile artery. The bulbar artery supplies the proximal shaft and is the first branch of the penile artery, which then divides into the dorsal and cavernosal arteries. The cavernosal artery enters and supplies the corpora cavernosa via several helicine arteries, which in turn flow into the sinusoids via multiple arterioles. The intercavernous septum is perforated, allowing for communication of blood (and injected pharmacological agents) across the midline. Emissary veins pierce the tunica albuginea to drain into the deep dorsal vein, via the spongiosal, circumflex and cavernosal veins [Citation4] ().
The penile erectile tissue, specifically the cavernous smooth musculature and the smooth muscles of the arteriolar and arterial walls, plays a key role in the sequence of events that brings to erection. In the flaccid state, smooth muscles are tonically contracted, letting a small amount of arterial flow for nutritional purposes. The blood partial pressure of oxygen (pO2) is about 35 mmHg. The flaccid penis is in a mild state of contraction, as shown by a further shrinkage following exposure to cold temperatures or after phenylephrine intracavernous injection. Sexual stimulation triggers the release of neurotransmitters from the cavernous nerve terminals. This results in relaxation of these smooth muscles and the following chain of events:
Dilatation of the arterioles and arteries which bring increases blood flow in both the diastolic and the systolic phases.
Trapping of the incoming blood by the expanding sinusoids.
Compression of the subtunical venular plexus between the tunica albuginea and the peripheral sinusoids. This results in a decreasing venous outflow.
Stretching of the tunica to its maximal capacity, which occludes the emissary veins between the inner circular and the outer longitudinal layers and further decrease of the venous outflow.
A pO2 of about 90 mmHg and an intracavernous pressure of about 100 mm/Hg raise the penis from the dependent position to the erect state (the full-erection phase).
A further pressure increase (to several hundred mm/Hg) with contraction of the ischiocavernosus muscles (rigid-erection phase).
Three phases of detumescence have been distinguished in animal studies. The first entails a transient intracorporeal pressure increase, indicating the beginning of smooth muscle contraction against a closed venous system. The second phase shows a slow pressure decrease, suggesting a slow reopening of the venous channels with resumption of the basal level of arterial flow. The third phase shows a fast pressure decrease with fully restored venous outflow capacity. Erection thus involves sinusoidal relaxation, arterial dilatation, and venous compression.
This process is dependent upon the parasympathetic nervous system, which induces smooth muscle relaxation allowing arterial pressure blood into the corpus cavernosum by nitric oxide (NO) action [Citation5]. NO is generated by three nitric oxide synthase (NOS) enzyme isoforms: neuronal, endothelial and inducible. The neuronal isoform appears to be the primary mediator of physiologic erection [Citation6]. Neuronal NO induces erections while shear stress also propagates the erectile response via endothelial NO. Regardless to the source, NO modulates smooth muscle cyclic GMP to induce relaxation in a paracrine fashion. Vascular relaxation in turn allows arterial blood to fill the corpora that, by distention, creates a venous seal to maintain erection.
Pathophysiology of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction
Two main pathways in LUTS lead to the development of symptoms in men: benign prostatic obstruction (BPO) and benign prostatic enlargement (BPE). In addition to this setting, detrusor overactivity/overactive bladder (OAB) can occur in both men and women. This dichotomy associates with voiding symptoms and/or storage symptoms.
Voiding symptoms are associated with BPO, which is linked to BPE because of BPH. Storage symptoms are more complex and do not appear to be BPH- or BPE-related because they manifest in men and women; more likely, these symptoms are associated with involuntary detrusor contractions or detrusor overactivity (DO) [Citation7,Citation8]. Involuntary detrusor contraction during the storage phase of the voiding cycle [Citation9] seems to lead to OAB symptoms. Storage LUTS may be associated with bladder dysfunction due to changes or alterations in afferent nerves or in interstitial cells within the bladder rather than BPE [Citation10,Citation11].
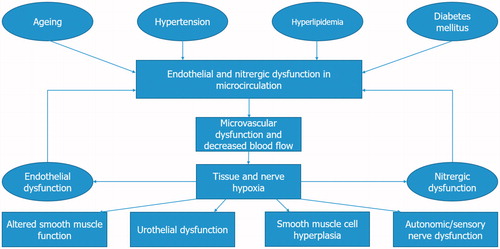
Four pathophysiological pathways might lead to increased risk of LUTS development. These include reduced nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling, chronic inflammation/steroid hormone imbalance/increased RhoA–Rho-kinase activity, autonomic hyperactivity, and pelvic atherosclerosis [Citation1,Citation12–14]. These factors can lead to reduced function of nerves and endothelium, alterations in smooth muscle tone, arterial insufficiency, reduced blood flow and hypoxia-related tissue damage, increased smooth muscle cell proliferation in the prostate, and bladder hypertrophy/noncompliance [Citation12].
The vascular system of the low urinary tract is regulated by smooth muscle cell relaxation, which respond to PDE5 inhibition. LUTS/BPH may develop from decreased oxygenation of lower urinary tract tissue, which might ensue with the above-mentioned risk factors. Atherosclerosis contributes also with remodeling of smooth muscle structure and function in the pelvic vasculature to its development [Citation15,Citation16] in penis [Citation17], prostate [Citation18], and bladder [Citation16]. This results in chronic ischemia of the low urinary tract often associated with LUTS/BPH [Citation19].
Moreover, three nerve systems are involved in physiopathology of LUTS/BPH: the pudendal, pelvic, and hypogastric nerves. The voiding process involves stimulation of the detrusor and inhibition of the parasympathetic innervation of the urethra and bladder neck hypogastric nerves, plus recruitment of motor neurons to the urethral sphincter [Citation20]. The storage process involves inhibition of the parasympathetic innervation of the detrusor muscle with urethral sphincter contraction via sympathetic innervation of the hypogastric nerves and recruitment of the pudendal nerves. Storage symptoms may be the result of bladder dysfunction due to changes or alterations in afferent nerves or in interstitial cells [Citation21].
Similar mechanisms have been studied in pathophysiology of ED and are strongly linked to this condition. The NO-cGMP pathway is important in smooth-muscle relaxation and erection of the penis. Activation of the RhoA/ROCK signaling pathway decreases smooth-muscle relaxation tone in corpora cavernosa. Increased sympathetic nervous system activity might affect smooth muscle and vascular tone via α1-adrenergic receptors in the penis. Finally, atherosclerosis can result in decreased perfusion/ischemia of penile arteries [Citation13,Citation14,Citation22]. All of these pathophysiological mechanisms are thought to contribute to the development of either ED or LUTS/BPH [Citation13], and can explain a link between these conditions ().
Etiology and clinical aspects of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction
The European Association of Urology (EAU) and the American Urological Association (AUA) [Citation23] guidelines define LUTS as storage (irritative) symptoms (daytime urinary frequency, urgency, and nocturia), voiding (obstructive) symptoms (straining, weak stream, intermittent stream, and incomplete emptying), or post-micturition symptoms (post-micturition dribbling) that affect the lower urinary tract [Citation23,Citation24].
The clinical diagnosis of LUTS/BPH is a multistep process used to eliminate prostate cancer, identify risk factors, and obtain physiological measures. Symptoms of LUTS/BPH are generally assessed using the International Prostate Symptom Score (IPSS) or AUA Prostate Symptom Index (AUA-SI); serum prostate specific antigen (PSA) levels; urinalysis; a transrectal ultrasound of the prostate; the measurement of the maximal urinary flow rate (Qmax) assessed by uroflowmetry; and the measurement of post-void residual volume assessed by post-void bladder ultrasound.
The definition of LUTS/BPH used in clinical studies and in the literature varies widely. Men with LUTS/BPH have generally been identified:
Histologically by having BPH;
With symptom severity assessed by total IPSS as being either mild (0–7), moderate (8–19), or severe (20–35);
With increased prostate size (BPE – defined as prostate volume ≥20 mL [Citation12]. Patients with prostate volume ≥30 ml have 3.5-times greater risk of having moderate-to-severe symptoms, three-times greater risk of acute urinary retention, and a significantly greater risk of requiring BPH-related surgery [Citation25,Citation26];
and with a Qmax of 4–15 mL/s, which is indicative of benign prostatic obstruction (BPO).
The large overlap of men with both LUTS and ED has shown the strong link between the two conditions, being age a known predictor of the combined phenotype and LUTS/BPH severity an even better predictor, thus establishing an independent link between LUTS/BPH and ED [Citation1].
According to the underlying causes, ED can be classified as ():
Table 1. Etiology of erectile dysfunction.
Psychogenic
Organic; this one further divided in nonendocrine and endocrine
In the past, ED was considered, in most cases, to be a purely psychogenic, but current evidence shows that more than 80% of cases have an organic etiology. The two milestone epidemiological studies (MMAS and EMAS) have studied this condition in men aged 40–80 years. However, the prevalence of ED in younger men is increasing even due to social awareness and overcoming taboo issues. In this context, a recent naturalistic study has demonstrated that one out of four men seeking medical help for ED is <40 years old [Citation27]. Currently, it is believed that most cases of ED in younger men have a psychological basis; this is often strengthened by sudden onset, good quality spontaneous or self-stimulated erections, major life events or previous psychological problems. However, recent studies have been brought that consider ED in younger men a possible spy of subclinical or future organic problems, linked to endothelial dysfunction, insulin resistance Peyronie’s disease, neurogenic disorders, medication side effects, early onset hypogonadism, dysthyroidism [Citation28–32]. Often, even organic causes lead in the end to a psychological component, regardless of the trigger event, since ED imposes negative effects on interpersonal relationships, mood and quality of life.
Psychogenic ED is also called adrenaline-mediated ED (noradrenaline-mediated or sympathetic-mediated ED). Stress, depression and anxiety are generally defined as heightened anxiety related to the inability to achieve and maintain an erection before or during sexual relations, and are commonly associated with psychogenic ED. This association is explained by the role of noradrenaline as the primary erectolytic (anti-erectile) neurotransmitter.
Among the nonendocrine causes, the vasculogenic mechanism is by far the most common one and can be divided into arterial inflow disorders and venous outflow disorders (defects in the veno-occlusive mechanism).The arterial changes related to atherosclerosis, diabetes, cigarette smoking and other vascular risk conditions can lead to arterial stenosis and changes in arterial wall (decreased elasticity), thus decreasing corpora cavernosa oxygenation and paving road to collagen deposition and decrease of smooth muscle/collagen ratio. This may eventually lead to inability of the cavernosa to compress the subtunical veins, causing secondary veno-occlusive dysfunction.
Veno-occlusive defects are, by themselves, uncommon. The pathophysiology of structurally based corporeal veno-occlusive dysfunction is related to increase in corporeal connective tissue content. It may be present alone in injuries in penile tunica albugineaor Peyronie’s disease, but it is often associated to arterial inflow disorders, neurogenic disorders with reduction in NO load, or psychological etiologies that involve an increase in orthosympatic activation (i.e. stress, depression, anxiety) [Citation33].
Other organic etiologies include neurogenic factors (affecting innervation and nervous function), with deficit in nerve signaling to the corpora cavernosa; this may be due to various conditions, such as spinal cord injury, multiple sclerosis, Parkinson disease, lumbar disc disease, pelvic fractures, traumatic brain injury, radical pelvic surgery (radical prostatectomy, radical cystectomy, abdominoperineal resection) and diabetic neuropathy. Upper motor neuron lesions (above spinal nerve T10) do not create local changes in the penis but can inhibit the central nervous system (CNS)-mediated control of the erection. Instead, sacral lesions (S2–S4 typically responsible for reflexogenic erections) cause functional and structural alterations due to the decreased innervation. These changes in innervation of cavernosal tissue lead finally to the reduction in NO load that is available to the smooth muscle. From this condition, chronic ischemia can occur, leading to reduced arterial inflow, and changes in smooth muscle/collagen ratio, resulting in veno-occlusive dysfunction [Citation34–37].
Among iatrogenic factors (caused by medical or surgical treatment), the most common is radical pelvic surgery. The damaged structures are usually nerves (of periprostatic plexus or cavernous nerves); sometimes damage is done to accessory pudendal arteries. A variety of drugs and medications has been studied in association with ED. However, a clear role in determining ED for many medications has been recently debated and results difficult to define. TOMHS compared five anti-hypertensive drugs with a placebo for changes in quality of life (sexual function was assessed by physician interviews). Chlorthalidone (a diuretic drug used in hypertension) had the greatest effect on sexual function two years after treatment, but the placebo achieved almost the same level at four years. Accordingly, chlorthalidone may potentiate ED earlier in those who are likely to develop the condition later in life (see “Epidemiology and risk factors of ED and LUTS/BPH-hypertension and cardiovascular disease” section of this chapter for more) [Citation38].
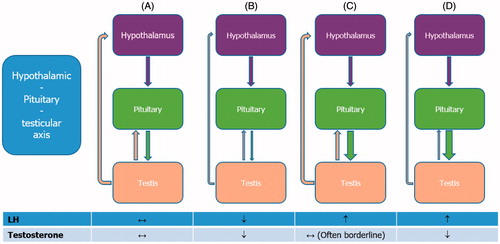
The endocrine factors leading to ED include low serum testosterone levels, since androgens are the major regulators of penile development and function. Few data have established a clear role of other hormones in ED. A role has been documented for thyroid hormones, prolactin, growth hormone, insulin-like growth factor 1, dehydroepiandrosterone, and oxytocin. Although these hormones play a part in the pathophysiology of erection, their epidemiological impact is likely to be small and must be confirmed through further studies. After testosterone, prolactin is the most commonly altered hormone in men with sexual dysfunction; its main effect is to inhibit gonadotropin secretion to induce hypogonadism. Thus, prolactin should be considered for further screening in ED patients with low serum testosterone and LH levels [Citation39] ().
Figure 3. Normal and pathological hypothalamic–pituitary–testicular axis. (A) Normally functioning hypothalamic–pituitary–testicular axis. Gonadotropin-releasing hormone (GnRH) stimulates the release of luteinizing hormone (LH). This triggers the testes to respond by secreting testosterone, which, in turn, exerts a negative feedback on the hypothalamus and pituitary gland. Both circulating LH and testosterone are within the normal range. (B) Central hypogonadism. The pituitary release of LH is impaired, the testes are no longer stimulated and testosterone production drops; both circulating LH and testosterone are decreased. (C) Subclinical or compensated hypogonadism. The testicular responsiveness to LH is impaired, testosterone production is maintained owing to overstimulation by LH; circulating testosterone is normal or borderline, whereas LH is increased. In this case, the system is driven to its maximal capacity and no further adjustment can be achieved. (D) Primary hypogonadism. In testicular failure, increases in LH serum levels can no longer sustain testosterone release by Leydig cells; circulating testosterone is low and LH is high.
Patients with combined LUTS/BPH and ED are more complex and heterogeneous to examine. They will seek medical care for the impact of LUTS on quality of life and/or management of ED. Thus, an integrated urological and andrological approach is essential to effectively treat the condition from all points of view. Typically, these patients are aged 50–80 years and often have both ED and LUTS/BPH. Thus, establishing an accurate medical and sexual history is essential to ensure the correct treatment. We suggest avoiding empirical treatment before establishing a correct diagnosis, as failure in first-step therapy may eventually lead to dropout. This is particularly true for elderly patients who can easier renounce to medical help for ED after the first failure. A practical set of signs and symptoms to look for is summarized in .
Table 2. Differences in symptoms of erectile dysfunction by etiology.
To accurately trace urological and sexual anamnesis, structured interviews and self-reported questionnaires come to help. These are composed of a set of standardized questions, which require a unique response. Among the most used ones, we mention IPSS, IIEF and IIEF-5 and Structured Interview on erectile dysfunction (SIEDY). The IPSS questionnaire is a validated, seven-part, self-administered questionnaire that is used to assess LUTS/BPH severity and response to treatment [Citation40]. The 15-item IIEF is a validated self-administered patient questionnaire that assesses ED [Citation41]. An abridged five-item version of the IIEF was developed (IIEF-5) to diagnose the presence and severity of ED. The five items selected are based on ability to identify the presence or absence of ED and on adherence to the National Institute of Health’s definition of ED [Citation42]. IIEF-5 is an excellent diagnostic test for detecting the presence and severity of ED, and should be administered more often by clinicians [Citation1]. SIEDY is a 13-item interview composed of three scales that identify and quantify important domains in men with ED (organic, scale 1; marital, scale 2; and intrapsychic, scale 3) [Citation43].
Physical examination must search for signs related to causes of ED, including chest evaluation (presence of gynecomastia – enlargement of the chest and/or mammalian button >2 cm; search for signs of chronic cardio-pulmonary diseases), distribution of body hair and androgenization grade. Evaluation of penis prostate and testes is mandatory to establish-related volumes: according to patient’s age, small testes and/or small prostate volume might imply hypogonadism. It is important to ask for eventual muscular force decrement, as well as a decrease in beard and body hair growth. Assessment of the peripheral vascular system is also important to determine the characteristics of the pulse, to ascertain the presence of an arterial bruit (a vascular sound that is associated with turbulent blood flow). Increased pulse rate (tachycardia) might suggest hyperthyroidism, whereas reduced pulse rate (bradycardia) might be evident in men with heart block (arrhythmia), hypothyroidism or in those who use certain drugs (e.g. β-blockers). Diminished or absent pulses in the various arteries examined could be indicative of impaired blood flow caused by atherosclerosis. The evaluation of the penis in the flaccid state might show the presence of Peyronie disease (involving palpable fibrous plaques), phimosis (congenital narrowing of the opening of the foreskin) or frenulum breve (whereby the tissue under the glans penis that connects to the foreskin is too short and restricts the movement of the foreskin), which can all contribute to ED. Measurement of blood pressure, waist circumference and body mass index should also be performed [Citation44].
A few biochemical and hormonal parameters are of value in patients with ED (hormonal parameters include essentially total testosterone, LH, prolactin, SHBG to assess free testosterone). However, levels of cholesterol, triglycerides, fasting glucose and glycosylated hemoglobin (HbA1c) are important determinants of cardiovascular and metabolic risk stratification. Total testosterone and SHBG for the evaluation of calculated free testosterone are sufficient parameters to rule out hypogonadism. Prolactin and thyroid hormone evaluation are limited to a subset of patients.
In the eventuality of abnormal biochemical or hormonal values, a second-line evaluation is necessary. If the fasting plasma glucose level is 100–126 mg/dl, or HbA1c is >5.7%, an oral glucose tolerance test can be used to exclude overt type 1 and type 2 diabetes mellitus. The Princeton III Consensus Panel has established criteria for performing further cardiovascular evaluation ().
Table 3. Princeton III consensus recommendations for risk stratification and cardiovascular evaluation for sexual activity.
Second-line evaluation
Ultrasound examination is a second-line test that can be used to examine penile structure and vasculature with penile Doppler ultrasonography, allowing for the examination of the cavernosal and dorsal penile arteries. The ultrasound scan can be performed during flaccidity (static) or following drug-stimulated erection (dynamic). Drugs commonly used to stimulate erection comprehend PgE1 derivatives, such as alprostadil, which, injected through a fine needle into the corpora cavernosa, cause smooth muscle relaxation, vasodilatation and increased blood inflow, leading to erection in physiological conditions. An adequate response to a trial of these agents confirms adequate arterial supply and veno-occlusive mechanism, and precludes the need for further investigation. Penile Doppler ultrasonography is used in those patients in whom arterial or venous insufficiency is suspected. Furthermore, penile Doppler sonography may be used to study the penile anatomy in patients with post-traumatic/post-surgical abnormalities where curative/reconstructive surgery is being considered.
At ultrasound, the corpora cavernosa appear as longitudinally orientated vascular beds of mixed echogenicity, with the tunica albuginea visualized as a thin echogenic envelope, usually 2 mm thick. In the absence of cavernosal fibrosis, the interface between the tunica and the underlying cavernosal tissue is quite distinct. The spongiosum is visualized on the ventral surface and is of slightly higher reflectivity than the cavernosa. The cavernosal arteries can be found within the corpora cavernosa at ultrasound as parallel hyperechoic lines. Variants in arterial anatomy exist in up to 20%, but they are not relevant to clinical practice. After intracavernosal injection of alprostadil, Doppler scan shows a change overtime of the spectral Doppler waves in a velocity/time curve to evaluate the cavernosal arteries both anatomically and functionally. A quiet, private and comfortable environment is essential for a good outcome of the test. Many patients will be anxious, and a detailed explanation of the procedure is of paramount importance. Informed consent should be obtained, especially with regard to the low risk of priapism following intracavernosal injection; this accounts for about 0.01% of the procedures, and is defined as prolonged and painful response of the penis to drug-stimulated erection (longer than 4 h), which requires urgent treatment. With patient positioned supine on the bed, an initial injection of alprostadil is done at the basis of left and/or right corpora cavernosa, with an angle of about 30 degrees and perpendicular to longitudinal axis of the penis. Before injection, eventual abstinence from antiplatelet drugs, if possible, is advised.
If the patient is pharmacologically naive, a small dose (5 mg of alprostadil) is initially given. If there has been a poor response to PDE5i agents previously, then up to the full dose (20 mg) may be given. Utilizing a high frequency linear probe, and once tumescence starts; a longitudinal scan enables visualization of the cavernosal artery at its root. A velocity gradient exists within the artery from the base to the tip, and reproducible and accurate measurements are best obtained at the penile base towards the peno-scrotal junction. Two-three minutes after injection, the cavernosal arteries should become more visible, and spectral measurement and image acquisition should begin at this stage. In addition, the quality of the erection should be assessed both objectively (by the operator) and subjectively (by the patient) and recorded. If the quality of the erection is insufficient, repeated injection can be made. Repeated Doppler measurements should occur at 5-min intervals until the maximal peak systolic velocity (PSV) and end-diastolic velocity (EDV) are judged to have been reached. The PSV cutoff is >35 cm/s and EDV is usually normal if negative or close to 0 cm/s, usually <5 cm/s. The waveform during erection follows four main phases. Following injection of pharmaco-stimulant, initial waveforms display elevation of velocities, especially the diastolic velocity, which reflects smooth muscle relaxation. The PSV then usually stabilizes after this and is sustained for at least 5 min. After this, intracavernosal pressure increases, and this phenomenon can be indirectly measured by a steady reduction in the end-diastolic velocity as increasing sinusoidal distension elevates IP and reduces venous outflow. As the IP continues to rise, the diastolic velocity diminishes and the systolic waveform narrows. When IP exceeds diastolic pressure, the diastolic waveform will reverse. As the maximal penile rigidity is achieved in the final phase, the intracavernosal pressure is equal to or greater than systolic, producing further narrowing of the systolic peak. In some cases, the systolic velocity may reduce to such an extent that there is a transient interruption to systolic flow. When the minimal diastolic velocity is reached, the Doppler study is finished (). The examination is completed by longitudinal and transversal gray scale scan to search for the presence of non-vascular abnormalities such as plaques, fibrosis or tunica albuginea defects.
Figure 4. Schematic representation of the main phases of cavernosal artery waveform during a penile Doppler ultrasound examination. (A) In basic conditions, the waveform appears hardly detectable in a velocity/time curve. (B) A few minutes after injection of alprostadil, the cavernosal artery inflow becomes well detectable. (C) The waveform continues to modify with increasing peak systolic velocity (PSV, cutoff >35 cm/s) and decreasing end-diastolic velocity (EDV, cutoff <5 cm/s), until the systolic velocity peaks, indicating the grade of arterial inflow. Acceleration time (AT, cutoff <100) narrows, and can be measured from the bottom to the top of the peak. (D) Later, diastolic flow reversal is shown, indicating an intact venous occlusion mechanism. (E) Intracavernosal pressure exceeds systolic pressure at maximal tumescence, causing reduction in the systolic velocity and narrowing of the systolic peak.

Conclusions
Low urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction share similar pathophysiological pathways (NO – cGMP signaling, increased sympathetic nervous system activity, atherosclerosis). They also have common risk factors, such as metabolic syndrome, obesity, hypertension [Citation45–47]. Furthermore, the IIEF-5 index seems to positively correlate with the IPSS score [Citation48]. These observations provide the rational for common therapeutic approaches. Interestingly, treatment with PDE5 inhibitors has been showed to improve LUTS in patients with BPH [Citation3,Citation49], probably ameliorating the endothelial function [Citation2]. Although the pathophysiologic role of the α-adrenoceptor system in the etiology of both ED and LUTS in patients with BPH has been demonstrated [Citation50], a meta-analytic study highlighted a worse negative impact of the combination therapy with 5α-reductase inhibitors plus α-blockers on ED compared to the single therapy [Citation51]. Therefore, patients with LUTS/BPH and ED may benefit from single therapy with α-blockers. Finally, long-term testosterone replacement therapy has shown to restore urinary and sexual function in hypogonadal men [Citation52]. Accordingly, its withdrawal has been associated with a worsening of LUTS and IIEF-5 index [Citation53]. In conclusion, common therapeutic approaches in the aging male suffering from LUTS/BPH and ED may be sought.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kirby M, Chapple C, Jackson G, et al. Erectile dysfunction and lower urinary tract symptoms: a consensus on the importance of co-diagnosis. Int J Clin Pract. 2013;67:606–618.
- Amano T, Earle C, Imao T, et al. Administration of daily 5 mg tadalafil improves endothelial function in patients with benign prostatic hyperplasia. Aging Male. 2017;22:1–6.
- Ko WJ, Han HH, Ham WS, et al. Daily use of sildenafil 50mg at night effectively ameliorates nocturia in patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: an exploratory multicenter, double-blind, randomized, placebo-controlled study. Aging Male. 2017;20:81–88.
- Patel DV, Halls J, Patel U. Investigation of erectile dysfunction. Br J Radiol. 2012;85:69–78.
- Rajfer JAW, Bush PA, Dorney FJ, et al. Nitric oxide as a mediator of smooth muscle relaxation of the corpus cavernosum in response to nonadrenergic-noncholinergic neurotransmission. N Engl J Med. 1992;326:90–94.
- Burnett AL. Nitric oxide control of lower genitourinary tract functions: a review. Urology. 1995;45:1071–1083.
- Reynard JM. Does anticholinergic medication have a role for men with lower urinary tract symptoms/benign prostatic hyperplasia either alone or in combination with other agents? Curr Opin Urol. 2004;14:13–16.
- Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49:651–658.
- Hashim H, Abrams P. Overactive bladder: an update. Curr Opin Urol. 2007;17:231–236.
- Parsons JK. Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol. 2011;21:1–4.
- Roosen A, Chapple CR, Dmochowski RR, et al. A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol. 2009;56:810–819.
- Park HJ, Won J, Sorsaburu S, et al. Urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) and LUTS/BPH with erectile dysfunction in Asian men: a systematic review focusing on tadalafil. World J Mens Health. 2013;31:193–207.
- Andersson KE, De Groat WC, McVary KT, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011;30:292–301.
- Gacci M, Eardley I, Giuliano F, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2011;60:809–825.
- Kolpakov V, Di Sciullo A, Nasuti M, et al. Reduced smooth muscle cell regeneration in Yoshida (YOS) spontaneously hypercholesterolemic rats. Atherosclerosis 1994;111:227–236.
- Azadzoi KM, Tarcan T, Siroky MB, et al. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol. 1999;161:1626–1635.
- Kostis JB, Jackson G, Rosen R, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol. 2005;96:85–93.
- Azadzoi KM, Babayan RK, Kozlowski R, et al. Chronic ischemia increases prostatic smooth muscle contraction in the rabbit. J Urol. 2003;170:659–663.
- Berger AP, Deibl M, Leonhartsberger N, et al. Vascular damage as a risk factor for benign prostatic hyperplasia and erectile dysfunction. BJU Int. 2005;96:1073–1078.
- Giuliano F, Ückert S, Maggi M, et al. The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur Urol. 2013;63:506–516.
- McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol. 2005;47:838–845.
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–1109.
- Rosen R, Carson C, Giuliano F. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol. 2005;47:824–837.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803.
- Arrighi HM, Guess HA, Metter EJ, et al. Symptoms and signs of prostatism as risk factors for prostatectomy. Prostate. 1990;16:253–261.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487.
- Capogrosso P, Colicchia M, Ventimiglia E, et al. One patient out of four with newly diagnosed erectile dysfunction is a young man — worrisome picture from the everyday clinical practice. J Sex Med. 2013;10:1833–1841.
- Salonia A, Castagna G, Saccà A, et al. Is erectile dysfunction a reliable proxy of general male health status? The case for the International Index of Erectile Function–Erectile Function domain. J Sex Med. 2012;9:2708–2715.
- Yao F, Liu L, Zhang Y, et al. Erectile dysfunction may be the first clinical sign of insulin resistance and endothelial dysfunction in young men. Clin Res Cardiol. 2013;102:645–651.
- Ludwig W, Phillips M. Organic causes of erectile dysfunction in men under 40. Urol Int. 2014;92:1–6.
- Sanders SA, Hill BJ, Janssen E, et al. General erectile functioning among young, heterosexual men who do and do not report condom-associated erection problems (CAEP). J Sex Med. 2015;12:1897–1904.
- Papagiannopoulos D, Khare N, Nehra A. Evaluation of young men with organic erectile dysfunction. Asian J Androl. 2015;17:11–16.
- Nehra A, Goldstein I, Pabby A, et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol. 1996;156:1320–1329.
- Leungwattanakij S, Pummangura N, Ratana-Olarn K. Penile enhancement using a porcine small intestinal submucosa graft in a rat model. Int J Impot Res. 2006;18:39–43.
- Ferrini MG, Davila HH, Kovanecz I, et al. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–435.
- Mulhall JP, Müller A, Donohue JF, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008;5:1126–1136.
- Ferrini MG, Kovanecz I, Sanchez S, et al. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6:415–428.
- Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;4:16003.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557.
- Grimm RH Jr, Grandits GA, Prineas RJ, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women: treatment of mild hypertension study (TOMHS). Hypertension. 1997;29:8–14.
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830.
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326.
- Althof SE, Rosen RC, Perelman MA, et al. Standard operating procedures for taking a sexual history. J Sex Med. 2013;10:26–35.
- Ghanem HM, Salonia A, Martin-Morales A. SOP: physical examination and laboratory testing for men with erectile dysfunction. J Sex Med. 2013;10:108–110.
- Aktas BK, Gokkaya CS, Bulut S, et al. Impact of metabolic syndrome on erectile dysfunction and lower urinary tract symptoms in benign prostatic hyperplasia patients. Aging Male. 2011;14:48–52.
- Demir O, Akgul K, Akar Z, et al. Association between severity of lower urinary tract symptoms, erectile dysfunction and metabolic syndrome. Aging Male. 2009;12:29–34.
- Yassin AA, Saad F, Gooren LJ. Metabolic syndrome, testosterone deficiency and erectile dysfunction never come alone. Andrologia. 2008;40:259–264.
- Nakamura M, Fujimura T, Nagata M, et al. Association between lower urinary tract symptoms and sexual dysfunction assessed using the core lower urinary tract symptom score and International Index of Erectile Function-5 questionnaires. Aging Male. 2012;15:111–114.
- Gacci M, Andersson KE, Chapple C, et al. Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2016;70:124–133.
- Yassin A, Saad F, Hoesl CE, et al. Alpha-adrenoceptors are a common denominator in the pathophysiology of erectile function and BPH/LUTS-implications for clinical practice. Andrologia. 2006;38:1–12.
- Favilla V, Russo GI, Privitera S, et al. Impact of combination therapy 5-alpha reductase inhibitors (5-ARI) plus alpha-blockers (AB) on erectile dysfunction and decrease of libido in patients with LUTS/BPH: a systematic review with meta-analysis. Aging Male. 2016;19:175–181.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199:257–265.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19:64–69.