Abstract
Background: The hormonal metabolism of adipose tissue differs across regions of fat. This issue has never been verified in male patients with coronary artery disease (CAD) with and without systolic heart failure (SHF).
Methods: We examined 90 male patients with CAD with and without SHF and 42 healthy controls.
Results: In patients with CAD with and without SHF, androgen receptor (AR) expression in adipose tissue of the lower leg was higher than AR expression of the thoracic wall and epicardial adipose tissue (EAT) (both p < .0001 for SHF patients and both p < .001 for patients without SHF). Expression of aromatase in adipose tissue of the lower leg among patients with CAD and SHF was higher than aromatase expression of the thoracic wall and EAT (p < .001 and p < .05, respectively), and in patients without SHF, it was higher only than aromatase expression of the thoracic wall (p < .05). There were no differences in expression of estrogen receptor (ER) between three regions of adipose tissue both in men with CAD with and without SHF.
Conclusions: In male patients with CAD, site-related differences of adipose tissue in expression of AR and aromatase are present regardless of coexisting SHF with the highest hormonal activity within peripheral subcutaneous adipose tissue.
Introduction
There is increasing evidence indicating a critical contribution of various organs and systems to the pathogenesis of HF syndrome [Citation1–4], including deranged body composition, for example abnormalities within adipose tissue. Adipose tissue has recently been described as one of the major endocrine gland that plays a role in hormonal response. There is growing evidence suggesting that low testosterone concentration may have its origin in disorders of fat metabolism. Moreover, decreased levels of testosterone coexist with comorbidities such as obesity, diabetes, insulin resistance, metabolic syndrome, and erectile dysfunction which underlie coronary artery disease (CAD) [Citation5–14]. The occurrence of steroid receptors and key enzymes of steroidogenesis in adipose tissue sustains the direct role of estrogens and androgens in adipose functions. Important element of ischemic HF pathophysiology is multiple anabolic deficiency. Patients with HF develop depleted levels of testosterone which unfavorably affect clinical status, accelerate disease progression, and lead to poor outcomes [Citation15]. Moreover, there is evidence that deranged estrogen metabolism seen in men with heart failure (HF) has negative clinical and prognostic consequences [Citation16]. However, the origin of these aforementioned hormone disturbances remains unclear. We hypothesize that adipose tissue as a source of steroid receptors and key enzymes of steroidogenesis (e.g. aromatase) and being a large body compartment might have an impact on circulating levels of anabolic hormones in HF. There is evidence that adipose tissue is not a homogeneous organ and hormonal activity of adipose tissue differs across diverse body regions and depends on the type of adipose tissue (visceral vs. subcutaneous). Particularly noteworthy is epicardial adipose tissue (EAT) as a unique type of visceral adipose tissue which is biologically more active than subcutaneous adipose tissue. The close anatomical proximity of the epicarial fat tissue with cardiac muscle and coronary arteries, as well as the lack of structures separating the myocardium and adipose tissue such as fascia [Citation17,Citation18], allow for possible paracrine interactions between these tissues. There are premises that in the course of CAD and HF, adipose tissue develops several functional and structural derangements, which directly and indirectly may contribute to the progression of these conditions. However, the pathophysiology of adipose tissue has not been comprehensively studied in male patients with CAD with or without coexisting SHF. The purpose of the present study was to evaluate expression of androgen and estrogen receptors (ERs) in various regions of adipose tissue and expression of aromatase as a key enzyme of steroidogenesis and their possible interplay with circulating anabolic hormones in a cohort of unselected men with CAD with and without CHF.
Methods
Study population
We examined male patients who were electively hospitalized or attended the outpatient clinic. The study included three group of patients: (1) healthy men (42 patients), (2) men with CAD without systolic heart failure (SHF) (49 patients), and (3) men with CAD with SHF with left ventricular ejection fraction (LVEF ≤45%) (41 patients).
Among studied groups of patients, hormonal indices in peripheral blood were assessed. Among studied groups of patients, we selected men in whom adipose tissue from three different areas (subcutaneous adipose tissue of lower leg, subcutaneous adipose tissue of thoracic wall, and EAT over the left anterior descending artery) was taken during cardiac surgery (patients with CAD with and without SHF). In healthy men, we selected patients who underwent orthopedic surgery in whom only subcutaneous adipose tissue of the lower leg was taken during surgery.
Inclusion criteria for group 3 were as follows: (1) LVEF ≤45% assessed by echocardiography, (2) symptoms of HF in New York Heart Association Functional Classification (NYHA) class I–III within ≥3 months preceding the study, (3) CAD confirmed by angiography (the presence at least one significant stenosis of coronary artery) and the presence of typical symptoms according to European Society of Cardiology (ESC) guidelines, and (4) clinical stability and unchanged medication within ≥1 month preceding the study. Inclusion criteria for group 2 were as follows: (1) LVEF >45% assessed by echocardiography, (2) CAD confirmed by angiography (presence at least 1 significant stenosis of coronary artery) and the presence of typical symptoms according to ESC guidelines, and (3) clinical stability and unchanged medication within ≥1 month preceding the study. Inclusion criteria for group 1 were as follows: (1) clinical stability within ≥1 month preceding the study and (2) the absence of cardiovascular disease.
Additional inclusion criteria are qualification for cardiac surgery for group 2 and 3, and qualification for orthopedic surgery for group 1. Exclusion criteria included: (1) acute coronary syndrome or/and coronary revascularization within ≥3 months preceding the study, (2) HF decompensation within ≥1 month preceding the study, (3) any hormonal treatment (at the time of the study or in the past), and (4) any acute/chronic illness that might influence hormonal metabolism
We prospectively identified 132 patients who were suitable for the study and who agreed to participate. The study protocol was approved by the local ethics committee, and all subjects gave written informed consent.
Methods
Study included clinical parameters, which were obtained from medical history, physical examination, laboratory, and echocardiographic measurements: (1) anthropometric data (weight [kg], height [cm], body mass index – BMI [kg/m2]); (2) data regarding severity and symptoms of CAD and HF according to Canadian Cardiovascular Society scale (CCS) and NYHA classification, respectively; (3) data regarding pharmacological treatment; (4) echocardiographic parameters (LVEF using the planimetric method by Simpson); (5) basic laboratory tests (morphology of blood cells, assessment of renal function – serum creatinine [mg/dL], estimated glomerular filtration rate [ml/min/1.73 m2] using Modification in Diet in Renal Disease (MDRD) equation, assessment of lipids [total cholesterol, LDL, HDL, TG – mg/dl]) evaluated using standard methods in local laboratory; and (6) hormonal status in peripheral blood (levels of total testosterone [TT], dihydrotestosterone [DHT], and total estradiol were measured by immunoassays (Diagnostic Products Corp, San Francisco, CA), estimated free testosterone [eFT] calculated with the validated equation of Vermeulen [Citation19], and free estradiol calculated with the equation of Sodergard et al. [Citation20]) assessed using standard methods in local laboratory; all applied devices and methods have updated quality certificates.
Preparation of tissues
The research material was secured as soon as possible after collection (approximately 30 min). Venous blood, in order to allow the assessment of concentrations of the steroid hormones and other factors, was centrifuged to obtain the serum, which was frozen at −80 °C. On the other hand, sections of body fat (peripheral subcutaneous adipose tissue of the lower leg, central subcutaneous adipose tissue of the thoracic wall, and EAT around the left anterior descending coronary artery) after sampling were immediately transferred into a container with ice, then frozen to a temperature of −80 °C.
RNA isolation
RNA was isolated from three different areas of adipose tissue using the kit “RNeasy Fibrous Tissue Mini Kit” (Qiagen, Warsaw, Poland).
cDNA synthesis
The reverse transcription reaction was carried out using a set of “SuperScript III First-Strand Synthesis System” (Invitrogen-Thermo Fisher Scientifc, Warsaw, Poland) with primer oligo(dT)20 using as a template RNA. Gene expression was analyzed by polymerase chain reaction (PCR) using the camera “iQ5 Optical System” (Bio-Rad, Warsaw, Poland). A reference gene used to determine relative levels of gene expression was the gene encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which is considered to be so-called housekeeping gene expressed at a constant level in most tissues.
Construction of primers for PCR
The mRNA sequences available in GenBank were used during construction of primers. In order to optimize conditions for PCR, it was performed at different concentrations of the primers and different temperatures, using the melting curve analysis of PCR products.
DNA electrophoresis on agarose gel
Sections of DNA fragments were performed by electrophoresis on a 1.5% agarose gel.
Statistical analysis
Normally distributed continuous variables were presented as means ± standard deviations. The intergroup differences for continuous variables were tested using the ANOVA test and Student’s t-test. Variables with a skewed distribution were expressed as medians with lower and upper quartiles. They were log-transformed in order to normalize their distribution. The categorical variables were expressed as numbers with percentages. The intergroup differences for the categorical variables were tested using the χ2 test. The associations between studied variables were tested using Spearman correlation. P < .05 was considered statistically significant. The following codes for statistically significance were accepted: *p < .05, **p < .01, ***p < .001, and ****p < .0001.
Results
Anabolic hormones pattern in peripheral blood in the study groups: healthy men and men with CAD with and without systolic HF
We identified 132 men among which 42 men were healthy without cardiovascular history (mean age: 50 ± 14 years), 49 men had CAD without SHF (mean age: 66 ± 9 years), and 41 men had CAD with SHF (mean age: 63 ± 8 years), who were suitable for the study and agreed to participate. The baseline clinical characteristics of 132 male patients included in the study are shown in . Hormonal indices are shown in . Patients with CAD with and without SHF had significantly lower free testosterone levels compared to healthy men (both p < .01), whereas TT levels were lower only in men with CAD without SHF compared to healthy men (p < .05). There were no differences between studied groups of men regarding DHT levels. Instead, total estradiol levels were higher in men with CAD with and without SHF as compared to healthy men (p < .01 and p < .05, respectively). Free estradiol was higher in men with CAD and SHF compared to healthy men (p < .05).
Table 1. Clinical characteristics of the study groups: healthy men and men with coronary artery disease with and without systolic heart failure.
Table 2. Hormonal indices in studied groups of patients.
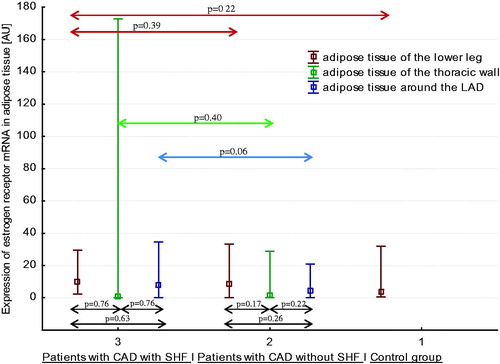
Estrogen receptor mRNA expression pattern in three areas of adipose tissue in healthy men, men with CAD without SHF, and men with CAD with SHF
Expressions of ER mRNA in three regions of adipose tissue in three studied groups of men are shown in . There were no differences in expression of ER mRNA between three regions of adipose tissue both in men with CAD and SHF, and in men with CAD without SHF. There were no differences between expression of ER mRNA in peripheral subcutaneous adipose tissue (of the lower leg) in healthy men, men with CAD without SHF, and men with CAD with SHF. Similarly, there was no differences between expression of ER mRNA in central subcutaneous adipose tissue (of the thoracic wall) and in EAT (around the left anterior descending artery) in men with CAD without SHF and in men with CAD with SHF.
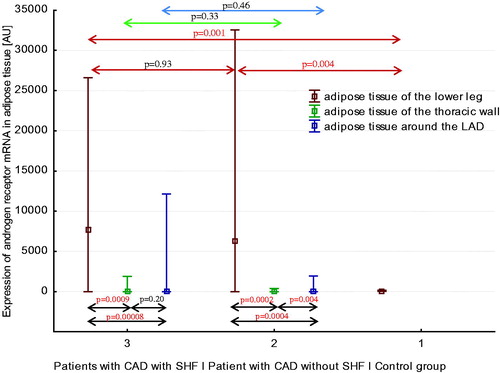
Androgen receptor mRNA expression pattern in three areas of adipose tissue in healthy men, men with CAD without SHF, and men with CAD with SHF
Expressions of androgen receptor (AR) mRNA in three regions of adipose tissue in three studied groups of men are shown in . Expression of AR mRNA in adipose tissue of the lower leg was significantly higher in men with CAD with and without SHF compared to healthy men. Among patients with CAD and SHF, expression of AR mRNA in adipose tissue of lower leg was higher than expression of AR mRNA in adipose tissue of the thoracic wall and in EAT (both p < .0001). Similarly, among men with CAD without SHF, expression of AR mRNA in adipose tissue of lower leg was higher than expression of AR mRNA in adipose tissue of the thoracic wall and in EAT (both p < .001). Additionally, in men with CAD without SHF, expression of AR mRNA in EAT was higher than expression of AR mRNA in adipose tissue of the thoracic wall (p < .01).
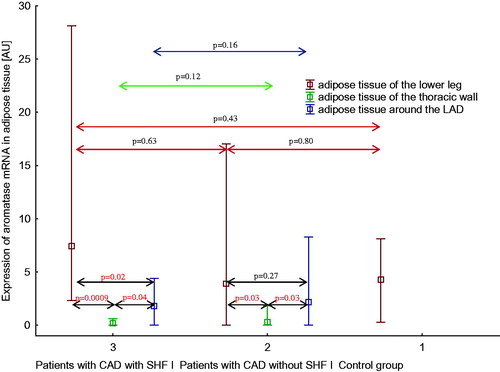
Aromatase mRNA expression pattern in three areas of adipose tissue in healthy men, men with CAD without SHF, and men with CAD with SHF
Expression of aromatase mRNA in three regions of adipose tissue in three studied groups of men is shown in . Among patients with CAD and SHF, expression of aromatase mRNA in adipose tissue of the lower leg was higher than expression of aromatase mRNA in adipose tissue of the thoracic wall and in EAT (p < .001 and p < .05, respectively). Expression of aromatase mRNA in EAT was higher than expression of aromatase mRNA in adipose tissue of the thoracic wall (p < .05). Among patients with CAD without SHF, expression of aromatase mRNA in adipose tissue of the lower leg was higher than expression of aromatase mRNA in adipose tissue of the thoracic wall (p < .05), but not in EAT. Expression of aromatase mRNA in EAT was higher than expression of aromatase mRNA in adipose tissue of the thoracic wall (p < .05). There were no intergroup differences regarding aromatase expression.
Interrelations between expression of ER mRNA in three areas of adipose tissue and the concentrations of the androgenic and estrogenic hormones in the peripheral blood in men with CAD with and without SHF
With respect to the hormonal parameters, there were only relevant associations with expression of ER mRNA in epicardial adipose. Testosterone levels (both total and free) were positively associated with expression of ER mRNA in EAT (r = 0.69, p < .01; and r = 0.65, p < .05, respectively). Additionally, expression of ER mRNA in adipose tissue of the lower leg was negatively associated with expression of this receptor mRNA in EAT (r = −0.35, p < .05).
Interrelations between expression of AR mRNA in three areas of adipose tissue and the concentrations of the androgenic and estrogenic hormones in the peripheral blood in men with CAD with and without SHF
Among hormonal parameters, there were only relationships between expression of AR mRNA in peripheral subcutaneous adipose tissue and testosterone and estradiol. Estradiol levels (both total and free) (r = 0.57, p < .01, and r = 0.59, p < .01, respectively) and testosterone levels (both total and free) (r = 0.58, p < .01, and r = 0.65, p < .01, respectively) were positively associated with expression of AR mRNA in adipose tissue of the lower leg. Additionally, expression of AR mRNA in adipose tissue of the lower leg was negatively associated with expression of this receptor mRNA in adipose tissue of the thoracic wall (r = −0.45, p < .01).
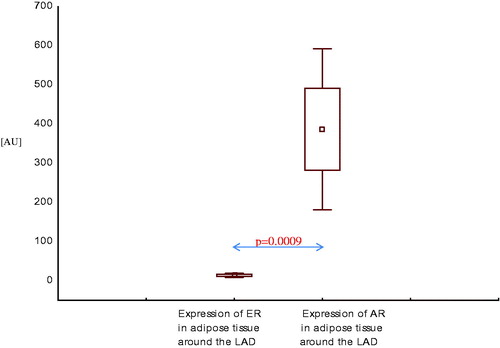
Comparison of expression of androgen and ER mRNA in three areas of adipose tissue in healthy men and in men with CAD with and without SHF
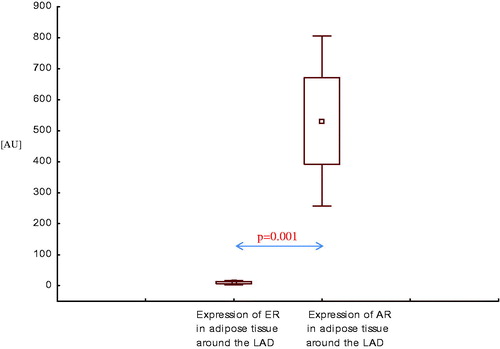
Expression of AR mRNA in adipose tissue of the lower leg was higher than expression of ER mRNA in this area, both in healthy men () and in men with CAD with SHF () and without SHF () (p < .001, p < .0001, and p < .001, respectively).
Figure 4. Expression of estrogen and androgen receptor mRNA in adipose tissue of the lower leg in healthy men.
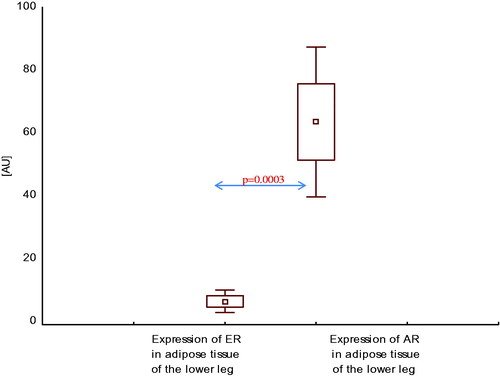
Figure 5. Expression of estrogen and androgen receptor mRNA in adipose tissue of the lower leg in men with coronary artery disease with systolic heart failure.
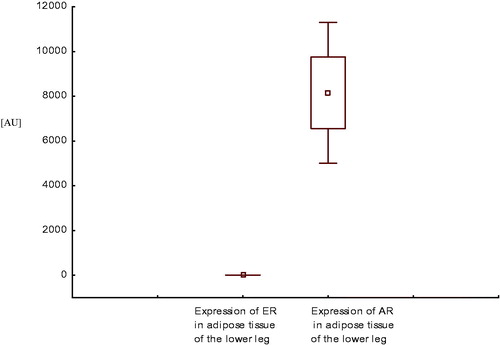
Figure 6. Expression of estrogen and androgen receptor mRNA in adipose tissue of the lower leg in men with coronary artery disease without systolic heart failure.
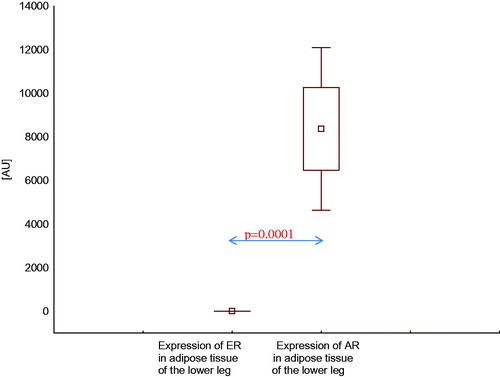
Similarly, expression of AR mRNA in adipose tissue of the thoracic wall was higher than expression of ER mRNA in this area, both in men with CAD with SHF () and without SHF () (p = .05, p = .02, respectively).
Figure 7. Expression of estrogen and androgen receptor mRNA in adipose tissue of the thoracic wall in men with coronary artery disease with systolic heart failure.
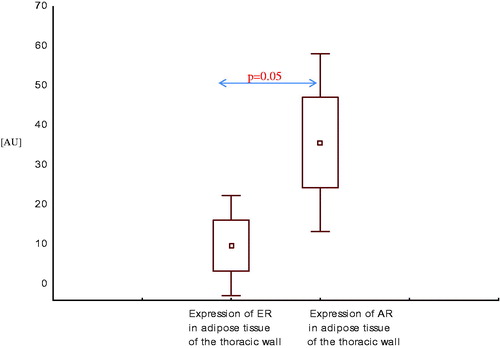
Figure 8. Expression of estrogen and androgen receptor mRNA in adipose tissue of the thoracic wall in men with coronary artery disease without systolic heart failure.
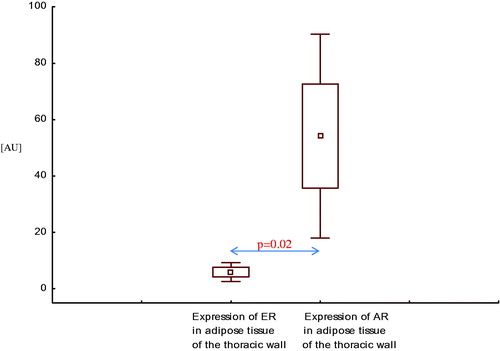
In adipose tissue around the left anterior descending artery (LAD), expression of AR mRNA was also higher than expression of ER mRNA, both in men with CAD with SHF () and without SHF () (p < .001, p < .01, respectively).
Discussion
Our study presents three major findings. We have demonstrated site-related differences of adipose tissue in expression of AR and aromatase in patients with CAD regardless of coexisting SHF. Among the three study areas of fat, expression of AR was highest in peripheral subcutaneous adipose tissue both in men with CAD with and without SHF. Among the three studied areas of fat, expression of aromatase was also highest in peripheral subcutaneous adipose tissue but only in men with CAD with coexisting SHF. In men with CAD without SHF, expression of aromatase was similarly high in EAT and in peripheral subcutaneous adipose tissue. In these group of patients also expression of AR was higher in EAT than in central subcutaneous adipose tissue, but significantly lower than in peripheral subcutaneous adipose tissue as mentioned above. These site-related differences of adipose tissue were not been observed in ER expression. Second, in the course of CAD (regardless of coexisting SHF), we have observed higher expression of AR in peripheral subcutaneous fat tissue as compared to healthy controls. Third, expression of AR across three studied areas of adipose tissue was higher than expression of ER in men with CAD regardless of coexisting SHF.
Deranged balance of serum concentrations of androgens and estradiol was observed in men with chronic SHF in our previous studies [Citation15,Citation16]. Our previous studies also confirmed that both low and high levels of estradiol in men with chronic SHF were predictors of poor prognosis [Citation16]. Recent study has confirmed testosterone/estradiol imbalance in peripheral blood not only in men with chronic SHF, but also in men without SHF, both suffered from CAD. High estradiol and low testosterone levels may be due to increased peripheral aromatization as reflection of expression of aromatase – a key enzyme of estrogen synthesis in men – which was significantly higher in peripheral adipose tissue as compared to central and EAT in men with SHF and to central adipose tissue in men without SHF. This finding may confirm the potential impact of peripheral expression of aromatase on circulating levels of sex steroid hormones in men with CAD. Additionally, high expression of aromatase in EAT in men with CAD without SHF confirms high metabolic activity of this unique fat depot and indicates the potential paracrine interactions of products of aromatase activity with myocardium.
Peripheral aromatization and related low testosterone concentrations and high estradiol levels are observed not only in ischemic heart disease but also above all in conditions predisposing to CAD, such as obesity or metabolic syndrome. Recent studies have confirmed that in the obese patients, peripheral aromatization resulted in excessive estrogen concentrations may suppress secretion of testosterone [Citation21]. Higher estradiol levels, as a mentioned above result of aromatization, were observed not only in the obese, but also in men with prediabetes with coexisting late-onset hypogonadism [Citation22]. Adipose tissue may contribute to low testosterone levels likewise in other mechanisms than aromatization. Adiposity with associated hyperinsulinism suppresses sex hormone binding globulin (SHBG) synthesis and therewith the levels of serum testosterone [Citation23]. Moreover, visceral fat cells secrete many cytokines which impair testicular steroidogenesis and therefore contribute to low testosterone concentrations [Citation23]. Taken together, recent data indicate that adipose tissue is not an inactive depot of reserve energy, but it is able to secrete molecules which may affect the hypothalamus–pituitary–testis axis [Citation21].
On the other hand, low TT concentrations which are inversely related to visceral obesity [Citation23] and components of the metabolic syndrome may be also a consequence of poor metabolic status [Citation24], which is observed for instance in the course of end-stage heat failure. This bidirectional relationship between testosterone and obesity with its consequences such as diabetes mellitus both type 2 and type 1, dyslipidemia, and cardiovascular disease was confirmed in other studies [Citation25].
Observed hypogonadal–obesity cycle shows that androgen deficiency contributes to increase in abdominal adipose tissue which further reduces the levels of circulating testosterone, resulting in progressively more severe hypogonadism [Citation26,Citation27].
Low testosterone concentrations in the aging male are associated with increased mortality [Citation6] and coexists with multitude of comorbidities in prevalence studies such as CAD, obesity, diabetes, insulin resistance, metabolic syndrome, and erectile dysfunction [Citation5–14]. Moreover, total and free testosterone is decreased in obese males in proportion to the degree of their obesity [Citation28,Citation29]. These metabolic disorders contribute to the development of atherosclerosis through the mechanism of inflammation, endothelial dysfunction, and increased intimal thickness [Citation30].
Recent data indicate that in some patients with late-onset hypogonadism, severity of erectile dysfunction is associated with an increased waist circumference, hyperglycemia, hypertriglyceridemia, hyperlipidemia, and a history of diabetes mellitus [Citation5]. These data suggest that erectile dysfunction may be a prognostic indicator for the development of cardiovascular morbidity in these patients [Citation5,Citation30]. Indeed, hypogonadism and erectile dysfunction have emerged as predictors of CAD [Citation31]. However, cardiovascular risk in case of erectile dysfunction/hypogonadism may be diminished by physically active lifestyle [Citation32].
There is increasing evidence of a beneficial effect of testosterone treatment on visceral fat, sustained weight loss, glycemic control, and other elements of the metabolic syndrome [Citation23,Citation25,Citation33,Citation34]. As shown in the previous studies, interruption of testosterone replacement therapy resulted in worsening of symptoms in hypogonadal men [Citation35]. In vivo studies in men have shown that testosterone affects triglyceride metabolism in visceral adipose tissue and regulates insulin sensitivity [Citation36]. It was shown that androgens activate the GLP-1 receptor and enhance B-cell function [Citation37]; moreover, additionally direct effects of testosterone on insulin signaling genes in adipose tissue, as well as fall in circulating free fatty acids, were described [Citation38]. In fact, withdrawing of testosterone supplementation in patients with hypogonadotropic hypogonadism increases insulin resistance [Citation39].
Similarly, androgen deprivation therapy used for the treatment of prostate cancer increases the risk of coronary heart disease, type 2 diabetes, and cardiovascular death [Citation14,Citation40,Citation41] and is associated with the decline of testosterone levels [Citation23,Citation42]. There is evidence that sex steroids are involved in the site specificities of adipose tissue metabolism. Steroid hormone actions are mediated by specific intracellular receptors in their target cells, as, for example, adipocytes.
Imbalance between testosterone and estradiol levels in peripheral blood may due to different density of androgen and ERs in peripheral adipose tissue. Our study has shown higher expression of AR than ER in each of the three studied areas of adipose tissue, and moreover, the density of AR was highest in peripheral adipose tissue in men with CAD with and without SHF. Low testosterone level in peripheral blood may be explained by its binding to AR which expression is high in peripheral adipose tissue. On the contrary, high level of estradiol in peripheral blood may be due to low density of ER as estradiol binding site. Moreover, interplay between androgen and ER was observed in breast cancer cells where AR directly inhibited the ER activity [Citation43]; therefore, there is possible interaction between androgen and ER also in adipose tissue, especially in the presence of significantly lower expression of ER compared to AR in our study. Additionally, not only testosterone but also estradiol may raise the level of AR what explains high peripheral expression of AR in adipose tissue. Similar observations were noted in cultured human prostatic stromal cells [Citation44]. Other hypothesis of diminished expression of ER in three areas of adipose tissue may include potential impact of high serum estradiol levels on saturation of ER so that it becomes redundant and its synthesis declines [Citation44]. We have studied subtype α of ER in adipose tissue which mainly mediates beneficial metabolic effects of estrogens such as anti-lipogenesis, improvement of insulin sensitivity and glucose tolerance, which may be impaired in the course of pathologic, inflammatory state as CAD. Low expression of ER in three studied areas of adipose tissue may, in turn, cause inflammation and fibrosis within adipose tissue and may lead to fat dysfunction.
Increased metabolic activity of peripheral adipose tissue in men with CAD measured by high expression of AR and aromatase may have impact on hormonal disturbances observed in peripheral blood. It seems that the presence of SHF does not affect additionally hormonal derangements within adipose tissue. Moreover, in men with CAD without SHF fairly hormonally active, in respect of expression of AR and aromatase, turned out to be EAT.
Study limitations
Analyzed cohort represents a subgroup of the population of men with CAD who in part consisted of patients with mainly mild-to-moderate SHF without severe left ventricular dysfunction; therefore, differences between group of patients with CAD without SHF could not be shown.
Conclusions
The present study demonstrates that in an unselected cohort of male patients with CAD, site-related differences of adipose tissue in expression of AR and aromatase are present regardless of coexisting SHF with the highest hormonal activity within peripheral subcutaneous adipose tissue. Observed diversity concerned expression of sex steroid receptors and key enzyme of steroidogenesis (aromatase) within various areas of adipose tissue can be a source of hormonal abnormalities which are present in peripheral blood in men with CAD.
Disclosure statement
The authors report no declarations of interest.
Additional information
Funding
References
- Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102.
- Coats AJS. Heart failure: what causes the symptoms of heart failure? Heart. 2001;86:574–578.
- Coats AJ, Clark AL, Piepoli M, et al. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S36–S39.
- Jankowska EA, Ponikowski P, Piepoli MF, et al. Autonomic imbalance and immune activation in chronic heart failure – pathophysiological links. Cardiovasc Res. 2006;70:434–445.
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194.
- Malkin CJ, Pugh PJ, Morris PD, et al. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821–1825.
- Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–917.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–283.
- Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299.
- Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–2701.
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75.
- Ponikowska B, Jankowska EA, Maj J, et al. Gonadal and adrenal androgen deficiencies as independent predictors of increased cardiovascular mortality in men with type II diabetes mellitus and stable coronary artery disease. Int J Cardiol. 2010;143:343–348.
- Schipf S, Haring R, Friedrich N, et al. Low total testosterone is associated with increased risk of incident type 2 diabetes mellitus in men: results from the study of health in Pomerania (SHIP)). Aging Male. 2011;14:168–175.
- Haidar A, Yassin A, Saad F, et al. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007;10:189–196.
- Jankowska EA, Biel B, Majda J, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837.
- Jankowska EA, Rozentryt P, Ponikowska B, et al. Circulating estradiol and mortality in men with systolic chronic heart failure. JAMA. 2009;301:1892–1901.
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543.
- Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672.
- Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810.
- Jankowska EA, Filippatos G, Ponikowska B, et al. Reduction in circulating testosterone relates to exercise capacity in men with chronic heart failure. J Card Fail. 2009;15:442–450.
- Jankowska EA, Drohomirecka A, Ponikowska B, et al. Deficiencies in circulating testosterone and dehydroepiandrosterone sulphate, and depression in men with systolic chronic heart failure. Eur J Heart Fail. 2010;12:966–973.
- Corona G, Forti G, Maggi M. Why can patients with erectile dysfunction be considered lucky? The association with testosterone deficiency and metabolic syndrome. Aging Male. 2008;11:193–199.
- Rabijewski M, Papierska L, Piątkiewicz P. The prevalence of prediabetes in population of Polish men with late-onset hypogonadism. Aging Male. 2014;17:141–146.
- Yassin AA, Saad F, Haider A, et al. The role of the urologist in the prevention and early detection of cardiovascular disease. Arab J Urol. 2011;9:57–62.
- Chen RY, Wittert GA, Andrews GR. Relative androgen deficiency in relation to obesity and metabolic status in older men. Diabetes Obes Metab. 2006;8:429–435.
- Saad F, Yassin A, Almehmadi Y, et al. Effects of long-term testosterone replacement therapy, with a temporary intermission, on glycemic control of nine hypogonadal men with type 1 diabetes mellitus – a series of case reports. Aging Male. 2015;18:164–168.
- Kapoor D, Malkin CJ, Channer KS, et al. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf). 2005;63:239–250.
- Cohen P. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt – a major factor in the genesis of morbid obesity. Med Hypotheses. 1999;52:49–51.
- Corona G, Mannucci E, Fisher AD, et al. Low levels of androgens in men with erectile dysfunction and obesity. J Sex Med. 2008;5:2454–2463.
- Zumoff B, Strain GW, Miller LK, et al. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab. 1990;71:929–931.
- Ho CH, Wu CC, Chen KC, et al. Erectile dysfunction, loss of libido and low sexual frequency increase the risk of cardiovascular disease in men with low testosterone. Aging Male. 2016;19:96–101.
- Tamler R, Deveney T. Hypogonadism, erectile dysfunction, and type 2 diabetes mellitus: what the clinician needs to know. Postgrad Med. 2010;122:165–175.
- Leoni LA, Fukushima AR, Rocha LY, et al. Physical activity on endothelial and erectile dysfunction: a literature review. Aging Male. 2014;17:125–130.
- Salman M, Yassin DJ, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20:45–48.
- Yassin A, Almehmadi Y, Saad F, et al. The author's reply: changing testosterone had no direct effect on HbA1c or weight in diabetic men when TRT was interrupted and then resumed. Clin Endocrinol. 2016;85:500–501.
- Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19:64–69.
- Mårin P, Odén B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80:239–243.
- Navarro G, Xu W, Jacobson DA, et al. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab. 2016;23:837–851.
- Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Dia Care. 2016;39:82–91.
- Yialamas MA, Dwyer AA, Hanley E, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–4259.
- Jones TH. Cardiovascular risk during androgen deprivation therapy for prostate cancer. BMJ. 2011;342:d3105.
- Mazur A, Westerman R, Werdecker A, et al. Testosterone and type 2 diabetes in men. Aging Male. 2014;17:18–24.
- Eri LM, Haug E, Tveter KJ. Effects on the endocrine system of long-term treatment with the luteinizing hormone-releasing hormone agonist leuprolide in patients with benign prostatic hyperplasia. Scand J Clin Lab Invest. 1996;56:319–325.
- Peters AA, Buchanan G, Ricciardelli C, et al. Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res. 2009;69:6131–6140.
- Smith P, Rhodes NP, Ke1 Y, et al. Upregulation of estrogen and androgen receptors modulate expression of FGF-2 and FGF-7 in human, cultured, prostatic stromal cells exposed to high concentrations of estradiol. Prostate Cancer Prostatic Dis. 2002;5:105–110.