Abstract
Objective: This study set out to assess effects of testosterone replacement therapy (TRT) on parameters of metabolic syndrome and vascular function in obese hypogonadal males with type 2 diabetes mellitus (DM2).
Study design: Fifty-five obese hypogonadal diabetic males on oral hypoglycemic treatment were enrolled into this one-year, double-blind, randomized, placebo-controlled clinical study. Group T (n = 28) was treated with testosterone undecanoate (1000 mg i.m. every 10 weeks) while group P (n = 27) received placebo.
Methods: Anthropometrical and vascular measurements – flow-mediated dilatation (FMD) and intima media thickness (IMT) – biochemical and hormonal blood sample analyses were performed at the start of the study and after one year. Derived parameters (BMI, HOMA-IR, calculated free testosterone (cFT) and bioavailable testosterone (BT)) were calculated.
Results: TRT resulted in reduction of HOMA-IR by 4.64 ± 4.25 (p < .001), HbA1c by 0.94 ± 0.88% points (p < .001), and an increase in FMD by 2.40 ± 4.16% points (p = .005).
Conclusion: TRT normalized serum testosterone levels, improved glycemic control and endothelial function while exerting no ill effects on the study population.
Introduction
Approximately, 50% of older (age >40 years) male patients with type 2 diabetes mellitus (DM2) have decreased testosterone (T) levels, which is much higher prevalence than in healthy population [Citation1]. T plays an important role in maintaining glycemic control [Citation2]. DM2 and insulin resistance (IR) reduce T biosynthesis, and vice versa, decreased T level increases IR and the occurrence of DM2 [Citation3,Citation4]. Low T is also associated with obesity, dyslipidemia, and hypertension, all of which increase the risk of cardiovascular disease (CVD) [Citation5].
Multiple studies have shown that testosterone replacement therapy (TRT) in obese hypogonadal diabetic male patients reduces plasma glucose, HbA1c, cholesterol, IR, inflammation, NAFLD, ameliorates osteoporosis and symptoms of hypogonadism and improves vascular function and morphology [Citation2,Citation6–9]. Other studies were unable to prove these effects or have shown opposite effects [Citation10–12].
Several studies on carotid artery atherosclerosis have demonstrated an inverse relationship between level of T and degree of atherogenesis [Citation8,Citation10]. Increased intima media thickness (IMT) is an early – potentially reversible – morphological marker of atherogenesis. Observational studies have demonstrated that TRT reduces the intima media thickening regardless of body mass index (BMI), increases coronary artery diameter and the rate of blood flow, and improves time to cardiac ischemia in men with coronary heart disease [Citation13]. Cross-sectional studies have shown the association of low T levels and endothelial dysfunction. Endothelial dysfunction is both an early functional marker of vascular disease and a facilitative process in the development of atherosclerosis [Citation14]. Androgen receptors (AR) have been localized in endothelial and vascular smooth muscle cells [Citation15]. It has been shown that AR expression is lower in elderly males with DM2 [Citation16]. Laboratory and clinical studies have found that T at normal concentrations acts as a direct vasodilator, independent of activity on AR [Citation17]. A prospective study has shown that TRT improves endothelial function [Citation18]. On the other hand, a double-blind placebo controlled study of three year duration could not show any changes in IMT or coronary artery calcification between testosterone gel and placebo-treated groups [Citation11].
Data on effects of TRT on morbidity and mortality are discordant. Some epidemiological studies have reported an increase in cardiovascular mortality related to TRT while others have not been able to establish a correlation between the two [Citation19,Citation20]. The Veterans study showed a significantly reduced mortality (by half) in men with low T who received TRT, compared to similar men who did not [Citation21,Citation22].
It is also well documented that TRT has beneficial effect on sexual function and quality of life [Citation23–25].
The main objective of this study was to shed more light upon the ubiquitous conundrum: “should TRT be used to treat hypogonadal DM2 patients?” and: “do benefits of TRT outweigh the risks associated with TRT?” We investigated the effects of TRT on IR, visceral obesity, glycemic control, lipid abnormalities, vascular function, and morphology in obese hypogonadal men with DM2.
Subjects and methods
Study population
This was a one-year, randomized, double-blind, placebo-controlled study of 55 obese DM2 male patients not treated with insulin, with confirmed untreated late-onset hypogonadism (LOH) who were registered at the Diabetic outpatient clinic of the General hospital Celje. LOH was defined as the presence of three sexual symptoms – decreased sexual interest, absent or rare morning erections and erectile dysfunction – in conjunction with total testosterone (TT) level <11 nmol/l and/or free testosterone (FT) level <220 pmol/l [Citation26].
Additional inclusion criteria were as follows: men aged >35 years, body mass index (BMI) ≥ 30 kg/m2, treated with oral antidiabetic medications.
Exclusion criteria were as follows: previously treated hypogonadism, insulin therapy, a history of current prostate or breast cancer, severe benign prostatic hyperplasia or elevated prostate-specific antigen (PSA >4.0 μg/l), severe heart failure, acute coronary event or procedure during the six months leading up to the study, chronic obstructive lung disease, hypothyroidism, severe obstructive sleep apnea (OSA), and active infection.
Study design and protocol
Inclusion and exclusion criteria were reviewed at the screening visit, where patients underwent clinical history and physical examination. We performed baseline clinical examination, anthropometric measurements, and took blood samples for biochemical tests.
Subjects were then randomized into two groups to receive either placebo (group P) or testosterone in the form of testosterone undecanoate (TU Nebido 1000 mg; Bayer AG) (group T) during the year of the study following the protocol: first injection of TU/placebo was administered at the first visit, second 6 weeks later (visit 2), and remaining injections each 10 weeks after the previous injection. Safety investigations – determination of hematocrit (Hct) and PSA – were performed every three months. Patients were not advised to introduce any dietary or other lifestyle changes during the study.
The cardiologist performed functional and morphological investigations at the baseline visit (visit 1) and after 12 months (visit 7) in order to evaluate endothelial function and early morphological atherosclerotic changes: ultrasound testing for FMD of the brachial artery and IMT measurement of carotid arteries.
After 12 months, all subjects were re-evaluated with a clinical history and examination, followed by biochemical and hormonal assessment and vascular measurements.
This study conformed to the principles outlined in the Declaration of Helsinki and was approved by the National ethical committee. Written informed consent was obtained from all subjects prior to their participation in the study.
Measurements
Fasting blood samples were taken between 7:00 and 11:00 a.m. to measure serum total T (TT), estradiol (E2), sex hormone binding globulin (SHBG), luteinizing hormone (LH), follicle-stimulating hormone (FSH), fasting plasma glucose (FPG), glycated hemoglobin A1c (HbA1c), fasting insulin, lipid profile (total cholesterol, HDL, LDL cholesterol, triglycerides), and routine blood tests (complete blood count, electrolytes, urea, creatinine, liver tests), prostate specific antigen (PSA), serum albumin.
BMI was calculated as weight in kilograms divided by square of height in meters. Patients’ waist circumference (WC) was measured.
The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated as:
Calculated free testosterone (cFT) and bioavailable testosterone (BT) levels were calculated from SHBG, serum albumin, and TT levels using the method of Vermeulen [Citation27].
Use of any oral hypoglycemic medication was permitted and patients continued on the same medication throughout the study without dose adjustments.
Methods
Ultrasonography of carotid arteries
All subjects underwent ultrasonography for evaluation of IMT and plaques. The thickening of the inner layers of the artery (intima and media) is the first perceptible and measurable morphological change in the course of atherosclerogenesis, which develops more rapidly in DM2 patients than it does in other people [Citation28]. Ultrasound examination of the carotid arteries was performed using the General Electric Logiq S7 Expert/Pro ultrasonic device, with 9L-D probe, the frequency band 3.1–10 MHz in B mode. IMT was measured on three sections of both carotid arteries: communis, internal and bulbus. At each investigated section, IMT measurements were performed three times and the average value for each section was subsequently calculated.
Assessment of endothelial function
Endothelial function was studied with brachial artery flow-mediated dilatation (FMD) and endothelium-independent nitroglycerine-mediated dilatation (NMD) according to the method established by Celermajer [Citation29]. After scanning the baseline artery diameter, the sphygmomanometer cuff was inflated to 50 mmHg above systolic blood pressure and kept for 5 min. Brachial artery diameter was measured again during reactive hyperemia one minute after cuff deflation. FMD (%) was defined as the percentage change of the artery diameter following reactive hyperemia relative to the baseline diameter.
NMD-induced dilatation was provoked by sublingual administration of 400 mg of glycerol-trinitrate (GTN), which acts as a nitric oxide (NO) donor. NMD (%) was expressed as the percentage change in the diameter after GTN administration relative to the baseline scan. All measurements were carried out by the same investigator.
Statistical methods
All values are expressed as mean ± SD in their respective units when normally distributed. Distribution of variables was checked using Shapiro–Wilk test. Changes between values are expressed in their respective units or percentage points (where applicable).
Independent samples t-test was used to compare inter-group differences, either the group means at specific time point or mean differences between consecutive time points. Paired samples t-test was used to compare intra-group differences between time points T0 and T1. The choice of test (Student or Welch) was governed by the result of Levene’s test of variance.
Pearson’s correlation was used to establish correlation direction and magnitude between TT level and other variables.
p values below .05 were considered statistically significant. Statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, IL).
Results
Fifty-five hypogonadal men, aged 40–70 years (mean 60.15 ± 7.23 years) with DM2 were included in this study. Patients in group P received placebo and group T received TU.
The baseline characteristics of placebo and testosterone group were not statistically different, the sole exception being IMT. Mean BMI of study population was 33.34 ± 4.07 kg/m2, and mean WC was 116.56 ± 4.97 cm. Mean TT level was 7.59 ± 1.71 nmol/l. All patients had lipid abnormalities, twenty-nine of them were receiving lipid-lowering medications throughout the study. Forty-nine patients had arterial hypertension and were receiving antihypertensives.
Effects of TRT on glycemic control
Changes in glycemic control are shown in and .
Figure 1. HbA1c values in obese hypogonadal DM2 patients before and after one year of TRT or placebo (intra- and inter-group differences and corresponding p values shown in ); HbA1c: glycated hemoglobin A1c (%), P: placebo group, T: testosterone group, time point T0: before and T1: after one year of study.
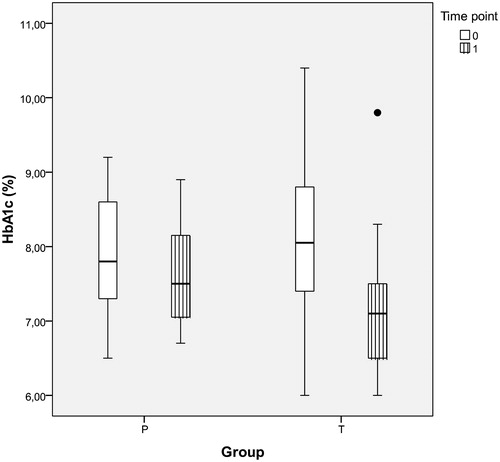
Table 1. Anthropometric and metabolic parameters in obese hypogonadal DM2 patients before in after one year of TRT or placebo.
One-year treatment effect of TRT on HbA1c (group T) was a reduction of mean HbA1c by 0.94 ± 0.88% points (p < .001). The mean difference in group P, while statistically significant, was mere 0.24% points (p = .004). Mean difference in HbA1c reduction between group T and group P was 0.70% points (p < .001) in favor of group T. Correlation between the levels of TT and HbA1c was moderately negative (r = −0.297, p = .028). Correlation between the mean changes of levels of TT and HbA1c from T0 to T1 was negative (r = −0.427, p = .001).
Both groups had elevated FPG levels at the beginning of the study. FPG decreased by 1.23 ± 1.25 mmol/l (p < .001) in group T by the end of the study. There was no change in mean FPG level in group P.
Effects of TRT on components of the metabolic syndrome and insulin sensitivity
HOMA-IR results are presented in and . The HOMA-IR decreased significantly in group T by 4.64 ± 4.25 (p < .001). Correlation between the level of TT and HOMA-IR was negative (r = −0.404, p = .002), and the correlation between mean changes of TT and HOMA-IR over the course of this study was also negative (r = −0.446, p = .001).
Figure 2. HOMA-IR in obese hypogonadal DM2 patients before and after one year of TRT or placebo (intra- and inter-group differences and corresponding p values shown in ); HOMA-IR (homeostasis model assessment insulin resistance index), P: placebo group, T: testosterone group, time point T0: before and T1: after one year of study.
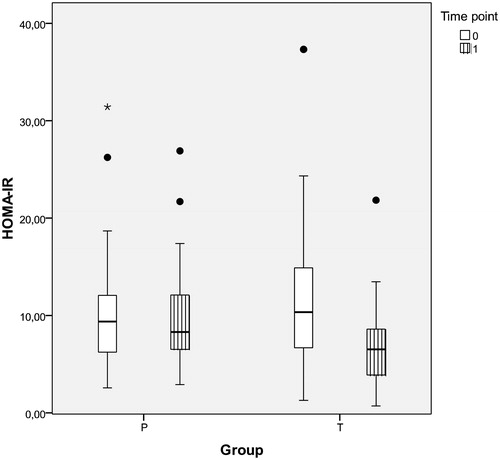
Plasma insulin level mean decreased by 8.52 ± 7.76 mE/l (p < .001) in group T after one year of TRT.
BMI and WC showed statistically significant reductions in both groups after one year of the study. No statistically significant difference between the two groups or the change of their respective mean anthropometric parameters could be shown.
No statistically significant changes were observed in either systolic or diastolic blood pressure levels following TRT.
There was a statistically significant decrease in mean total cholesterol levels in both groups (); reduction by 0.70 ± 0.57 mmol/l (p < .001) in group T and by 0.42 ± 0.78 mmol/l (p = .010) in group P.
No statistically significant changes were observed in levels of LDL-, HDL-cholesterol and TG.
Testosterone levels
TT, cFT, and calculated BT concentrations increased significantly in group T. TT level increased from 7.24 ± 1.97 nmol/l to 17.04 ± 3.07 nmol/l (p < .001) after one year of TRT (), pushing all group T patients well into eugonadal range. There was a small but statistically significant change in TT level (from 7.96 ± 1.34 nmol/l to 9.83 ± 1.51 nmol/l, p < .001) in group P as well.
Table 2. Testosterone levels in obese hypogonadal DM2 patients before and after one year of TRT or placebo.
No statistically significant change in SHBG levels has been observed in either group throughout the one year course of this study.
Vascular studies
The effects of TRT on FMD and IMT are presented in and . FMD improved by 2.40 ± 4.16% points (p = .005) in group T after one year of testosterone treatment. There was no change in FMD observed in group P. Correlation between TT and FMD was positive (r = 0.349, p = .009). Correlation between mean changes of TT and FMD was likewise statistically significant and positive (r = 0.267, p = .049).
Figure 3. FMD in obese hypogonadal DM2 patients before and after one year of TRT or placebo (intra- and inter-group differences and corresponding p values shown in ); FMD (flow mediated dilatation, %), P: placebo group, T: testosterone group, time point T0: before and T1: after one year of study.
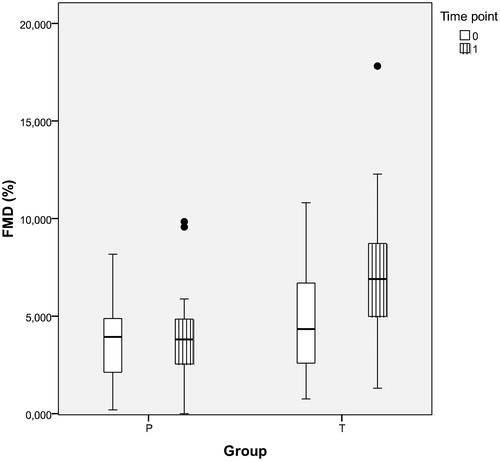
Table 3. FMD and IMT results in obese hypogonadal DM2 patients before and after one year of TRT or placebo.
No statistically significant differences in NMD have been observed in either group at either time point, as expected.
IMT has decreased at statistically significant levels in both groups after one year (), however, the decrease observed in group T (by 0.10 ± 0.06 mm, p < .001) was twice that of group P (decrease by 0.05 ± 0.09 mm, p = .006).
Safety investigations
Throughout one year of the study levels of hemoglobin (Hb), red blood cell (RBC) counts and hematocrit (Hct) increased, but Hct values never exceeded the upper safety limit of 52%. The mean Hct has increased by 2.14 ± 2.42% points (p < .001) in group T. As expected, there were no Hct changes observed in group P.
Levels of PSA increased by 0.22 ± 0.39 μg/l (p = .006) in group T. No PSA increase was noted in group P. One group T study participant was enrolled with pre-existing (diagnosed) benign prostate hyperplasia and was under permanent supervision of a urologist; his basal PSA level was 4.0 μg/l, and did not change.
No adverse health effects related to TRT have been observed in study population throughout the one year of our study.
Discussion
This one-year double-blind placebo controlled study investigated the effect of TRT on IR, visceral obesity, glycemic control, lipid abnormalities, vascular function, and morphology in obese hypogonadal men with DM2.
We have shown that one year of TRT improved insulin sensitivity and glycemic control and reversed endothelial dysfunction in obese hypogonadal men with DM2.
In several studies, an intervention with T reduced body weight, BMI, WC, and fat mass in obese men [Citation30–32] and vice versa, T levels increased in men who succeeded in losing weight, BMI, and visceral fat mass either by dieting, physical activity, or bariatric surgery [Citation3]. Prospective observational studies of hypogonadal subjects receiving long-acting TU showed almost 5% decrease in body weight in the first year of treatment and greater than 13% decrease after 5 years [Citation33,Citation34]. Testosterone invariably increases lean body mass, an effect that is not achieved by any anti-obesity or antidiabetic medication [Citation35].
On the other hand, Hoyos et al. [Citation36] failed to show any effect of TRT (using testosterone undecanoate) during 18 weeks, on overall weight or the number of participants with metabolic syndrome but observed increased insulin sensitivity, reduced liver fat, and decreased arterial stiffness.
Our results show significant reduction in BMI, WC, and body weight in both placebo and TRT groups after one year. Reduction in WC, which is a practical indicator of visceral adiposity, implies that weight loss was predominantly from the visceral fat. Even though our study population was not given any new instructions regarding diet or lifestyle, we speculate that there was a general positive effect of participating in the study, predominantly psychological one, that lead to improvement of antropometric parameters and consequently to slight increase in T levels in P group.
It is known that TRT improves IR at multiple levels through reduction of visceral adiposity. AR numbers are greater in visceral adipocytes than in subcutaneous adipose tissue [Citation37]. T inhibits lipoprotein lipase activity and thus reduces triglyceride uptake into adipocytes [Citation38]. An anti-inflammatory effect of TRT has also been demonstrated, showing that T reduces inflammatory cytokines (TNF-α, IL-1b) and increases anti-inflammatory cytokine IL-10 [Citation39]. Several studies of direct effects of T on cultured adipocytes and adipose tissue suggest that TRT might decrease adipogenesis [Citation40,Citation41].
A large, multi-centric, randomized, double-blind, placebo-controlled TIMES2 study (Testosterone Replacement in Men with Metabolic Syndrome or Type 2 Diabetes), carried out in eight European countries, including 220 hypogonadal men with DM2, showed that IR (measured with HOMA-IR) decreased significantly over the course of 12 months. In addition to IR reduction, the proportion of body fat, total cholesterol, LDL-cholesterol, and HbA1c also decreased.
Similarly, TU-treated men showed significant reduction of HOMA-IR and FPG in comparison with placebo group in our study. This was corroborated by the negative correlation between TT and HOMA-IR. Our findings are in accordance with several studies, that demonstrated a significant reduction of IR in hypogonadal men, with or without DM2, following TRT [Citation2,Citation42,Citation43]. Corona et al. showed that throughout the course of TRT there was a significant effect of T on fasting glycemia and HOMA-IR, as well as body composition, increase in lean mass in particular, which may contribute to the improved metabolism. TRT was associated with a reduction of FPG and IR. Increased muscle mass is responsible for more favorable glucose metabolism associated with T [Citation44].
Testosterone plays an important role in maintaining glycemic control. DM2 and IR reduce testosterone biosynthesis and vice versa, decreased T levels increase IR and the occurrence of DM2 [Citation45]. Many observational studies have shown a negative correlation between T and IR in men [Citation3,Citation46]. A strong indication of this correlation is an increase in IR in patients with prostate cancer treated with androgen deprivation therapy (ADT). ADT also worsens glycemic control in men with DM2 or increases the risk of development of DM2 [Citation47,Citation48] and cardiovascular disease [Citation49].
In hypogonadal men, the effects of TRT on glycemic control are somewhat diverse. Some studies replacing T in hypogonadal men with DM2 found no effect on glycemic control [Citation50–52] while others found that TRT improved FPG and/or HbA1c [Citation6,Citation7,Citation39]. No improvement in glucose levels was observed in trials using oral T preparations; however, the use of transdermal and parenteral preparations significantly improved fasting glycemia [Citation53]. The advantage of using i.m. TU is full control over administration of T, thus ensuring better adherence to the therapy than in studies using oral or transdermal T preparations [Citation54].
It is well known that a reduction in IR in DM2 results in an improvement of glycemic control. Our patients presented a statistically significant reduction in FPG and HbA1c after one year of TRT, correlation coefficient again indicating negative and moderate relation between the levels of TT and HbA1c. Results of this study indicate that TRT improves IR, which ameliorates FPG and HbA1c levels.
Little or no effects of TRT observed by some studies may in part be due to short treatment duration. Diagnosis of hypogonadism allows only a short placebo-controlled study design because of the unpleasant signs and symptoms of the disease [Citation52].
Available data regarding the impact of TRT on lipid profiles are inconsistent. Some studies have shown a reduction of HDL- and an increase of LDL-cholesterol levels [Citation55], others an increase in HDL and a reduction of LDL levels [Citation56], often accompanied by a reduction in total cholesterol [Citation57]. Allan et al. [Citation31] have not noticed any significant changes in lipid profile after one year of TRT.
Our study has shown a significant decrease in mean total cholesterol levels in both groups after one year, which can – at least in part – be attributed to the use of lipid lowering therapy by a sizeable proportion of our study population. However, the magnitude of the change is greater in TRT group. No statistically significant changes have been observed in LDL-, HDL-cholesterol, and triglyceride levels.
There is no evidence that TRT has significant effects on blood pressure either from meta-analysis or single clinical trials [Citation58]. No statistically significant changes were observed in either systolic or diastolic blood pressure in our patients following TRT.
TT, cFT, and calculated BT concentrations in group T (TU group) have increased significantly after first year of our study. TT increased from 7.24 ± 1.97 nmol/l at baseline to 17.04 ± 3.07 nmol/l (p < .001) as the result of TRT. Saad et al. also used testosterone undecanoate in their one year study and observed a TT increase to a lesser degree – from 7.6 ± 2.1 nmol/l to 13.2 ± 3.5 nmol/l [Citation58]. The BLAST study has also shown similar increase in TT to our study [Citation59].
Serum SHBG levels did not change significantly in our study after one year of TRT. One could reasonably expect an increase in SHBG since BMI and HOMA-IR are in negative correlation with hepatic synthesis of SHBG [Citation60]. The explanation for this could be that any increase in SHBG due to improvements in IR may have been offset by the increase in circulating free testosterone, as number of SHBG binding sites for testosterone is reduced [Citation61].
Similarly, the Moscow study by Kalinchenko et al. [Citation9] has not shown any significant change of SHBG in 184 hypogonadal men, despite increases in plasma total (TT) and free testosterone. An increase of SHBG had been expected because features of metabolic syndrome had improved. It is well known that decreased SHBG is linked to IR, obesity, metabolic syndrome and DM2 [Citation62–64].
We showed that one year TRT has improved endothelial function (assessed by FMD) in obese hypogonadal men with DM2 implying that TRT might attenuate atherosclerotic process. Significant increase in FMD/NMD ratio indicates that brachial artery dilation improved due to amelioration of endothelial function rather than of improvement of vascular smooth muscle function of the arterial wall.
Endothelial dysfunction is both an early functional marker of vascular disease and a facilitative process in the development of atherosclerosis [Citation65]. Some authors stated that endothelial dysfunction could be considered an early marker for silent coronary artery disease [Citation66]. Other studies investigating the relationship between T levels and FMD has shown varying results [Citation11,Citation14,Citation16,Citation56]. Studies using testosterone enanthate [Citation10,Citation12] in young hypogonadal men without metabolic syndrome or diabetes did not observe any improvements in FMD.
Above mentioned variability in studies’ findings can be attributed to differences in study design, duration of treatment, dosage, use of different formulations of T, and the scale of T level change from baseline to the level reached in each respective study.
Our findings are in accordance with Mazo et al. [Citation67], who showed improvement of FMD after 6 months of TRT using testosterone undecanoate.
The improvement of endothelial function following TRT could be due to direct effects of testosterone. Androgen receptors are present on the vessel wall [Citation68] in endothelial and vascular smooth muscle cells [Citation69]. TRT stimulates activity of endothelial progenitor cells (EPCs) [Citation70]. Hypogonadal men had depletion in ex-vivo-determined circulating EPCs, which were subsequently restored by pharmacological treatment with T. T moderates regulation of vascular tone by increasing vasodilatation in coronary arteries of patients with coronary artery disease [Citation71], implying its beneficial effect on cardiovascular system. Hypogonadal men exhibit increased levels of endothelin-1, a major vasoconstrictor. TRT has been proven to reduce the levels of endothelin-1 [Citation72].
IMT of peripheral arteries, carotid arteries in particular, is used as a preclinical morphological marker of atherosclerosis [Citation73]. The reduced level of T is associated with increased carotid artery IMT independently of the presence of other cardiovascular risk factors. TRT has been shown to reduce the IMT regardless of BMI [Citation74], while some studies have investigated effect of TRT on IMT with varying results [Citation33,Citation43]. A pilot study in severely obese men has shown obvious improvements in IMT, endothelial function and metabolic parameters in treated patients following 54 weeks of TRT using TU [Citation33]. Similarly, Aversa et al. have shown marked improvements in IMT after one year of TRT using TU [Citation43]. On the other hand, Basaria et al. could not show any changes in early markers of atherogenesis in a double-blind, placebo-controlled, three-year trial [Citation11].
Our data show that IMT has improved at statistically significant levels in both groups after 12 months of TRT. The improvement in group T (TRT) was twice that of group P (placebo). Results indicate that other factors were also affecting IMT in addition to TRT, lipid-lowering medication (HMG-CoA reductase inhibitors) being the most likely culprit along with overall weight loss and improvement in glycemic control.
Concerns have been raised regarding potential increase in the risk of cardiovascular events (CV) in men with heart disease when treated with TRT – myocardial infarction in particular [Citation19,Citation71]. Very high risk of CV events due to TRT was implied in the controversial Vigen study [Citation19], however, as Morgentaler [Citation75] and others have noted, the odds ratio for CV events as a result of TRT has been severely exaggerated, the raw data revealing diametrically opposing figure – “a lower percentage of CV adverse events in the T-treated group compared to untreated men”. Some other epidemiological trials showed a significantly reduced mortality (by half) in men with low T who received TRT as compared to similar men who did not [Citation21,Citation22].
Observational study The RHYME (The Registry of Hypogonadism in Men), carried out in six European countries in men with hypogonadism over the period of two years has shown that the rates of CV were within the expected range, with no evidence of an increased risk in patients treated with TRT compared to those not receiving the treatment [Citation76].
Conclusion
Our study on obese hypogonadal male patients with DM2 has shown that TRT can exert clinical benefits on glycemic control, IR, and endothelial function.
According to the Endocrine Society Clinical Practice Guidelines [Citation77], successful weight loss combined with optimization of glycemic control is the first-line approach for normalization of circulating T levels in majority of obese men with DM2. On the other hand, the adherence to lifestyle changes in these patients is poor, making this approach rather unsuccessful. However, whether the weight loss without the accompanying TRT – even if successful – can fully reverse the hypothalamic-pituitary-testicular (HPT) axis impairment is currently not known.
In selective cases, especially when obese patients with DM2 present symptoms of hypogonadism combined with low T levels TRT can be useful.
When TRT is being considered, it is important that diagnostic criteria for hypogonadism are confirmed, contraindications excluded, and that treatment process is monitored according to the guidelines [Citation77].
Acknowledgments
Bayer Pharma AG (Berlin, Germany) provided testosterone and placebo without influencing the study protocol.
No other potential conflicts of interest relevant to this study exist. The funder played no role in study design, data analysis, or in writing of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–1840.
- Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906.
- Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–2353.
- Dandona P, Dhindsa S, Chaudhuri A, et al. Hypogonadotrophic hypogonadism in type 2 diabetes, obesity and the metabolic syndrome. Curr Mol Med. 2008;8:816–828.
- Dandona P, Dhindsa S, Chandel A, et al. Hypogonadotropic hypogonadism in men with type 2 diabetes. Postgrad Med. 2009;121:45–51.
- Jones TH. Effects of testosterone on type 2 diabetes and components of the metabolic syndrome. J Diabetes. 2010;2:146–156.
- Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care. 2011;34:828–837.
- Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7.
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double‐blinded placebo‐controlled Moscow study. Clin Endocrinol (Oxf). 2010;73:602–612.
- Zitzmann M, Brune M, Nieschlag E. Vascular reactivity in hypogonadal men is reduced by androgen substitution. J Clin Endocrinol Metab. 2002;87:5030–5037.
- Basaria S, Harman S, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314:570–581.
- Bernini G, Versari D, Moretti A, et al. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab. 2006;91:1691–1697.
- Malkin CJ, Pugh PJ, Morris PD, et al. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598.
- Zitzmann M, Vorona E, Wenk M, et al. Testosterone administration decreases carotid artery intima media thickness as a marker of impaired vascular integrity in middle-aged overweight men. J Mens Health. 2009;6:243.
- Cao J, Li J, Hao W, et al. Correlation of sex hormone and androgen receptor with diabetes mellitus in elderly men. Aging Male. 2011;14:162–167.
- Jones RD, Pugh PJ, Jones TH, et al. The vasodilatory action of testosterone: a potassium-channel opening or a calcium antagonistic action? Br J Pharmacol. 2003;138:733–744.
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029.
- Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013;310:1829–1836.
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. Gong Y, editor. PLoS One. 2014;9:e85805.
- Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058.
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733.
- Panach-Navarrete J, Martínez-Jabaloyas JM. The influence of comorbidities on the aging males’ symptoms scale in patients with erectile dysfunction. Aging Male. 2017;20:146–152.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19:64–69.
- Almehmadi Y, Yassin D-J, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194.
- Tajar A, Huhtaniemi IT, O’Neill TW, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European male aging study (EMAS). J Clin Endocrinol Metab. 2012;97:1508–1516.
- de Ronde W, van der Schouw YT, Pols HAP, et al. Calculation of bioavailable and free testosterone in men: a comparison of 5 published algorithms. Clin Chem. 2006;52:1777.
- Irie Y, Katakami N, Kaneto H, et al. Maximum carotid intima-media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis. 2012;221:438–444.
- Raitakari OT, Celermajer DS. Flow-mediated dilatation. Br J Clin Pharmacol. 2000;50:397–404.
- Svartberg J, Agledahl I, Figenschau Y, et al. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20:378.
- Allan CA, Strauss BJG, Burger HG, et al. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93:139–146.
- Salman M, Yassin D-J, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20:45–48.
- Francomano D, Bruzziches R, Barbaro G, et al. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Investig. 2014;37:401–411.
- Hoyos CM, Yee BJ, Phillips CL, et al. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531–541.
- Yassin A, Almehmadi Y, Saad F, et al. The Author's Reply: Changing testosterone had no direct effect on HbA1c or weight in diabetic men when TRT was interrupted and then resumed. Clin Endocrinol (Oxf). 2016;85:500–501.
- Hoyos Camilla M, Killick R, Yee Brendon J, et al. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo‐controlled trial. Clin Endocrinol (Oxf). 2012;77:599–607.
- O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277–284.
- Mårin P, Lönn L, Andersson B, et al. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab. 1996;81:1018–1022.
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318.
- Quarta C, Mazza R, Pasquali R, et al. Role of sex hormones in modulation of brown adipose tissue activity. J Mol Endocrinol. 2012;49:R1–R7.
- Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108:272–280.
- Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669–1675.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–3503.
- Corona G, Giagulli VA, Maseroli E, et al. Therapy of endocrine disease: Testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–R116.
- Morton A. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1903–1903.
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75.
- Wang H, Sun X, Zhao L, et al. Androgen deprivation therapy is associated with diabetes: evidence from meta‐analysis. J Diabetes Investig. 2016;7:629–636.
- Basaria S, Muller DC, Carducci MA, et al. Relation between duration of androgen deprivation therapy and degree of insulin resistance in men with prostate cancer. Arch Intern Med. 2007;167:612–613.
- Choong K, Basaria S. Emerging cardiometabolic complications of androgen deprivation therapy. Aging Male. 2010;13:1–9.
- Basu R, Man CD, Campioni M, et al. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care. 2007;30:1972.
- Lee C-H, Kuo S-W, Hung Y-J, et al. The effect of testosterone supplement on insulin sensitivity, glucose effectiveness, and acute insulin response after glucose load in male type 2 diabetics. Endocr Res. 2005;31:139–148.
- Corona G, Giagulli VA, Maseroli E, et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Investig. 2016;39:967–981.
- Saad F, Gooren L, Haider A, et al. An exploratory study of the effects of 12 month administration of the novel long-acting testosterone undecanoate on measures of sexual function and the metabolic syndrome. Arch Androl. 2007;53:353–357.
- Morgentaler A, Dobs AS, Kaufman JM, et al. Long acting testosterone undecanoate therapy in men with hypogonadism: results of a pharmacokinetic clinical study. J Urol. 2008;180:2307–2313.
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle‐aged men: a meta‐analysis. Clin Endocrinol (Oxf). 2005;63:280–293.
- Maggio M, Snyder PJ, De Vita F, et al. Effects of transdermal testosterone treatment on inflammatory markers in elderly men. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2014;20:1170–1177.
- Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons from the testosterone trials. Endocr Rev. 2018;2017–00234. DOI: 10.1210/er.2017-00234
- Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J Endocrinol. 2014;220:R37–R55.
- Hackett G, Cole N, Bhartia M, et al. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11:840–856.
- Osuna C JA, Gómez-Pérez R, Arata-Bellabarba G, et al. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52:355–361.
- Rastrelli G, Corona G, Cipriani S, et al. Sex hormone‐binding globulin is associated with androgen deficiency features independently of total testosterone. Clin Endocrinol (Oxf). 2017;88:556–564.
- Saad F, Aversa A, Isidori AM, et al. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev. 2012;8:131–143.
- Traish AM, Haider A, Doros G, et al. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–329.
- Siddiqui K, Al-Rubeaan K, Nawaz SS, et al. Serum sex hormone binding globulin (SHBG) relation with different components of metabolic syndrome in men with type 2 diabetes. Horm Metab Res. 2018;50:138–144.
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126.
- Corrado E, Rizzo M, Coppola G, et al. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb. 2010;17:1–11.
- Mazo E, Gamidov S, Sotnikova E. Effects of different treatments on endothelial function in patients with erectile dysfunction and hypogonadism. Ter. Arkh. 2008;80:59–63.
- Fujimoto R, Morimoto I, Morita E, et al. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169–174.
- Lin AL, Gonzalez R, Shain SA. Androgen directs apparent cytoplasmic and nuclear distribution of rat cardiovascular androgen receptors. Arterioscler Thromb Vasc Biol. 1985;5:659.
- Foresta C, Caretta N, Lana A, et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab. 2006;91:4599–4602.
- Webb CM, McNeill JG, Hayward CS, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690.
- Kumanov P, Tomova A, Kirilov G, et al. The relationship of plasma endothelin and testosterone levels in male hypogonadism. Fertil Steril. 2004;82:S297.
- Hak AE, Witteman JCM, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639.
- Poredos P, Kek A, Verhovec R. Morphological and functional changes of the arterial wall in subject at risk of atherosclerosis and in patients with peripheral arterial occlusive disease. Vasa. 1997;26:271–276.
- Morgentaler A, Miner MM, Caliber M, et al. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90:224–251.
- Rosen RC, Wu FCW, Behre HM, et al. Registry of hypogonadism in men (RHYME): design of a multi-national longitudinal, observational registry of exogenous testosterone use in hypogonadal men. Aging Male. 2013;16:1–7.
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1–30.

