Abstract
Premature mortality in Russia is a major socio-economic problem, especially from acute cerebrovascular diseases which constitute 21.4% of the total mortality and is a considerable contributor to chronic disability. Risk of vascular catastrophe is higher in males than females, thought, in part, due to anti-atherosclerotic effects of oestrogens in females whilst an associated age-related deficiency of testosterone is observed in men. Clinical symptoms such as high blood pressure, changes in lipid profile, insulin resistance, obesity, and blood coagulation factors often accompany declining testosterone in males and reduced total testosterone is considered a cardiovascular risk factor. In the present study, the prevalence of hypogonadism in men who had suffered ischaemic stroke was evaluated along with the efficacy of testosterone undecanoate injections (TU) in patients with testosterone deficiency and type-2 diabetes (T2DM) in the acute phase of hemispheric ischaemic stroke. Hypogonadism was present in 66.3% of patients with ischaemic stroke, 50% with T2DM, and 26.3% without T2DM, respectively. TU treatment, at both the 2 and 5-year observation points, demonstrated significant improvements in biochemical, physical, and mental parameters. This supports that testosterone deficiency is a contributing factor in ischaemic events and that long-term testosterone therapy could play an important role in patient recovery.
Introduction
Premature mortality within the Russian Federation is a major socio-economic problem, especially from acute cerebrovascular diseases which constitute 21.4% of the total death rate. Likewise, chronic disability arising from stroke affects 3.2 per 10,000 of the population, and is the primary cause of disability [Citation1]; the economic impact of cerebrovascular disease is clear with the rate of developing a stroke at 525 per 100,000 of the population. This proves fatal in 39% of cases whilst only 20−22% of those surviving are able to return to work [Citation2]. Repeated ischaemic strokes, the frequency of which range from 13% to 28% over the course of 5 years, are also a major cause of the disability [Citation3].
The observable probability of haemorrhagic stroke occurring in men is 30% higher than in women between the age of 45–64, with a reduction in this difference at an older age [Citation4] thought to be a consequence of sex-linked hormone deficiency. In general, men suffer vascular catastrophes at a younger age than women, as the protective, anti-atherosclerotic effect of oestrogens is present up to the onset of menopause [Citation5]. However in men, an age-related deficiency in androgens can occur [Citation6,Citation7] the frequency of which is approx. 8% at an age of 40−49 years, 29% at 50−59, 44% at 60−69, and more than 70% when over 70 [Citation8].
A reduction in the levels of total testosterone, which accompany clinical symptoms of androgen deficiency, has been recorded in 45% of patients with isolated arterial hypertension (AH), in 66.6% of patients with a combination of ischaemic heart disease (IHD) and AH, in 70% of patients with a combination of IHD and type 2 diabetes (T2DM), and in 100% of patients suffering from diabetes, obesity and IHD at the same time [Citation9]. Also, age-related androgen deficiency has a negative effect on the risk factors associated with cerebrovascular disease, including blood pressure (BP), lipid profile, obesity, and blood coagulation factors [Citation10]. A number of studies suggesting that low total testosterone concentration maybe a biomarker for T2DM and is involved in the increase risk of mortality associated with T2DM [Citation11,Citation12]. It is role as a biomarker has also been highlighted by Rabijewski et al. [Citation13] who showed its high prevalence in prediabetic men. Along with T2DM, androgen deficiency also has a role in type 1 diabetes having a negative effect on glycaemic control, further it may worsen the biochemical risk profile of cardiovascular disease [Citation14–19].
The risk of developing a stroke increases sharply when a patient has diabetes [Citation20] with statistical studies in recent years, showing more than 10% of patients with T2DM die as a result of the disruption of the cerebral circulation [Citation21]. In this case, it is considered that the deficiency of sex hormones contributes to the development of insulin resistance and diabetes, and may be regarded as a component of metabolic syndrome in men [Citation10].
Studies carried out previously demonstrated the high prevalence of age-related hypogonadism in patients who had suffered a stroke [Citation22]. At that time, only isolated studies investigated the effect of androgen deficiency in men on the development of stroke and its correction. The presence of a low level of both total and free T in men who suffered a stroke was corroborated in the Tromsø Study [Citation23], where it was observed that the morphological changes in the walls of the arteries occur due to androgen deficiency which can lead to the development of ischaemic stroke [Citation24]. In earlier studies that assessed the effect of sex hormones in the development of stroke, the role of T in disrupting the cerebral circulation was evaluated as neutral, which could be the result of measuring only total rather than bioavailable T [Citation25–27]. Conversely, the occurrence of androgen deficiency in men with acute cerebral disease has also been demonstrated. Dash et al. [Citation28] showed a low level of serum T in eight out of nine men during the acute phase of stroke. Furthermore, in a larger study of 144 men with stroke, an inverse dependence of the severity and magnitude of stroke and the 6-month fatality rate on the level of free and total T was noted, which could not be accounted for by the risk factors of stroke, including age, BP, diabetes, IHD, smoking, and atrial fibrillation [Citation29].
Nevertheless, ambiguity remains regarding the role of T in stroke. On the one hand, the protective effect T has on brain cells has been documented; however, the possibility of a detrimental effect of T has been observed due to an increased size of stroke lesion in the presence of [Citation30]. Other studies have demonstrated a possible role for testosterone therapy (TTh) as a treatment for stroke due to an increased restoration of brain function, suggesting a possible therapeutic role of androgen receptor activation [Citation31]. This hypothesis is supported by clinical studies demonstrating the use of drugs to correct androgen deficiency thus lowering the risk factors of vascular diseases [Citation32,Citation33], as well as the significance of TTh in the recovery period after stroke, leading to an increase in muscle strength, mobility, and an overall improvement in quality of life of the patients [Citation34].
The aim of the present work, some of which has been previously presented in the Russian language [Citation20], was to study the prevalence of hypogonadism in men who had suffered ischaemic stroke, as well as to evaluate the efficacy of TU injections in patients with testosterone deficiency and T2DM in the acute phase of hemispheric ischaemic stroke.
Materials and methods
This study contained 154 male patients aged from 52 to 69 years (mean 61.4 ± 4.1) in whom hemispheric ischaemic stroke had occurred for the first time. The ischaemic nature of the stroke was diagnosed from the clinical picture of the disease and confirmed by the results of MRI analysis.
Apart from the conventional tests, body mass index (BMI) and the average systolic and diastolic arterial pressures (SAP and DAP) were determined for evaluating the somatic status. The National Institutes of Health Stroke Scale (NIHSS) was used for the dynamic assessment of the severity of the condition and the degree of neurological deficit. Patients with severe stroke were not included in the study because of the impossibility of predicting a long-term observation. Muscle strength was evaluated on a 5-point scale and the level of depression on the Beck scale. Levels of glycated haemoglobin (HbA1c), cholesterol, triglycerides, low-, and high-density lipoproteins (LDL and HDL) were determined by blood analysis.
Study of the androgen status included clinical evaluation of symptoms using the “Aging Male Symptoms (AMS)” standard questionnaire; the results of which were evaluated on a points scale, and laboratory confirmation of the deficiency. The levels of total T (normal 12−33 nmol/L) and sex hormone-binding globulin (normal 15−60 nmol/L) were determined, and the level of free T was estimated by calculation (lower limit of normal 255 pmol/L). During the acute phase, all of the patients were prescribed reperfusion, neuroprotective, and antioxidant therapy, as well as physical therapy and electrophoresis with proserin or magnesium sulphate. All patients received treatment with anti-hypertensive and anti-coagulatory drugs as secondary prophylactic agents.
In order to assess the efficacy of testosterone treatment, 102 men with T2DM and hypogonadism who had suffered ischaemic stroke took part in the second stage of the study. They were divided into a group receiving TU (n = 72) and a control group (n = 30) group. All patients gave informed consent for participation in the study. TU (1000 mg) was given by intramuscular injection at 1 week and 6 weeks after the development of stroke, and subsequently every 12 weeks for 2 years. After 5 years, a follow-up study was undertaken; 47 patients remained on testosterone (group Ta), and 25 patients had stopped testosterone treatment (group Tb). All 30 patients in the control group (CTRL) were followed up to 5 years.
Results
Hypogonadism was diagnosed in 102 out of the 154 men with ischaemic stroke (66.3%). In patients with T2DM, androgen deficiency was prevalent in 50% of patients whilst it was only prevalent in 26.3% in those without T2DM. The testosterone and control groups of patients with T2DM and hypogonadism did not differ significantly by age, clinical characteristics, and androgen status or biochemical blood analysis ().
Table 1. Comparative properties of testosterone and control groups before the start of treatment.
At the 2- and 5-year intervals, improvements in clinical symptoms of hypogonadism were recorded in patients receiving TTh ( and ). BMI had significantly decreased from 32.0 ± 3.1 at baseline to 26.4 kg/m2±2.1 (p < .01) after 2 years (27.2 ± 2.6; 5 years; p = .02) (). An increase in the levels of total T (baseline 9.2 ± 1.4 nmol/L to 16.2 ± 1.5 after 2 years (p < .001) (17.8 ± 0.8; 5 years) and free T (baseline 137.4 ± 11.7 pmol/L to 268.4 ± 10.7 after 2 years (p < .005) (314.2 ± 14.4; 5 years (p < .005) were recorded () as well as a significant decrease in HbA1c levels from 7.2 ± 2.1% at baseline to 6.6 ± 1.3% (p < .005) at 2 years and 6.9 ± 0.8% (p < .005) at 5 years ().
Figure 1. (a) BMI decreased from baseline over the 5-year period after TU treatment, after ceasing TTH, BMI returned to near baseline (Tb group). (b) A significant increase in total T is observed from baseline over the 5-year period compared with the control group. (c) A significant increase in free T is observed from baseline over the 5-year period after TU treatment. (d) A significant decrease is observed at both the 2- and 5-year intervals after TU treatment. (e) After TTH treatment, a significant decrease in total cholesterol levels are observed over the first 2 years. (f) After TTH treatment, a significant decrease in triglyceride levels is observed over the first 2 years. (g) After TTH treatment, a significant decrease in LDL cholesterol levels is observed over the first 2 years. (h) No significant change in HDL cholesterol levels are observed over the course of the study in any group observed.
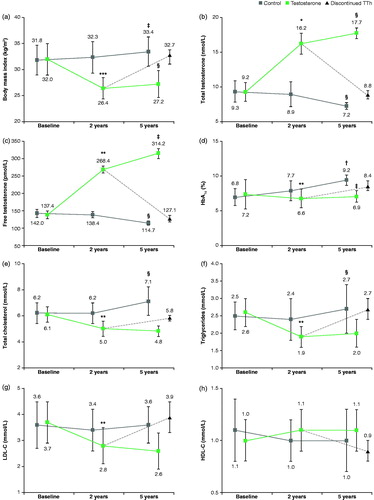
Table 2. Comparative properties of testosterone and control groups 2 years after stroke.
Table 3. Comparative properties of testosterone and control groups 5 years after stroke (differences between 2 and 5 years after the treatment).
Significant reductions in the levels of cholesterol, triglycerides, LDL, and HDL were observed after 2 years with the exception of HDL levels. Cholesterol decreased from 6.1 ± 0.6 mmol/L at baseline to 5.0 ± 0.6; p < .005 after 2 years (4.8 ± 0.4; 5 years; ) whilst triglycerides reduced from 2.6 ± 0.4 mmol/L to 1.9 ± 0.3; p < .005 at 2 years (2.0 ± 0.4; 5 years; ) and LDL from 3.7 ± 0.8 mmol/L at baseline to 2.8 ± 0.7; p < .005 after 2 years (2.6 ± 0.7; 5 years; ). The level of HDL showed no change across the study from 1.0 ± 0.2 mmol/L at baseline to 1.1 (±0.2; p > .05) at 2 and 5 years, respectively (); with the exception of HDL, there was a significant difference between the 5-year end point and baseline but no significance between the 2- and 5-year observation points, reflecting the stabilizing nature of the treatment.
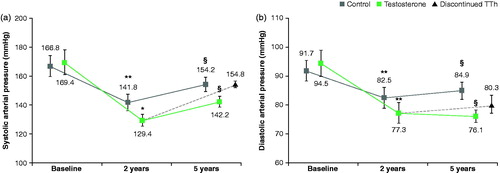
The SAP and DAP levels had decreased significantly at both the 2 and 5-year follow-ups with SAP decreasing from 169.4 ± 8.5 mm Hg to 129.4 ± 4.2 (p < .001) at 2 years before increasing to 142.2 ± 3.7 (p < .01) at 5 years (); despite this increase between 2 and 5 years, decrease from baseline was still statistically significant. Similarly, DAP decreased from 94.5 ± 4.5 mm Hg at baseline to 76.1 ± 2.2 (p < .01) after 5 years (77.3 ± 3.5 at 2-years) ().
Figure 2. (a) After TTH treatment, a significant decrease in SAP is observed over the first 2 years. (b) After TU treatment, a significant decrease in SAP is observed over the first 2 years.
In muscle strength scores, those within the TTh group showed an increase between baseline and 2 years (3.5 ± 0.3 to 4.1 ± 0.3; p > .005); however, at 5 years, this score had reduced back down to 3.5 ± 0.5. Within the control group, the muscle strength points averaged 3.1 (±0.4) at baseline with an increase at 2 years to 3.4 ± 0.1 before a decrease at 5 years to 2.9 ± 0.4. This decrease across both groups could be a consequence of ageing and it is of interest that those who stopped TTh (group Tb) had a greater decrease in score from baseline (3.5 ± 0.3) at 5 years (2.8 ± 0.4) suggestive of a positive role for TTh in increasing muscle strength ().
Figure 3. (a) A significant increase in muscle strength was observed between baseline and the 2-year follow-up. (b) A significant decrease in AMS was observed between baseline and the 2-year follow-up with further significant improvements observed at 5 years. (c) A significant decrease in Beck depression scores was observed between baseline and the 2-year follow-up with further significant improvements observed at 5 years.
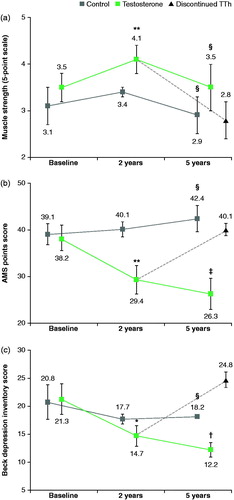
Both AMS and Beck depression inventory (BDI) scores showed statistically significant improvements over both 2 and 5-year-interval follow-up periods. AMS scores decreased from 38.2 ± 2.7 at baseline to 29.4 ± 1.8 (p < .005) after 2 years and again to 26.3 ± 3.3 (p < .005) at 5 years. Interestingly, those who ceased TTh (group Tb) showed an increased AMS score of 40.1 ± 1.3 (p < .005) at the 5-year follow-up (). Depression scores decreased from 21.3 ± 3.7 at baseline to 14.7 ± 1.8 (p < .001) after 2 years followed by another decrease to 12.2 ± 1.3 (p < .001) after 5 years; as with the AMS scores, those ceased TTh (group Tb) showed an increase in scores, from baseline (21.3 ± 3.7 to 24.8 ± 1.4 (p < .001) after 5-year follow-up ().
With the control group, apart from SAP and DAP, no significant improvements were observed across the study time period ( and ).
After 2 years, repeated ischaemic stroke occurred in three (7.1%) patients in the TTh group and in five (16.6%) in the control group. Twelve (28.6%) patients from the TTh group and two (6.6%) from the control group returned to work (). In all, 47 of 72 patients in the TTh group received TU regularly with all 72 patients of the TTh group alive at the end of the study. The remaining 25 patients stopped the regular treatment because of its high cost and thus were excluded from the study. In the control group, 24 patients were alive, 3 dead from repeated stroke, 2 from myocardial infarction, and 1 from lung cancer (mortality 20.0%) at the end of the study. All patients reached pensionable age (60 years in Russia) with three patients from the TTh group remaining in work, and none continuing work in the control group ().
Figure 4. (a) A significant difference in the rate of reoccurring stoke is observed between the TU and control group after 2 years. (b) A significant difference in the rate of mortality is observed between those that remain on TTH and those who are either in the control group or have ceased treatment at the 5-year follow-up.
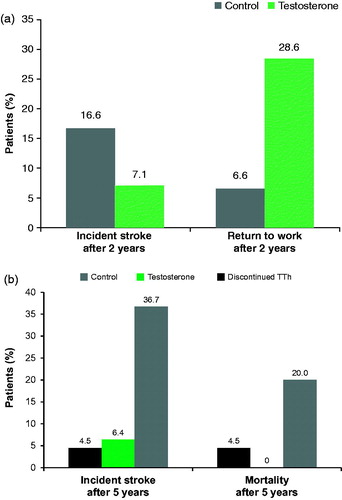
After 5 years, repeated ischaemic stroke occurred in 3 of the 47 patients (6.4%) although none were fatal, in the group that had ceased T treatment, 1 patient had a fatal stroke (4.5%). In the control group, 11 (36.6%) patients had a repeated stroke with 6 deaths reported (3 stroke, 1 myocardial infarction, 1 lung cancer; ).
Discussion
It has been postulated that testosterone deficiency plays an important role in ischaemic events [Citation35–37], although this remains ambiguous. For example, the Atherosclerosis Risk in Communities study (ARIC), after controlling for atherosclerotic risk factors, concluded there was no association between endogenous T and the incident rate of clinical stroke or ischaemic brain changes [Citation38]. However whilst ambiguity does remain, it is known that patients with T2DM have a 2–4 times increased risk of cardiovascular morbidity and mortality compared with individuals without T2DM [Citation39]; therefore developing appropriate strategies for the long-term management of patients with both low T levels and T2DM is an area for clinical consideration.
In the present study, a high prevalence of hypogonadism was recorded in men who have suffered an ischaemic stroke, with frequency increasing when patients also have T2DM. For men with hypogonadism who had suffered an ischaemic stroke, the correction of the deficiency with TU during the acute phase of the disease helps to increase muscle strength, the ability to work, and improves the patients’ quality of life. Furthermore, reductions in BP, the levels of cholesterol, triglycerides, and LDL, body weight, and improved glycaemic control, known risk factors for CVD, were also observed; especially in the first 2 years of treatment. Improvements in these biochemical parameters have been observed in a number of other studies where TU has been used for TTh in addition to highlighting the safety and effectiveness use of long-term TU administration as an important clinical consideration [Citation40–44].
Our results indicate that long-term TTh with TU has a positive effect on risk factors of repeated ischaemic stroke and, in the absence of contraindications, can be used effectively for treatment. Our observations mirror other findings of the protective effects of testosterone with regard to vascular function. It has been shown that higher levels of endogenous T levels aid the functional recovery of stroke patients [Citation45]. In this study, 111 male stroke patients showed those with higher T levels had a higher functional independence measure at discharge. Webb et al. [Citation46] examined the effect of oral TU on 22 men randomly assigned to be given either TU or placebo in a crossover study over an 8-week period. In unobstructed coronary arteries, an increase in perfusion was observed with a decrease in arterial stiffness and a reduction in high-density lipoproteins recorded. The authors concluded that these effects may explain the positive actions exogenous T has on exercised induced myocardial ischaemia. Haider et al. [Citation47] reported that cardiometabolic parameters such as lipid pattern, glycaemic control, blood pressure, heart rate, and pulse pressure were all shown to significantly improve in a study of 77 men diagnosed with hypogonadism after a mean follow-up time of 7.3 years who were continuously treated with TU injections in 3-monthly intervals after an initial 6-week interval. Much larger studies further support our findings. Anderson et al. [Citation48] examined the effect of TTh on 4736 men divided into groups who had persistent low (<212 ng/dl n = 801), normal (212–742 ng/dl n = 2241), and high (>742 ng/dl n = 1694) T levels with normalization associated with a reduction in major adverse cardiovascular events (MACE) over a 3-year period. Using a cohort of 44335 men divided into two groups, patients who had previously been prescribed TTh (8808, 19.8%) and those who had no prior TTh (35,527, 80.2%), Cheetham et al. [Citation49] showed the rate of composite cardiovascular events were 23.9 vs 16.9 per 1000 person-years over a median follow-up of 3.4 years in the never-TTh and ever-TTh groups, respectively. Similar results were obtained by Sharma et al. [Citation50]. In this retrospective study of 83010 subjects with low T levels, which utilized propensity score-weighted Cox proportional hazard models for the association of TTh with all-cause mortality, myocardial infarction (MI), stroke, and a composite endpoint to compare risk between the following groups: TRT treatment resulting in normalization of T levels (Group 1–43931), TTh treatment without normalization of T levels (Group 2–25701), and an untreated control group (Group 3–21380). The all-cause mortality [hazard ratio (HR): 0.44] risk of MI (HR: 0.76) and stroke (HR: 0.64) were significantly lower in Group 1 whose testosterone levels had been normalized (median age 66 years) after a mean follow-up period of 6.2 years compared with the control group (median age 66 years, follow-up period of 4.7 years). Similar results were obtained between groups 1 and 2 with the all-cause mortality (HR: 0.53), risk of MI (HR: 0.82), and stroke (HR: 0.70) significantly lower in Group 1. However, no difference in MI or stroke risk was observed between groups 2 (non-normalized TTh) and 3 (untreated). Additionally, a number of studies have highlighted the protective effects TTh has against myocardial ischaemia due to delayed time to ischaemia [Citation51–53], reduced exercise myocardial ischaemia [Citation51,Citation53], and a decrease in coronary stenosis [Citation54,Citation55] further supported by studies showing no adverse effects on cardiovascular health when using T treatment for erectile dysfunction [Citation56,Citation57]. However, not all studies have reported positive effects of TTh in relation to ischaemic events with Vigen et al. [Citation44] reporting a significant increase in risk of mortality, MI, and stroke in 1223 patients undergoing TTh compared with a control group of 7486 patients; however, this study was criticized by many experts and medical societies for its flawed methodology. Corona et al. [Citation58] in a meta-analysis of randomized, controlled studies concluded that the available data do not support an increased CV risk related to TTh.
A number of mechanisms have been proposed in relation to the protective effects of T in cardiovascular disease (CVD) [Citation59]. A beneficial effect on vascular tone, the degree of blood vessel constriction relative to its maximal diameter which can be a contributing factor in ischaemic events in the presence of atherosclerotic plaques, has been indicated. The vascular endothelium is important for vascular health [Citation60], and low T levels in men with CVD risk factors have been shown to be associated with endothelial dysfunction independent of other factors [Citation61]. Furthermore, studies examining the effect of TTh showed an improvement in endothelial function in men with CVD [Citation62]. It has been proposed that endogenous T improves vascular tone by regulating the expression of voltage-gated calcium channels [Citation63] with physiologically administrated doses of T shown to act via a endothelial-derived hyperpolarizing factor modulation [Citation64]. T beneficial effects on coronary blood flow may also be a contributing factor in the reduction of ischaemic events. In men with CVD, short-term administration of physiological concentrations of T caused increased blood volume, flow, and coronary artery dilation [Citation65] with a decrease also seen in ischaemic episodes and angina attacks in men with CVD undergoing long-term TTh [Citation66]. This supports English et al. [Citation51] who showed an improvement of myocardial ischaemia during exercise testing in men with stable chronic angina on long-term TTh. A further beneficial factor could be attributed to T positive effects on coronary atheroma events which have been shown in a number of epidemiological studies [Citation67]. T is thought to reduce atherogenesis via the inhibition of tumour necrosis factor-α-induced vascular cell adhesion molecule-1 expression [Citation68,Citation69]. However, whilst physiological normal levels of T can have a beneficial effect on CVD and increasing these levels to within normal range by TTh can maintain these beneficial effects, high concentrations of T can also have a detrimental effect especially in young adults who have had a previous ischaemic event [Citation70]. This is supported by the large French Three-City prospective cohort study (3650 men aged >65 years) who concluded that both high and low plasma testosterone levels are associated with an increased risk of ischaemic events in elderly men but maintaining an optimal range of plasma testosterone may confer cardiovascular protection [Citation71]. Of interest is Yassin et al. conclusions from a recent study [Citation72] which showed cessation of treatment resulted in a reversion back to reduced T levels indicating a need for life-long treatment.
In conclusion, in T2DM patients and androgen deficiency, TTh therapy represents a useful clinical tool to manage ischaemic events in this subset of patients whilst having a potentially positive effect in their mobility and the overall quality of life. Although it should be noted that, whilst in the present study TTh has had a positive effect, ambiguity still remains and also, due to the potential detrimental effects of T at higher concentrations, normal physiological T levels should be maintained through careful titration of administered doses. In addition, as highlighted in a review of cardiovascular risk and TTh, many of previously reported venous thrombotic events can be attributed to undiagnosed thrombophilia-hypofibrinolysis, thus anamnestic screening for thrombophilia before starting treatment should be considered [Citation58]. Whilst there remains a need for long-term placebo-controlled trials into the safety of TTh, this study suggests that with careful monitoring, testosterone-deficient patients with T2DM and cardiovascular risk may benefit from TTh.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Vereshchagin NV, Varakin YY. Epidemiology of stroke in Russia: results and epidemiological aspects of the problem. Stroke. Supplement to: Korsakov J Neurol Psychiatry 2001;1:34–40.
- Skoromets TP, Skoromets AA, Skoromets TA. Neural diseases. Moscow: Medpress-inform 2010; 2010. p. 552.
- Rosamund W, et al. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee 2008;146.
- Shevchenko OP, Praskurnichii EA, Yakhno NN, et al. Arterial hypertension and cerebral stroke. Moscow: Reafarm; 2001. p. 192.
- Sullivan JM, Fowlkes LP. The clinical aspects of estrogen and the cardiovascular system. Obstet Gynocol. 1996;87:36S–343.
- Morley JE, Charlton E, Patrick P, et al. Validation of screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49:1239–1242.
- Corona G, Forti G, Maggi M. Why can patients with erectile dysfunction be considered lucky? The association with testosterone deficiency and metabolic syndrome. Aging Male. 2008;11:193–199.
- Mazur A, Westerman R, Werdecker A, et al. Testosterone and type 2 diabetes in men. Aging Male. 2014;17:18–24.
- Dedov II, Kalinchenko SY. Age-related androgen deficiency in men. Moscow: Prakticheskaya Meditsina; 2006. p. 239.
- Vertkin AL, Morgunov LY, Arinina EN, et al. Testosterone deficiency and somatic disease. Lechashchii Vrach (Practitioner). 2006;10:34–38.
- Schipf S, Haring R, Friedrich N, et al. Low total testosterone is associated with increased risk of incident type 2 diabetes mellitus in men: results from the Study of Health in Pomerania (SHIP). Aging Male 2011;14:168–175.
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194.
- Rabijewski M, Papierska L, Piątkiewicz P. The prevalence of prediabetes in population of Polish men with late-onset hypogonadism. Aging Male. 2014;17:141–146.
- Haidar A, Yassin A, Saad F, et al. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007;10:189–196.
- Saad F, Yassin A, Almehmadi Y, et al. Effects of long-term testosterone replacement therapy, with a temporary intermission, on glycemic control of nine hypogonadal men with type 1 diabetes mellitus – a series of case reports. Aging Male. 2015;18:164.
- Yassin AA, Saad F, Haider A, et al. The role of the urologist in the prevention and early detection of cardiovascular disease. Arab J Urol. 2011;9:57–62.
- Ho CH, Wu CC, Chen KC, et al. Erectile dysfunction, loss of libido and low sexual frequency increase the risk of cardiovascular disease in men with low testosterone. Aging Male. 2016;19:96–101.
- Leoni LA, Fukushima AR, Rocha LY, et al. Physical activity on endothelial and erectile dysfunction: a literature review. Aging Male. 2014;17:125–130.
- Smith ML, Honoré Goltz H, Ahn S, et al. Correlates of chronic disease and patient-provider discussions among middle-aged and older adult males: implications for successful aging and sexuality. Aging Male. 2012;15:115–123.
- Morgunov LIu, Denisova IA, Rozhkova TI, et al. Androgenic deficit and its treatment in stroke male patients with diabetes mellitus type II. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111:21–24.
- Amos AF, McCathy DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14:1–85.
- Dedov II, Mel’nichenko GA, Fadeev VV. Endocrinology. Moscow: Geotar-Media; 2008. p. 432.
- Svartberg J, Midtby M, Bønaa KH, et al. The associations of lifestyle factors and chronic disease with testosterone in men. The Tromsø Study. Eur J Endocrinol. 2003;149:145–152.
- Dockery F, Bulpitt CJ, Donaldson M, et al. The relationship between androgens and arterial stiffness in older men. J Am Geriatr Soc. 2003;51:1627–1632.
- Taggart H, Sheridan B, Stout RW. Sex hormone levels in younger male stroke survivors. Atherosclerosis. 1980;35:123–125.
- Goncharov NP, Katsiya GV, Dobracheva AD, et al. Androgen deficiency and problems in its diagnosis using current non-isotopic methods for determining testosterone. Problemy Endokrinoklogii (Problems in Endocrinology). 2008;54:30–39.
- Goncharov NP, Katsiya GV. Current methods for determining testosterone. problems and their solution. Andrologiya i Genital’naya Khirurgiya (Andrology and Genital Surgery). 2008;2:27–37.
- Dash RJ, Sethi BK, Nalini K, et al. Circulating testosterone in pure motor stroke. Funct Neurol. 1991;6:29–34.
- Jeppesen LL1, Jørgensen HS, Nakayama H, et al. Decreased serum testosterone in men with acute ischeamic stroke. Arterioscler Thromb Vasc Biol. 1996;16:749–754.
- Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–2464.
- Pan Y, Zhang H, Acharya AB, et al. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043:195–204.
- Sheilor IM, Zilov AV. Possible ways of using testosterone to correct metabolic syndrome and obesity in men. Consilium Medicum. 2007;9:71–74.
- Traish AM, Haider A, Haider KS, et al. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Therap. 2017;22:414–433.
- Abbasi A, Mattson DE, Cuisinier M, et al. Hyposomatomedinemia and hypogonadism in hemiplegic men who live in nursing homes. Arch Phys Med Rehabil. 1994;75:594–599.
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340.
- Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217.
- Pongkan W, Chattipakorn SC, Chattipakorn N. Roles of testosterone replacement in cardiac ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2016;21:27–43.
- Srinath R, Gottesman RF, Hill Golden S, et al. Association between endogenous testosterone and cerebrovascular disease in the ARIC study (Atherosclerosis Risk in Communities). Stroke. 2016;47:2682–2688.
- Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9:25.
- Yassin AA, Nettleship J, Almehmadi Y, et al. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia. 2016;48:793–799.
- Haider A, Yassin A, Doros G, et al. Effects of long-term testosterone therapy on patients with "diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515.
- Conaglen HM, Paul RG, Yarndley T, et al. Retrospective investigation of testosterone undecanoate depot for the long-term treatment of male hypogonadism in clinical practice. J Sex Med. 2014;11:574–582.
- Tan WS, Low WY, Ng CJ, et al. Efficacy and safety of long-acting intramuscular testosterone undecanoate in aging men: a randomised controlled study. BJU Int. 2013;111:1130–1140.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–3503.
- Momosaki R, Abo M, Watanabe S, et al. Effects of testosterone levels on functional recovery with rehabilitation in stroke patients. Neurol Med Chir (Tokyo). 2014;54:794–798.
- Webb CM, Elkington AG, Kraidly MM, et al. Effects of oral testosterone treatment on myocardial perfusion and vascular function in men with low plasma testosterone and coronary heart disease. Am J Cardiol. 2008;101:618–624.
- Haider A, Yassin A, Haider KS, et al. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag. 2016;14:251–261.
- Cheetham TC, An J, Jacobsen SJ, et al. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–499.
- Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–2715.
- Anderson JL, May HT, Lappé DL, et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol. 2016;117:794–799.
- English KM, Steeds RP, Jones TH, et al. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911.
- Malkin CJ, Pugh PJ, Morris PD, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90:871–876.
- Rosano GM, Leonardo F, Pagnotta P, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670.
- Mathur A, Malkin C, Saeed B, et al. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161:443–449.
- Webb CM, Adamson DL, de, et al. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol. 1999;83:437–439.
- Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7.
- Salman M, Yassin DJ, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20:45–48.
- Vigen R, O'Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836.
- Corona G, Dicuio M, Rastrelli G, et al. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is 'new'? J Investig Med. 2017;65:964–973.
- Kelly DM, Jones TH. Testosterone and cardiovascular risk in men. Front Horm Res. 2014;43:1–20.
- Webb CM, Collins P. Testosterone and coronary artery disease in men. Maturitas. 2010;67:15–19.
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029–1034.
- Kang SM, Jang Y, Kim Ji, et al. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol. 2002;89:862–864.
- Bowles DK, Maddali KK, Ganjam VK, et al. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2091–H2098.
- Gonzales RJ, Krause DN, Duckles SP. Testosterone suppresses endothelium-dependent dilation of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2004;286:H552–H560.
- Cornoldi A, Caminiti G, Marazzi G, et al. Effects of chronic testosterone administration on myocardial ischemia, lipid metabolism and insulin resistance in elderly male diabetic patients with coronary artery disease. Int J Cardiol. 2010;142:50–55.
- Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb. 1994;14:701–706.
- Nathan L, Shi W, Dinh H, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci USA. 2001;98:3589–3593.
- Hatakeyama H, Nishizawa M, Nakagawa A, et al. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett. 2002;530:129–132.
- Quillinan N, Deng G, Grewal H, et al. Androgens and stroke: good, bad or indifferent? Exp Neurol. 2014;259:10–15.
- Soisson V, Brailly-Tabard S, Helmer C, et al. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: the French 3C cohort study. Maturitas. 2013;75:282–288.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19:64–69.

