Abstract
Background: Sarcopenia is a pathophysiological condition diffused in elderly people; it represents a social issue due to the longer life expectancy and the growing aging population. It affects negatively quality of life and it represents a risk factor for other pathologies, such as diabetes, cardiovascular disease, and obesity. No silver bullet exists to hinder sarcopenia, but it may be counteracted by physical exercise, nutrition, and a proper endocrine milieu. Indeed, we aim to analyze the scientific literature to give to clinician effective advices to counteract sarcopenia.
Main text: Physical exercise, proper nutrition, optimized hormonal homeostasis represent the three pillars to fight sarcopenia. Physical exercise represents the most effective remedy to face sarcopenia, in particular if it is combined with a proper diet and with an adequate endocrine milieu. Consistency in training, adequate daily protein intake and eugonadism seems to be the keys to fight sarcopenia. The combination of these three pillars might act synergistically.
Conclusions: Optimization of these factors may increase their efficiency; however, scientific data may be sometimes confusing so far. Therefore, we aim to give practical advices to clinician to identify and to highlight the most important aspects in each of these three factors that should be addressed.
1. Background
Sarcopenia is an age-related syndrome defined by the loss of muscle mass and strength. Such a process is remarkable in several pathological conditions: chronic diseases, sarcopenic obesity, prolonged immobilization. However, sarcopenia represents also a physiological condition of aging. According to the different cutoffs used to evaluate the presence of sarcopenia and to the age of the evaluated population, the prevalence ranges from 10 to 20% in people older than 65 years old. These numbers are slightly higher in women [Citation1]. At the moment, a worldwide accepted definition of sarcopenia does not exist, but the European Working Group on Sarcopenia in Older People (EWGSOP) suggests that diagnosis of sarcopenia may be considered in presence of a) reduced muscle mass and b) low muscle strength or reduced exercise performance. A relationship between muscle mass, defined as cross-sectional area and muscle strength may be hypothesized but it is neither consistent nor simple [Citation2].
In young people, the average number of muscle fibers ranges from 400,000 to 900,000 and the number of motor units ranges from 125 to 325. In the eighth decade, the number of muscle fibers and of motor units range from 200,000 to 350,000 and from 1 to 125, respectively. Such a loss regards every muscle, but muscles with a prevalence of type 2 fiber are more interested in this process. Even regenerative processes of muscle tissue become slower and slower in aging; indeed, in satellite cells a progressive decrease of proliferative capacity and a reduced ability to fuse with preexisting myofibers has been observed [Citation3]. In aging the density of satellite cells surrounding type II muscle fibers decreases whereas an increase in density of satellite cells surrounding type I muscle fibers has been observed. Also, myonuclear domain that is the portion of cytosol regulated by a myonucleus, decreases in both muscle fibers. Even, the rate of protein synthesis decreases in type I and II muscle fibers [Citation4,Citation5]. From a physiopathological point of view, sarcopenic subjects show a net loss of muscular mass that seems to be due to a decrease muscle protein synthesis (MPS) and an increase in muscle protein breakdown (MPB). On the contrary, in healthy elderly subjects, MPB does not appear to be altered. PI3K-Akt pathway and mammalian target of rapamycin complex 1 (mTORC1) acting downstream and apoptosis promoted by ubiquitin ligases activated by forkhead box class O (FoxO), such as E3 ubiquitin, MuRF-1, and atrogin, are considered the main factors altering MPB [Citation6]. Insulin inhibits proteolysis activating PI3K-AKT-mTORC1 pathway; following on in insulin-resistant state, a common condition in elderly, MPB is enhanced [Citation7,Citation8]. Being insulin itself a vasodilator hormone, insulin resistance contributes to decreased vascular flow, which worsens insulin effects on muscle and limits the flow of nutrients and oxygen to muscle decreasing MPS and increasing MPB [Citation9]. Therefore, even micro and macroangiopathy, as frequently seen in elderly, play a pathophysiological role in sarcopenia. Serum insulin-like growth factor I (IGF-1), branched chain amino acids (BCAA), phosphatidic acid (PA), and mechanical stimulus are able to activate mTORC1 with different mechanisms; whereas glucocorticosteroids, myostatin, and reactive oxygen species (ROS) are negative regulators of mTORC1 [Citation10]. Consequently, GH deficit, malnutrition, immobilization, physical inactivity, and long-term treatment with corticosteroids as seen in chronic pathologies are risk factors for sarcopenia [Citation11]. Immobilization should be considered as a consequence of bone fracture, mainly due to osteoporosis in elderly men [Citation12–14]. Prevalence of osteoporosis increases as a function of age occurring in approximately 50% of men over 70 years in specific populations and affecting quantitative and qualitative bone structure [Citation14–16]. Chronic pathologies and aging are also linked to a pro-inflammatory state with an increased secretion of molecules, such as TNF-alfa, IL-1, and IL-6. These cytokines contribute to increase skeletal insulin resistance and muscle proteolysis activating ubiquitin-proteasome system, suppressing Akt/mTORC1 pathway, and antagonizing IGF-1 action on muscle [Citation6,Citation17]. TNF-α plays a crucial role in promoting muscle apoptosis activating the extrinsic apoptotic pathway with the engagement of caspase-8 and caspase-3 [Citation18]. Aging is associated to an increase in ROS production accompanied by an impairment in antioxidant systems that brings about an imbalance in redox state affecting mTORC1 pathway and damaging the structure of lipids and proteins. In particular, peroxidation of phospholipids of the inner mitochondrial membrane impairs mitochondrial respiratory chain and, in particular, supramolecular organization of the respiratory supercomplex I1III2 causing metabolic dysfunction of mitochondria. Such a dysfunction leads to lower respiratory capacity resulting in reduced ATP production and in increased ROS production perpetuating a vicious cycle. Oxidative stress represents also a causal factor in promoting a chronic inflammatory state by means of activation of redox-sensitive transcription factor NF-kB; in turns, inflammation produces ROS activating a vicious cycle [Citation19].
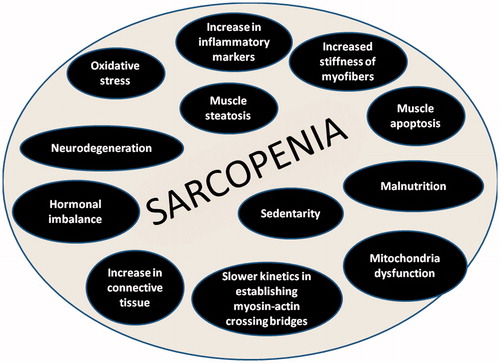
In evaluating muscle tissue both in quantitative and in qualitative terms many hypothesis have been suggested to explain the sarcopenia: increase in connective tissue, muscle steatosis, impaired muscle metabolism, increase in inflammatory markers (i.e. TNF-α and IL-6), increase stiffness of myofibers, a slower kinetics in establishing myosin-actin crossing bridges, increased oxidative stress, mitochondria dysfunction, hormonal imbalance, and decreased capillary flow [Citation20–24] (). All these para-physiological age-related changes affect not only muscle mass but also the intrinsic contractile properties of muscle fibers. This represents the reason, besides the prevalent loss of type 2 motor units, because strength and/or physical performance declines much more faster than muscle mass [Citation25].
Nevertheless, the most effective means to prevent or to slow down the development of sarcopenia are represented by: physical exercise, nutrition, and optimized endocrine milieu. The aim of this review is to highlight how and to what extent these parameters may affect or prevent sarcopenia considering synergistic or additive effects when present. Indeed, this review should be able to give to clinicians practical, simple, and effective advices to counteract sarcopenia. Scientific literature may provide a great amount of input regarding the roles of physical exercise, nutrition, and endocrine axis in the process of sarcopenia; however, clinicians should have a clear idea about which parts should be considered the most important ones in order to make them a priority. Counteracting sarcopenia should a time-effective process and for such a reason it is of paramount importance to organize scientific data in terms of priority.
2. Physical exercise
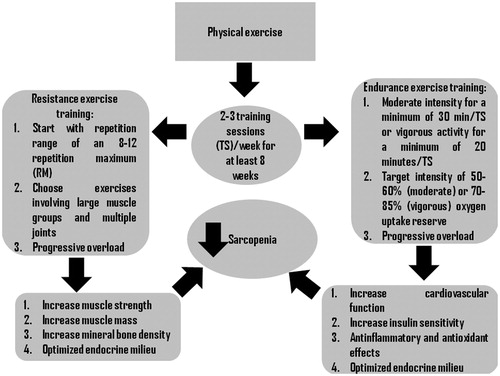
Physical exercise is a type of planned, structured, and repetitive motor activity that if suitably conducted and individualized, is aimed, in maintaining, improving, or restoring the state of individual well-being, psycho-physical health, and motor skills [Citation26]. Physical exercise, due to its anabolic effects, offers the most significant gain against sarcopenia, confirmed by skeletal muscle mass loss induced by sedentarity [Citation27–29]. It may act on skeletal muscle through different mechanism: activation of mTORC1 decreased expression of pro-inflammatory mediators, reduced oxidative stress, increased mitochondrial biogenesis, increased ratio IGF-1/myostatin, and increased response to insulin [Citation11,Citation17,Citation30].
Taking in mind that sarcopenia is defined by reduced muscle mass and strength, the best physical exercise to prevent its onset and development is represented by resistance exercise training (RET), whereas aerobic exercise training (AET) is more suitable to maintain and/or increase aerobic and cardiovascular fitness [Citation30–33]. Nevertheless, chronic AET seems to have significant effects on mitochondrial efficiency and biogenesis and the up-regulation of peroxisome proliferator-activated receptor gamma coactivator 1-α (PCG-1 α) appears to be crucial in this process. Indeed, PGC-1α activity is modulated by upstream signaling mediators, such as AMP-activated protein kinase (AMPK), p38 mitogen-activated protein kinases (MAPK), sirtuin 1 (SIRT1), and p-cAMP response element-binding protein (CREB) that are up-regulated by physical exercise. Following on, chronic AET appears to reduce myonuclear apoptosis and to increase IGF-1 levels [Citation30]. Recommendations regarding AET in elderly suggest to perform moderate-intensity activities for at least 30 min/d up to 60 min/d in bouts of at least 10 min each to total 150–300 min/week or at least 20–30 min/d or more of vigorous-intensity to total 75–150 min/week [Citation26]. Taking into account the ability of RET to stimulate net muscle protein anabolism, it should be considered as the first training option [Citation34]. It is not fully understood how RT increases muscle protein anabolism but it can hypothesize that mTORC1 plays a pivotal role, is a master regulator of different biological pathways activated by RT (i.e. MAPK and IGF-1/PI3K/Akt) [Citation30,Citation35].
Consistency in performing physical exercise is the keyword to obtain significant results; indeed, in the elderly muscle recovery after immobilization or detraining is slower compared to young people [Citation36]. For untrained individuals 2–3 training sessions (TS) weekly may be adequate whereas 3–4 TS·w−1 are recommended for intermediate subjects.
However, in order to obtain the best results, it is compulsory to evaluate training variables [Citation37]. Concerning specific parameters of volume and intensity, such as number of repetitions and sets, length of training session, loads (% of 1 repetition maximum) length of recovery phases; it seems that they have an absolute importance [Citation37]. However, the most important parameter appears to be the length of training schedule that should be at least of eight weeks with three or four sessions weekly [Citation38]. Van Roie et al. suggest that to counteract sarcopenia increasing muscle hypertrophy a mixed-low load training schedule consisting in 1 × 80–100 repetitions at 20% of 1 repetition maximum (RM) followed by 1 × 10–20 repetitions at 40% of 1 RM seems to represent the best tradeoff [Citation39,Citation40]. Otherwise, Cadore et al. suggest that to perform moderate-high intensity (65–80% of 1 RM) to maximize strength gains whereas lower intensity and high velocity of movement are necessary to increase power. Muscle power training should be performed at low-moderate intensity (40–60% of 1 RM). It is crucial to include specific power training because muscle power seems to be a significant predictor of functional performance in elderly affecting positively quality of life. Consequently, training schedule should be performed 3 times a week, with three sets of 6–20 repetitions and an intensity ranging from 40 to 60% of 1 RM in order to increase strength and power [Citation41]. Following on, it is worthy to note that mixing training intensity may provide the best results in terms of performance and appeal. Other recommendations to maximize training’s efficiency are represented by starting a training schedule choosing a load corresponding to a repetition range of an 8–12 repetition at 60–70% of 1 RM and exercises involving large muscle groups and multiple joints [Citation42].
Even simple exercises involving eccentric strength may be effective in preserving muscle strength and speed [Citation43,Citation44].
Progressive overload is the principle key to develop strength and muscle mass; indeed elderly men maintain a significant but slower neuromuscular ability to overcompensate training stimulus compared to young people (). Consequently, elderly men are less responsive to a training schedule and longer periods of training are required to stimulate adaptation process. However, taking into account that the decrease in number and in size of muscle fibers seems to be an effect of motor spinal neuronal loss, such ability assumes an outstanding role [Citation45].
Although physical exercise exerts many positive effects, elderly people may unable or unwilling to perform dynamic training schedule; for such a reason, the results of electrical muscle stimulation (EMS) on global strength should be carefully evaluated. Few scientific evidence is present in literature regarding the effectiveness of Whole Body Vibration (WBV), representing a novel technique able to induce a muscular stimulus by means of vibrations [Citation46]. EMS and WBV may be useful in the elderly as they do not require a full active effort; indeed, in both techniques muscle stimulation comes mainly from an exogenous source [Citation47–49]. WBV should be performed at medium frequency and medium duration to exert the best results [Citation50]. Following on, in our aim to highlight priorities it should be considered that a simple and practical home exercise program has been effective in improving muscle mass and walking speed in elderly men [Citation51].
Mixing AET and RT may represent a valid option to maximize the pros of each training; however, this combination may induce lower strength and power gains compared to strength training alone (i.e. interference effect). Nevertheless, this effect of AET on strength and power may occur only in muscle groups that are requested to produce both strength and endurance performance [Citation41]. Rotating activities may be the key to mix AET and RT successfully.
Finally, it should be considered that optimal process of adaption to different kinds of stimulations induced by physical exercise needs a good hormonal milieu [Citation52]. Indeed, any kind of hormonal deficit may prevent supercompensation process [Citation53].
3. Nutrition
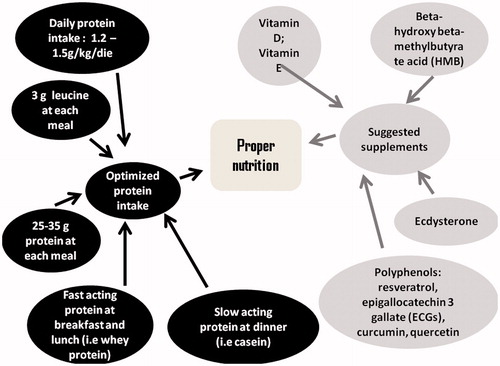
Besides physical exercise, also nutrition should be carefully evaluated and correct diet may be able to stimulate muscle anabolism and to limit muscle catabolism. Scientific evidence seems to be consistent in supporting the hypothesis that Mediterranean diet may exert positive effects on muscle tissue; its high quantity of fruits and vegetables represents a rich source of antioxidant substances that may limit oxidative stress, representing a mechanism responsible of sarcopenia. Even a significant daily intake of extra virgin olive oil may represent a good source of hydroxytyrosol (HT), a mitochondrial antioxidant with pleiotropic effects [Citation54,Citation55]. HT appears to activate AMPK and is also effective in reducing muscle damage, inflammation, and secondary immune deficiency in strenuous physical exercise [Citation56,Citation57]. Moreover, in Mediterranean diet the high amount of bluefish provides omega 3 fatty acids, which may augment muscle anabolic response to different stimuli (i.e. physical exercise, hormonal therapies, proteins), decreasing inflammation, oxidative stress, and insulin resistance [Citation58–61]. Proteins represent the most important macronutrient in counteracting muscle mass loss in the elderly; however, daily protein intake in the elderly may be not higher than 0.6 g/kg/die. Recent studies support the hypothesis that a daily protein intake of at least 1.2–1.5 g/kg/die may be necessary and such intake should be taken in different assumptions to counterbalance the reduced anabolic responsiveness to protein intake showed by elderly () [Citation62,Citation63]. Each assumption should provide at least 25 g of protein, as the optimal protein dosage to stimulate muscle protein synthesis is about 35 g [Citation64,Citation65]. Nevertheless, it seems that the amount of total protein, but not the pattern of protein intake, is of importance with respect to maximizing anabolic response; in this case the amount needed to maximally stimulate protein anabolism (i.e. synthesis minus breakdown), should be in accord with the higher end of the acceptable macronutrient distribution range (35% of total calories) [Citation66,Citation67]. Moreover, it would be useful to assume rapidly absorbed protein, such as whey protein, at each meal whereas a good amount of slowly absorbed protein, such as casein, should be assumed at bedtime to stimulate GH anabolic action and to prevent catabolism induced by nocturnal fasting. As regards amino acid composition of proteins, it would be useful to assume proteins rich in leucine, which seems to be the best amino acid in terms of anabolic properties [Citation68]. An intake of 3 g of leucine at each meal has been proposed by different authors as an efficient nutritional strategy to maintain muscle mass. It should consider that 100 g of meal or fish provide 1.4–1.7 g of leucine [Citation69].
Caloric restriction represents a significant anti-aging intervention reducing the activation of extrinsic and intrinsic apoptotic pathways by means of a reduced expression of inflammatory cytokines. 40% reduction in daily caloric intake reduces the apoptotic signaling pathway triggered by TNG-α and increases muscular IL-15 level and IL-15 receptor α chain which downregulate apoptosis promoted by TNF-α.
Even micronutrients and supplements appear to play a role against sarcopenia, such as vitamin E, vitamin D, β-hydroxy-β-methylbutyrate (HMB), resveratrol, epigallocatechin 3 gallate (ECGs), creatine (Cr), and CoQ10 [Citation19,Citation70–74]. In particular vitamin E, for its antioxidant properties; vitamin D, involved in muscle hypertrophy and whose deficiency has been associated to a higher prevalence of sarcopenia [Citation17,Citation75]; HMB and its acid form [Citation76] seem to increase protein synthesis and decrease protein degradation through activation of different pathway and stimulating skeletal muscle satellite cell activation [Citation77]; resveratrol, ECGs and other polyphenols (i.e. quercetin and curcumin) share strong antioxidant capacity and stimulate mitochondrial biogenesis [Citation78]. In particular, resveratrol seems to sustain anabolic process induced by strength training and to mimic caloric restriction [Citation79]. Cr shows dual roles in sarcopenia acting as a mitochondrial oxidant and as an anabolic agent stimulating mRNA expression of muscle regulatory factors, such as Myo-D, Myf-5, MRF-4, and myogenin, and of IGF-1 and to repress that of myostatin [Citation80]. Cr (Cr) administration increases creatine phosphate (CrP) ratios improving the cellular calcium handling and homeostasis and maintains an adequate MRC activity thus preventing ROS leakage [Citation19]. CoQ 10 acts as an electron and proton shuttle following two-electron redox reaction and reversible conversion from oxidized form (ubiquinol) to the reduced form (ubiquinol); ubiquinol protects mitochondrial lipid from peroxidation and CoQ10 shows anti-inflammatory effect through the inhibition of NF-kb nuclear translocation [Citation19,Citation81].
A growing interest is present regarding anabolic effects of ecdysterone, a promising selective estrogen receptor beta agonist [Citation82,Citation83] (). Last but not least, it seems that alkali-supplemented diets decrease urinary nitrogen excretion expressed as ratio to same day nitrogen intake, reducing muscle protein breakdown [Citation84,Citation85]. High-protein diet may increase the amount of acidic byproducts affecting negatively musculoskeletal system; the introduction of alkaline salts in diet may result in a more favorable environment for muscle conservation. Low-grade metabolic acidosis appears to stimulate proteolysis pathways. This stimulus can be triggered by an increased production of glucocorticosteroids, such as cortisol [Citation86,Citation87].
4. Hormones
An optimized endocrine milieu provides significant gains to counteract loss of muscle mass in elderly and for such a reason many supplements and/or drugs have been suggested to be helpful in restoring hormonal level and consequently muscle mass [Citation88] ().
Aging affects endocrine axis but the main hormones involved in muscle tissue are: testosterone, growth hormone, dehydroepiandrosterone, and insulin.
Table 1. Suggested blood test for endocrine evaluation.
4.1. Growth hormone (GH)
Growth hormone deficit (GHD) may affect negatively muscle mass but it should be remembered that reduced secretion of GH is much less frequent than sex hormones deficiency. Aging brings about a decrease in GH secretion by about 14% for each decade until an overt GH deficit in some subjects. In particular, the mixture of aging, obesity, and inactive lifestyle may provoke an overt GH deficit. In fact, obesity and overweight are associated to a reduced GH response to stimulatory tests [Citation89]. Indeed, it has been suggested to use different cutoffs for stimulatory GH test to define GHD according to BMI [Citation90,Citation91]. However, in order to diagnose GHD it has been suggested to perform two different stimulatory tests. The most used stimulatory tests are represented by: insulin tolerance test (ITT) and GHRH + arginine, keeping in mind that glucagon stimulatory test may use when ITT is contraindicated [Citation92]. Also, physical exercise may increase GH secretion and the amount of GH secretion depends on training status, volume, intensity, and duration of physical efforts; moreover, a reduction in physical exercise may bring about a decreased GH secretion.
IGF-I value is not approved to diagnose GHD, but it is useful to monitor replacement therapy targeting the top-end of the reference range. GH replacement therapy should be initiated with low dose (i.e. 100–200 mcg/die) increasing slowly according to clinical response and serum IGF-I value [Citation92,Citation93].
Although the mechanism by which GH affects positively protein synthesis are not completely clear; it seems that GH stimulates IGF-I synthesis, which in turns, via phosphorylation, stimulates several hypertrophic pathways including glycogen synthase kinase-3β and mTORC1. Moreover, IGF-I, reduces net protein breakdown by inactivation of FoxO transcription factors 1, 3, and slow down apoptosis in many cells. IGF-I may also increase protein synthesis rate through the activation of the mitogen-activated protein kinase cascade. Indeed, glucocorticosteroids induce protein degradation reducing IGF-I levels [Citation94,Citation95].
In subjects with an overt GHD, GH replacement therapy may bring about an overall increase in fat-free mass and a simultaneous decrease in fat mass. However, such an increase in fat-free mass should be evaluated as it may not be represented by muscle mass; indeed, GH replacement therapy may increase intracellular water and protein synthesis in connective tissue rather than in muscle tissue. Indeed, prolonged GH replacement therapy barely affects muscle mass and/or strength both in healthy subjects and in adults with GH deficiency [Citation96,Citation97]. In particular, in GH-deficient adults, GH replacements therapy may increase exercise performance but not muscle strength [Citation98,Citation99].
4.2. Dehydroepiandrosterone-sulfate (DHEA-S) and dehydroepiandrosterone (DHEA)
Aging affects also adrenal efficiency and decreased serum level of DHEA-S and DHEA are often observed in elderly, showing often serum concentrations three or four times lower than those observed in young men. DHEA has been often presented as “fountain of youth” and its use is increasing worldwide [Citation100]. Physiologically, DHEA is a weak androgen and it acts also as a precursor of more biologically powerful androgens. It seems that its conversion to active androgens or estrogens is crucial to bring about positive effects on muscle mass and body composition [Citation101,Citation102]. Its administration in subjects showing a real deficit may have beneficial effects on humor and well-being, and a small significant effect may be observed on muscle mass and strength [Citation103]. A study regarding DHEA administration in elderly men and women also showed a better adaption to a strength training schedule [Citation104,Citation105]. However, these effects on serum androgens levels and muscle mass have not been confirmed by other studies and the supplementation with DHEA in treatment of sarcopenia does not seem to be recommended [Citation106,Citation107].
4.3. Insulin
Even insulin secretion and sensitivity are affected by aging exerting negative effects not only on glucose metabolism but also on muscle protein anabolism being these processes independent. Insulin has a powerful anabolic and anti-catabolic effect on muscle mass and its deficit and/or low peripheral sensitivity deprive muscle tissue of both an anabolic and an anticatabolic factor [Citation108–110]. These complex actions of insulin are regulated by an interplay between amino acids (AA), muscle blood flow, microvascular recruitment, and insulin levels. Indeed, insulin seems to increase the MPS activating mTORC1 and p38 MAPK when AA delivery to the skeletal muscle is increased; AA availability, exercise, muscle perfusion, and insulin sensitivity are the main factors to guarantee AA delivery to skeletal muscle. Nevertheless, the anabolic properties of insulin are predominantly driven by its ability to attenuate skeletal MPB especially when AA are scarce [Citation7,Citation111]. A higher percentage of insulin resistance (IR) has been observed in sarcopenic subjects compared to non-sarcopenic ones regardless the presence of obesity [Citation112,Citation113]. IR may play a big role in age-related anabolic resistance in association with decreased peripheral perfusion [Citation114]. Insulin enhances the vasodilatory effect of acetylcholine and the lack of its signal besides other factors may affect negatively peripheral perfusion impairing the flow of nutrients and hormones to muscle fibers [Citation115,Citation116]. Indeed, the increase in muscle blood flow induced by hyperinsulinemia seems to be crucial to promote MPS guaranteeing both adequate amino acid delivery and insulin bioavailability [Citation9,Citation110]. IR also blunts the anticatabolic properties of insulin at great extent and it is likely to be related to impaired insulin signaling of muscle protein metabolism and endothelial dysfunction [Citation111]. Even a reduction in insulin secretion as observed in overt type 2 diabetes has been associated to the presence of sarcopenia [Citation117].
Notwithstanding, it is not thinkable to suggest insulin administration to hinder muscle mass loss rather than an active lifestyle and proper nutrition to maintain or recover an adequate insulin sensitivity [Citation118,Citation119].
4.4. Testosterone
Male hypogonadism seems to play an important role in sarcopenia in elderly. In such a population, it is quite common to find both low serum level of testosterone, defined as late-onset hypogonadism (LOH), and sarcopenia. Hypogonadism consists in a clinical and biochemical syndrome defined by a serum testosterone level below 8 or 11 nmol/L with a serum-free testosterone below 220 pmol/L [Citation120]. Equilibrium dialysis is the gold standard for free T measurement but may not be routinely available, alternatively, measurement of serum SHBG, albumin, and a reliable measurement of total testosterone allows for the determination of the calculated free T level [Citation121].
Since the third decade, serum testosterone level decrease by 1%/year with a simultaneous increase of Sex Hormone Binding Globulin by 1.7–2.6%/year [Citation122]. Concerning prevention and treatment of sarcopenia, testosterone administration in hypogonadal men may be helpful. Theoretically, to stimulate anabolic process, the testosterone replacement therapy (TRT) should be titrated to reach medium-high serum testosterone level inside the reference range. However, side effects should be carefully evaluated during TRT, especially if the established target is defined as above [Citation123]. In order to maximize efficiency of TRT, guaranteeing the highest level of serum testosterone without any side effects it would be advisable to use transdermal testosterone gel [Citation124].
Testosterone plays a crucial role in maintenance and development of muscle mass [Citation125]. From a physiological point of view, testosterone increases both cross-sectional area and myonuclei numbers, so that the ratio of numbers of myonuclei and muscle fiber area, and consequently the volume of cytoplasm controlled by a single myonucleus (i.e. nuclear domain) remain unchanged to support protein synthesis in both type 1 and 2 muscle fibers [Citation126–128]. Testosterone administration combined with strength training appears to be able to increase muscle fiber area by means of both hyperplasia and hypertrophy. Taken in mind that myonuclei in adult muscle fiber are not able to undergo mitosis, the increase in myonuclei number has to consider an external source, such as satellite cells and/or stem cells [Citation129]. Evidence demonstrate that testosterone may provoke a significant increase in fetal and embryonic myosin isoforms and an increase in satellite cells number even if expressed both for millimeter of muscle fiber and direct count or as percentage of myonuclear number [Citation126,Citation129].
The increased number of myonuclei by means of satellite cells activation remains even after a period of detraining and/or disuse playing a role in facilitating a rapid muscle strength recovery after a period of detraining by means of cellular memory [Citation130].
Although no evidence regarding an improvement in intrinsic contractile muscle property is present, a change in muscle architecture has been observed. In particular, a significant increase in muscle pennation and a slight decrease in muscle fiber length represent the most evident changes [Citation131–133].
Testosterone administration brings about an increase in muscle mass and a decrease in fat mass [Citation123,Citation131,Citation132,Citation134,Citation135]. Indeed, androgens are able to inhibit the commitment of multipotent adipose-tissue-derived stem cell into preadipocytes and the differentiation of subcutaneous abdominal preadipocytes into adipocytes [Citation136].
Testosterone is able to promote the commitment of pluripotent mesenchymal cells into myogenic lineage inhibiting at the same time their commitment into adipocyte lineage. Such a theory has been validated demonstrating that mesodermic pluripotent stem cells present in the muscle tissue are able to differentiate in myogenic lineage after androgen administration, explaining at cellular level the repartitioning effect of androgens on body mass [Citation137].
Adequate serum testosterone level is necessary to guarantee the physiological adaptation process induced by training [Citation138]. Indeed, muscle tissue in elderly people remain responsive to anabolic action of testosterone; such an effect may be also predictable as an increase in muscle mass is strictly related to serum testosterone level which in turn is directly correlated to testosterone dose administered [Citation131].
Finally, low serum testosterone level is associated with metabolic impairments, such as obesity, insulin resistance, metabolic syndrome, dyslipidemia, hypertension which affect negatively muscle mass and strength; otherwise, TRT is able to restore metabolic homeostasis creating a more favorable environment for increasing muscle mass and strength [Citation139]. Hypogonadism is also a risk factor for osteoporosis and bone fractures; common consequences of bone fractures are bed rest and immobilization which in turns represent severe risk factors for sarcopenia. Preventing bone fractures with administration of TRT in hypogonadal men should be accurately evaluated by clinician [Citation140].
5. Conclusions
In the pursuit of priority, we tried to summarize the enormous amount of data regarding sarcopenia in order to highlight the most important aspects of physical exercise, nutrition, and hormonal balance in order to hinder sarcopenia. Physical exercise, in particular consistent resistance/strength training, and proper nutrition, placing emphasis on adequate total protein intake and timing, are effective strategies not only to counteract directly sarcopenia but also to prevent the onset and the development of risk factors for sarcopenia, such as obesity, diabetes, chronic low grade inflammatory state, cardiovascular accidents, and hormonal deficit. In our review, we have just dealt with the effects of physical exercise and nutrition separately, however, it should be highlighted that their combination guarantees better results thanks to a synergic effect [Citation108,Citation141,Citation142]. Even in the case of overt hormonal deficiency, a proper replacement therapy is recommended not only because hormones act directly on muscle mass and performance but also because a good hormone milieu represents a requisite for adaptive process of supercompensation to training-induced stimulus.
Furthermore, preventing and treating chronic conditions, such as diabetes, obesity, hypertension, dyslipidemia, which may favor the onset of sarcopenia, should be considered by clinician. Diagnosis and therapy of osteoporosis should be carefully considered as osteoporosis itself represents a risk factor for bone fracture and, consequently for immobilization [Citation143–145]. Last but not least, it should be considered that physical exercise and nutrition exert their positive effects also on endocrine milieu increasing insulin sensibility, acute post-exercise GH, and basal sex hormones levels, in particular in overweight and obese subjects. Therefore, physical exercise, nutrition, and hormones appear to influence each other promoting a virtuous cycle.
Disclosure statement
Authors declare no conflict of interest.
References
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on Sarcopenia in older people. Age Ageing. 2010;39:412–423.
- Jones EJ, Bishop PA, Woods AK, et al. Cross-sectional area and muscular strength: a brief review. Sports Med. 2008;38:987–994.
- Snijders T, Parise G. Role of muscle stem cells in sarcopenia. Curr Opin Clin Nutr Metab Care. 2017;20:186–190.
- Faulkner JA, Davis CS, Mendias CL, et al. The aging of elite male athletes: age-related changes in performance and skeletal muscle structure and function. Clin J Sport Med. 2008;18:501–507.
- Velders M, Diel P. How sex hormones promote skeletal muscle regeneration. Sports Med. 2013;43:1089–1100.
- Xia Z, Cholewa J, Zhao Y, et al. Targeting inflammation and downstream protein metabolism in sarcopenia: a brief up-dated description of concurrent exercise and leucine-based multimodal intervention. Front Physiol. 2017;8:434.
- Everman S, Meyer C, Tran L, et al. Insulin does not stimulate muscle protein synthesis during increased plasma branched-chain amino acids alone but still decreases whole body proteolysis in humans. Am J Physiol Endocrinol Metab. 2016;311:E671–e677.
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176.
- Timmerman KL, Lee JL, Fujita S, et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59:2764–2771.
- Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8:405.
- Yoon MS. mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol. 2017;8:788.
- Galic E, Krpan D, Mirat J, et al. Diversity of bone cell activity as a histomorphometric feature of idiopathic osteoporosis in men. Aging Male. 2010;13:18–24.
- Miller LE, Pierson LM, Pierson ME, et al. Age influences anthropometric and fitness-related predictors of bone mineral in men. Aging Male. 2009;12:47–53.
- Liu M, Zhang Y, Cheng X, et al. The effect of age on the changes in bone mineral density and osteoporosis detection rates in Han Chinese men over the age of 50. Aging Male. 2014;17:166–173.
- Tungjai M, Kaewjaeng S, Jumpee C, et al. Bone mineral density at distal forearm in men over 40 years of age in Mae Chaem district, Chiang Mai Province, Thailand: a pilot study. Aging Male. 2017;20:170–174.
- Boyanov M, Bakalov D, Boneva Z. Bone mineral density in men with and without the metabolic syndrome. Aging Male. 2009;12:62–65.
- Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. 2015;12:22–26.
- Marzetti E, Calvani R, Bernabei R, et al. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty-a mini-review. Gerontology. 2012;58:99–106.
- Guescini M, Tiano L, Genova ML. The combination of physical exercise with muscle-directed antioxidants to counteract sarcopenia: a biomedical rationale for pleiotropic treatment with creatine and coenzyme Q10. Oxid Med Cell Longev. 2017;2017:7083049.
- Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc. 2003;51:323–330.
- Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332.
- Miller MS, Bedrin NG, Callahan DM, et al. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol. 2013;115:1004–1014.
- Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369.
- Bian AL, Hu HY, Rong YD, et al. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur J Med Res. 2017;22:25.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med. 2006;61:1059–1064.
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American college of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530.
- Suetta C, Hvid LG, Justesen L, et al. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–1180.
- Gianoudis J, Bailey CA, Daly RM. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos Int. 2015;26:571–579.
- Hamer M, Stamatakis E. Screen-based sedentary behavior, physical activity, and muscle strength in the English longitudinal study of ageing. PLoS One. 2013;8:e66222.
- Ziaaldini MM, Marzetti E, Picca A, et al. Biochemical pathways of Sarcopenia and their modulation by physical exercise: a narrative review. Front Med. 2017;4:167.
- Strasser B, Keinrad M, Haber P, et al. Efficacy of systematic endurance and resistance training on muscle strength and endurance performance in elderly adults–a randomized controlled trial. Wien Klin Wochenschr. 2009;121:757–764.
- Peterson MD, Rhea MR, Sen A, et al. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–237.
- Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American college of sports medicine and the American heart association. Med Sci Sports Exerc. 2007;39:1435–1445.
- Chen HT, Chung YC, Chen YJ, et al. Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J Am Geriatr Soc. 2017;65:827–832.
- Fry CS, Drummond MJ, Glynn EL, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skeletal Muscle. 2011;1:11.
- Landi F, Marzetti E, Martone AM, et al. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:25–31.
- Borde R, Hortobagyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45:1693–1720.
- Silva NL, Oliveira RB, Fleck SJ, et al. Influence of strength training variables on strength gains in adults over 55 years-old: a meta-analysis of dose-response relationships. J Sci Med Sport. 2014;17:337–344.
- Van Roie E, Delecluse C, Coudyzer W, et al. Strength training at high versus low external resistance in older adults: effects on muscle volume, muscle strength, and force-velocity characteristics. Exp Gerontol. 2013;48:1351–1361.
- Van Roie E, Walker S, Van Driessche S, et al. Training load does not affect detraining’s effect on muscle volume, muscle strength and functional capacity among older adults. Exp Gerontol. 2017;98:30–37.
- Cadore EL, Pinto RS, Bottaro M, et al. Strength and endurance training prescription in healthy and frail elderly. Aging Dis. 2014;5:183–195.
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708.
- Lim JY. Therapeutic potential of eccentric exercises for age-related muscle atrophy. Integr Med Res. 2016;5:176–181.
- Hoppeler H. Moderate load eccentric exercise; a distinct novel training modality. Front Physiol. 2016;7:483.
- Aagaard P, Suetta C, Caserotti P, et al. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64.
- Rogan S, de Bruin ED, Radlinger L, et al. Effects of whole-body vibration on proxies of muscle strength in old adults: a systematic review and meta-analysis on the role of physical capacity level. Eur Rev Aging Phys Act. 2015;12:12.
- Kemmler W, Bebenek M, Engelke K, et al. Impact of whole-body electromyostimulation on body composition in elderly women at risk for sarcopenia: the training and electro stimulation trial (TEST-III). Age (Dordr). 2014;36:395–406.
- Kern H, Barberi L, Lofler S, et al. Electrical stimulation counteracts muscle decline in seniors. Front Aging Neurosci. 2014;6:189.
- Machado A, Garcia LD, Gonzalez-Gallego J, et al. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand J Med Sci Sports. 2010;20:200–207.
- Wei N, Pang MY, Ng SS, et al. Optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of people with age-related muscle loss (sarcopenia): a randomized controlled trial. Geriatr Gerontol Int. 2016;17:1412–1420.
- Maruya K, Asakawa Y, Ishibashi H, et al. Effect of a simple and adherent home exercise program on the physical function of community dwelling adults sixty years of age and older with pre-sarcopenia or sarcopenia. J Phys Ther Sci. 2016;28:3183–3188.
- Di Luigi L, Rossi C, Sgro P, et al. Do non-steroidal anti-inflammatory drugs influence the steroid hormone milieu in male athletes? Int J Sports Med. 2007;28:809–814.
- Di Luigi L, Romanelli F, Sgro P, et al. Andrological aspects of physical exercise and sport medicine. Endocrine. 2012;42:278–284.
- Hashemi R, Motlagh AD, Heshmat R, et al. Diet and its relationship to sarcopenia in community dwelling Iranian elderly: a cross sectional study. Nutrition. 2015;31:97–104.
- Kim J, Lee Y, Kye S, et al. Association of vegetables and fruits consumption with sarcopenia in older adults: the fourth Korea national health and nutrition examination survey. Age Ageing. 2015;44:96–102.
- Hao J, Shen W, Yu G, et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J Nutr Biochem. 2010;21:634–644.
- Feng Z, Bai L, Yan J, et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: regulatory effects of hydroxytyrosol. Free Radic Biol Med. 2011;50:1437–1446.
- Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–412.
- Di Girolamo FG, Situlin R, Mazzucco S, et al. Omega-3 fatty acids and protein metabolism: enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:145–150.
- Lalia AZ, Dasari S, Robinson MM, et al. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging. 2017;9:1096–1129.
- Ticinesi A, Meschi T, Lauretani F, et al. Nutrition and inflammation in older individuals: focus on vitamin D, n-3 polyunsaturated fatty acids and whey proteins. Nutrients. 2016;8:186.
- Dardevet D, Remond D, Peyron MA, et al. Muscle wasting and resistance of muscle anabolism: the “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci World J. 2012;2012:269531.
- Cruz-Jentoft AJ. Sarcopenia: what should a pharmacist know? Farm Hosp. 2017;41:543–549.
- Genaro Pde S, Pinheiro Mde M, Szejnfeld VL, et al. Dietary protein intake in elderly women: association with muscle and bone mass. Nutr Clin Pract. 2015;30:283–289.
- Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev. 2013;71:195–208.
- Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake. Nutrients. 2016;8:359.
- Kim IY, Deutz NEP, Wolfe RR. Update on maximal anabolic response to dietary protein. Clin Nutr. 2017;17:30203.
- Borack MS, Volpi E. Efficacy and safety of leucine supplementation in the elderly. J Nutr. 2016;146:2625S–2629s.
- Leenders M, van Loon LJ. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr Rev. 2011;69:675–689.
- Khor SC, Abdul Karim N, Ngah WZ, et al. Vitamin E in sarcopenia: current evidences on its role in prevention and treatment. Oxid Med Cell Longev. 2014;2014:914853.
- Tieland M, Brouwer-Brolsma EM, Nienaber-Rousseau C, et al. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr. 2013;67:1050–1055.
- Kim MK, Baek KH, Song KH, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the fourth Korea national health and nutrition examination surveys (KNHANES IV) 2009. J Clin Endocrinol Metab. 2011;96:3250–3256.
- Alway SE, Myers MJ, Mohamed JS. Regulation of satellite cell function in sarcopenia. Front Aging Neurosci. 2014;6:246.
- Di Luigi L. Supplements and the endocrine system in athletes. Clin Sports Med. 2008;27:131–151.
- Ceglia L, Niramitmahapanya S, da Silva Morais M, et al. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98:E1927–E1935.
- Malafarina V, Uriz-Otano F, Malafarina C, et al. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas. 2017;101:42–50.
- Wilson JM, Fitschen PJ, Campbell B, et al. International society of sports nutrition position stand: beta-hydroxy-beta-methylbutyrate (HMB). J Int Soc Sports Nutr. 2013;10:6.
- Teixeira J, Chavarria D, Borges F, et al. Dietary polyphenols and mitochondrial function: role in health and disease. Curr Med Chem. 2017;24. DOI:10.2174/0929867324666170529101810
- Alway SE, McCrory JL, Kearcher K, et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J Gerontol A Biol Sci Med. 2017; 72:1595–1606.
- Louis M, Van Beneden R, Dehoux M, et al. Creatine increases IGF-I and myogenic regulatory factor mRNA in C(2)C(12) cells. FEBS Lett. 2004;557:243–247.
- Hargreaves IP. Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol. 2014;49:105–111.
- Parr MK, Botre F, Nass A, et al. Ecdysteroids: a novel class of anabolic agents? Biol Sport. 2015;32:169–173.
- Parr MK, Zhao P, Haupt O, et al. Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone. Mol Nutr Food Res. 2014;58:1861–1872.
- Ceglia L, Dawson-Hughes B, Increasing alkali supplementation decreases urinary nitrogen excretion when adjusted for same day nitrogen intake. Osteoporosis Int. 2017;28:3355–3359.
- Ceglia L, Harris SS, Abrams SA, et al. Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J Clin Endocrinol Metab. 2009;94:645–653.
- Frassetto L, Morris RC Jr, Sellmeyer DE, et al. Diet, evolution and aging–the pathophysiologic effects. Eur J Nutr. 2001;40:200–213.
- Carnauba RA, Baptistella AB, Paschoal V, et al. Diet-induced low-grade metabolic acidosis and clinical outcomes: a review. Nutrients. 2017;9:538.
- Di Luigi L, Baldari C, Pigozzi F, et al. The long-acting phosphodiesterase inhibitor tadalafil does not influence athletes’ VO2max, aerobic, and anaerobic thresholds in normoxia. Int J Sports Med. 2008;29:110–115.
- Glynn N, Agha A. Diagnosing growth hormone deficiency in adults. Int J Endocrinol. 2012;2012:972617.
- Ghigo E, Aimaretti G, Corneli G, et al. Diagnosis of adult GH deficiency. Pituitary. 2008;18:1–16.
- Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH research society in association with the European society for pediatric endocrinology, Lawson Wilkins society, European Society of endocrinology, Japan endocrine society, and endocrine society of Australia. Eur J Endocrinol. 2007;157:695–700.
- Molitch ME, Clemmons DR, Malozowski S, et al. Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609.
- Gasco V, Caputo M, Lanfranco F, et al. Management of GH treatment in adult GH deficiency. Best Pract Res Clin Endocrinol Metab. 2017;31:13–24.
- Laghi F, Adiguzel N, Tobin MJ. Endocrinological derangements in COPD. Eur Respir J. 2009;34:975–996.
- Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292:E1647–E1655.
- Appelman-Dijkstra NM, Claessen KM, Roelfsema F, et al. Long-term effects of recombinant human GH replacement in adults with GH deficiency: a systematic review. Eur J Endocrinol. 2013;169:R1–14.
- Ehrnborg C, Ellegard L, Bosaeus I, et al. Supraphysiological growth hormone: less fat, more extracellular fluid but uncertain effects on muscles in healthy, active young adults. Clin Endocrinol (Oxf). 2005;62:449–457.
- Rubeck KZ, Bertelsen S, Vestergaard P, et al. Impact of GH substitution on exercise capacity and muscle strength in GH-deficient adults: a meta-analysis of blinded, placebo-controlled trials. Clin Endocrinol (Oxf). 2009;71:860–866.
- Widdowson WM, Gibney J. The effect of growth hormone (GH) replacement on muscle strength in patients with GH-deficiency: a meta-analysis. Clin Endocrinol (Oxf). 2010;72:787–792.
- Samaras N, Samaras D, Frangos E, et al. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuvenation Res. 2013;16:285–294.
- Corona G, Rastrelli G, Giagulli VA, et al. Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebo-controlled trials. J Clin Endocrinol Metab. 2013;98:3615–3626.
- Ceci R, Duranti G, Rossi A, et al. Skeletal muscle differentiation: role of dehydroepiandrosterone sulfate. Horm Metab Res. 2011;43:702–707.
- Morales AJ, Haubrich RH, Hwang JY, et al. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol. 1998;49:421–432.
- Rutkowski K, Sowa P, Rutkowska-Talipska J, et al. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs. 2014;74:1195–1207.
- Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291:E1003–E1008.
- Brown GA, Vukovich MD, Sharp RL, et al. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. J Appl Physiol. 1999;87:2274–2283.
- Brown GA, Vukovich MD, Reifenrath TA, et al. Effects of anabolic precursors on serum testosterone concentrations and adaptations to resistance training in young men. Int J Sport Nutr Exerc Metab. 2000;10:340–359.
- Timmerman KL, Dhanani S, Glynn EL, et al. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr. 2012;95:1403–1412.
- Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000;85:69–79.
- Fujita S, Rasmussen BB, Cadenas JG, et al. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–E754.
- Abdulla H, Smith K, Atherton PJ, et al. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016;59:44–55.
- Kwon SS, Lee SG, Lee YH, et al. Homeostasis model assessment of insulin resistance in a general adult population in Korea: additive association of sarcopenia and obesity with insulin resistance. Clin Endocrinol. 2017;86:44–51.
- Kim TN, Park MS, Lim KI, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean sarcopenic obesity study. Clin Endocrinol (Oxf). 2013;78:525–532.
- Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229:R67–R81.
- Sabatini S, Sgro P, Duranti G, et al. Tadalafil alters energy metabolism in C2C12 skeletal muscle cells. Acta Biochimica Polonica. 2011;58:237–241.
- Westerbacka J, Bergholm R, Tiikkainen M, et al. Glargine and regular human insulin similarly acutely enhance endothelium-dependent vasodilatation in normal subjects. Arterioscler Thromb Vasc Biol. 2004;24:320–324.
- Tanaka K, Kanazawa I, Sugimoto T. Reduction in endogenous insulin secretion is a risk factor of sarcopenia in men with type 2 diabetes mellitus. Calcif Tissue Int. 2015;97:385–390.
- Barzilai N, Huffman DM, Muzumdar RH, et al. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322.
- Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am. 2013;42:333–347.
- Tajar A, Huhtaniemi IT, O’Neill TW, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European male aging study (EMAS). J Clin Endocrinol Metab. 2012;97:1508–1516.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15.
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202.
- Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688.
- Layton JB, Meier CR, Sharpless JL, et al. Comparative safety of testosterone dosage forms. JAMA Intern Med. 2015;175:1187–1196.
- Baldari C, Di Luigi L, Emerenziani GP, et al. Is explosive performance influenced by androgen concentrations in young male soccer players? Br J Sports Med. 2009;43:191–194.
- Kadi F, Eriksson A, Holmner S, et al. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31:1528–1534.
- Kadi F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol. 2008;154:522–528.
- Sinha-Hikim I, Artaza J, Woodhouse L, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–E164.
- Eriksson A, Kadi F, Malm C, et al. Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem Cell Biol. 2005;124:167–175.
- Egner IM, Bruusgaard JC, Eftestol E, et al. A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids. J Physiol. 2013;591:6221–6230.
- Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181.
- Storer TW, Magliano L, Woodhouse L, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485.
- Blazevich AJ, Giorgi A. Effect of testosterone administration and weight training on muscle architecture. Med Sci Sports Exerc. 2001;33:1688–1693.
- Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5α-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012;307:931–939.
- Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7.
- Sinha-Hikim I, Roth SM, Lee MI, et al. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–E205.
- Singh R, Artaza JN, Taylor WE, et al. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088.
- Kvorning T, Andersen M, Brixen K, et al. Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo-controlled, and blinded intervention study. Am J Physiol Endocrinol Metab. 2006;291:E1325–E1332.
- Choong K, Basaria S. Emerging cardiometabolic complications of androgen deprivation therapy. Aging Male. 2010;13:1–9.
- Basurto L, Zarate A, Gomez R, et al. Effect of testosterone therapy on lumbar spine and hip mineral density in elderly men. Aging Male. 2008;11:140–145.
- Kim HK, Suzuki T, Saito K, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23.
- Martone AM, Marzetti E. Exercise and protein intake: a synergistic approach against Sarcopenia. BioMed Res Int. 2017;2017:2672435.
- Martin-Fernandez M, Martinez E, Diaz-Curiel M, et al. Effects of PTH (1–84) on bone quality in a validated model of osteoporosis due to androgenic deprivation. Aging Male. 2014;17:42–50.
- Montero M, Serfati D, Luna S, et al. The effectiveness of intermittent rat parathyroid hormone (1–34) treatment on low bone mass due to oestrogen or androgen depletion in skeletally mature rats. Aging Male. 2010;13:59–73.
- Shigehara K, Konaka H, Koh E, et al. Effects of testosterone replacement therapy on hypogonadal men with osteopenia or osteoporosis: a subanalysis of a prospective randomized controlled study in Japan (EARTH study). Aging Male. 2017;20:139–145.