Abstract
Objective
To analyze pathobiology of ischiocavernosus (IC) and bulbospongiosus (BS) muscles in long-term diabetic male rats and its implication on erectile dysfunction (ED).
Methods
Male rats were grouped into control and diabetic rats (received single injection of 60 mg/kg bw. of streptozotocin [STZ]). At 120th day, the animals were subjected to various analyses like serum hormone, penile reflex, electromyography of IC and BS muscles, after euthanasia IC and BS muscles were processed for morphological, histology, histometric analysis, immunostaining and immunoblotting synaptophysin, nNOS and NADPH diaphorase histochemistry.
Results
Significant reduction in serum hormone level, penile reflex, reduced action potential or activity in both these muscles and wide range of histological alterations were observed in STZ rats. Muscles showed significant reduction in the diameter, volume and numerical density of the fiber in both muscles of STZ rats. Synaptophysin, nNOS and NADPH diaphorase were significantly reduced in diabetic animal IC and BS.
Conclusion
Severe neuromuscular circuitry alteration in IC and BS. Study concludes that degenerative changes in IC and BS may play a major role in ED in diabetic condition. Indicating diabetic-induced postsynaptic neuronal degeneration along with impaired motor action of the muscle and severe muscle degeneration affecting ED.
Introduction
Aging and other metabolic disorders like diabetes presents numerous comorbid conditions that lead to various systemic pathologies like erectile dysfunction (ED) in male [Citation1,Citation2]. One of the major predisposing factors for ED in men has been linked to low testosterone levels [Citation3], studies do indicate that testosterone supplementation improves or restore erectile function in these men [Citation4,Citation5]. Apart from psychological [Citation6] and others like vitamin D deficiency are correlated with ED in men with type 2 diabetes mellitus [Citation7] and altered inflammatory markers do show strong association in development of ED [Citation8]. Even though several treatment approaches exist but optimal treatment is complex, because of its multifarious nature.
ED in males is a topping list of male-related problems nowadays and it affects approximately 18 million men which worsen the quality of life in diabetic patients [Citation9–11]. Directly or indirectly diabetes mellitus causes the impaired sexual behavior, ED, etc. [Citation12,Citation13]. Both type I and type II diabetes mellitus have adverse effects on male sexual and reproductive functions in human and animals [Citation13,Citation14], which include ED, decreased libido, potency, ejaculation difficulties and infertility [Citation15,Citation16].
Nitrous oxide (NO) plays a key role in penile erection by initiating smooth muscle relaxation following sexual stimulation. Impaired NO activity and endothelial dysfunction have been described in diabetic animal models as well as in human volunteers [Citation9,Citation13]. Reduction in the number of nitric oxide synthase (NOS) containing neurons and the impaired NOS activity would lead to reduce in both neurogenic- and endothelium-mediated smooth muscle relaxations [Citation17]. The degeneration, alteration in the conduction velocity and decreased levels of neuronal NOS were seen in the diabetic rat dorsal nerve of penis, major pelvic ganglion [Citation18,Citation19] and dorsal root ganglion [Citation20]. It has been reported that reduction in the NO production via reduced NOS production in para ventricular nucleus induces the ED in streptozotocin-induced diabetic rat [Citation21] and also there was reduction in the neuronal size and population of spinal nucleus of the bulbocavernosus in the lumbar spinal cord [Citation22]. Our previous studies demonstrated that the diabetes-induced alteration in penile morphology and microarchitecture [Citation23,Citation24].
The initiation and maintenance of penile erection depend on nerve control of the vasculature [Citation25], concurrently, the integrity of the striated muscle system, i.e. ischiocavernosus (IC) and bulbospongiosus (BS) is mandatory for rigid erection. The penile bulb is surrounded by the BS, the penile crura and proximal part of the shaft by the IC [Citation26,Citation27]. The BS arises from the perineal body, and its anterior fibers end in a tendinous expansion, which extends over the dorsal aspect of the penis covering the dorsal vessels [Citation26]. The BS assists in penile erection by compressing the erectile tissue of the penile bulb and the deep dorsal vein of the penis [Citation28]. The IC arises from the ischial tuberosity and ramus, and its fleshy fibers end in an aponeurosis attached to the sides and undersurface of the crus penis [Citation26]. The contractions of muscles on corporal tissue facilitate elevation of intracavernous pressure (ICP) that seems to be a reflex and mediated through the corpus cavernosum, which apparently leads to rigid erection [Citation29]. Changes in the evoked response amplitude would indicate a defect in the reflex pathway. In the rigid erection phase, ICP may increase well above the systolic pressure due to IC and BS muscle contraction [Citation30]. It has been noted that surgical removal of IC and BS leads to ED [Citation31], indicating importance of IC and BS in penile erection.
Erection involves, IC – straightening, lengthening and flexion of the penis toward abdomen, BS – Erection of glans. Thus, these muscles exert a greater force on the penile erection. During the rigid erection phase, the flow in the internal pudendal artery is almost zero and in the cavernous artery is not measurable [Citation28,Citation32] and creates supra-systolic ICP. The correlation of these muscles in diabetic-induced ED is not clearly documented. The objective of the present study was to analyze the structural and functional status of perineal muscles (IC and BS) in correlation to erectile function in long-term diabetic rats.
Materials and methods
Animal
Male Wistar albino rats (Rattus norvegicus) were used for this study. Male rats of body weight (b.w.) 225–250 g were selected. They were housed individually in separate standard cages and maintained under standard laboratory conditions (temperature 24–28 °C, relative humidity 60–70%, and 12-h light-dark cycle) with free access to solid pellet diet and water ad libitum throughout the study. The study was approved by institutional animal ethics committee. Animal maintenance were according to the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines for laboratory animal facility in India [Citation33]. Details of the maintenance were given elsewhere [Citation34].
Experimental design and induction of diabetes
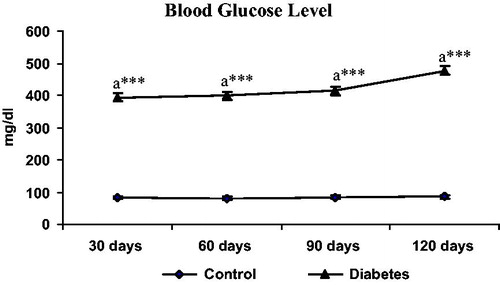
In this present study, animals were grouped as (n = 12), group I – control (received 0.1 M Citrate buffer) and group II – diabetes induced by single injection of streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO, USA) 60 mg/kg b.w. in 0.1 M citrate buffer. At the 4th day after injection, the blood glucose level was estimated, animals that showed above 250 mg/dl were considered as diabetic. Details of this procedure were given elsewhere [Citation23,Citation24].
Estimation of blood glucose level
Serum blood glucose level was estimated in control and diabetic animals at every 30 days interval. The blood was collected from retro-orbital venous plexus and blood glucose level was determined by using the glucometer (ACCU CHEK active, Roche, Germany).
Analysis of pituitary testicular axis
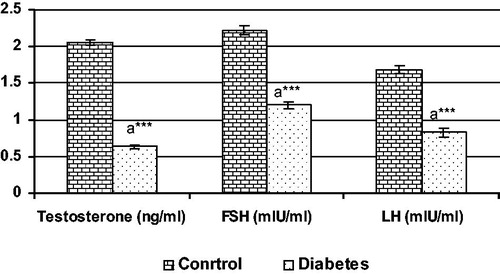
At the end of the experimental period, the blood was collected from control and diabetic animals through retro-orbital venous plexus by using the heparinized capillary tube. Serum was separated through centrifuge at 3000 rpm and it was used to estimate serum testosterone, follicular-stimulating hormone (FSH) and luteinizing hormone (LH) by radioimmunoassay.
Evaluation of penile reflexes
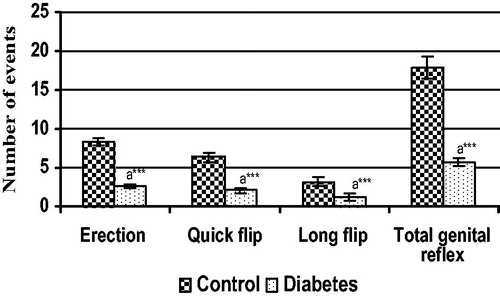
The potency was studied according to the method described in our earlier work [Citation24,Citation34]. On the 120th day, the test for penile reflexes was carried out by placing the animal on its back in a glass cylinder partial restraint. The preputial sheath was pushed behind the glands by means of thumb and index finger and held in this manner for a period of 30 min, which elicited a cluster of genital reflexes. The following components were recorded: Erections (E) – characterized by the engorgement of the penis with blood and by the accompanying extension and rigidity, Quick Flips (QF) – referred to a flaring of the head of the penis into a cup-like configuration, Long Flips (LF) – sharp involuntary dorsiflexion of the penis toward the abdomen with a rapid return to resting and Total Genital Reflex (TGR) sum of total reflex and erection index.
Electromyography
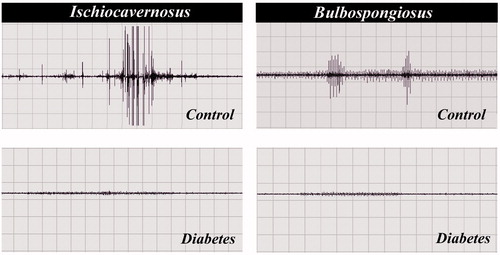
The electromyography (EMG) of IC and BS was done according to the methods described by Holmes et al. [Citation35] with some modifications. At the end of the 120th day, animals were anesthetized by using the ketamine (80 mg/kg body weight). Vertical incision was made at groin region between medial aspect of thigh and scrotum; identify the IC and BS muscle then clean the superficial fascia then the IC and BS were exposed. Three bipolar stainless steel loop electrodes were used in this study. Two electrodes were used as active and reference electrodes, it was placed on the surface of the exposed muscle at the level of belly and tendon regions, the third electrode was placed on lumber subcutaneous connective tissues served as a ground electrode. The recording signals were fed to high impedance preamplifier, displayed on a polygraph. Then the foreskin of the penis was retracted and the reflex-induced muscular action potential was recorded. The EMG pattern was compared between the groups.
Tissue harvesting
At the end of 120th day, animals were sacrificed by overdose of anesthesia (i.p), and followed by transcardial perfusion using 4% paraformaldehyde in 0.1M phosphate-buffered saline (PBS). IC and BS were dissected out and post-fixed in same fixative for further analysis.
Morphological and histological study
The organs removed were photographed and various gross measurements like volume, length, width, etc., were noted after being defatted. Volume was measured by the water displacement method and weight was measured by using the analytical balance. Length, width, and breadth were measured with the help of vernier caliper. Fixed muscle tissues were dehydrated in graded alcohol series, cleared in xylene and embedded in paraffin wax. Tissues were sectioned at longitudinally and transverse at 7 µm thickness. The sections were stained with hematoxylin and eosin.
Morphometric and stereological investigations
Stained slide was used to evaluate the quantitative measurement of histological alterations between the control and diabetic. Conventional stereological principles and accepted morphometric procedures, as described elsewhere [Citation25,Citation36], were used in the present study to obtain quantitative information. Systematic uniform random sampling protocol was adapted to avoid bias in selecting the sections/fields. For the quantitative using stereology, a 1-cm2 grid of square lattice containing 121 intersections used in this study. Diameter and numerical density of the muscle fibers and volume of the fiber component were estimated. The data are expressed in relative value.
Immunohistochemistry
The unfixed fresh tissues were frozen sectioned by using the cryostat microtome (Leica Microsystems, Wetzlar, Germany). The sections were stored at –20 °C until staining. After washing with PBS, slides were incubated with 3% H2O2 in methanol at room temperature for 15 min to quench endogenous peroxidase activity. The slides were incubated with blocking solution (1% bovine serum albumin) for 30 min at room temperature. Then, the sections were incubated overnight with mouse polyclonal anti-synaptophysin and nNOS antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, the sections were incubated with secondary antibody conjugated with FITC and Cy3 (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature, then washed with PBS, the nucleolus was counterstained with DAPI for 10 min and washed with PBS and mounted in anti-fade solution and the edges were sealed with nail polish. Photographs were taken by using epi-fluorescent microscope (Nikon, Japan) with appropriate filters.
Immunoblotting
Perineal muscles (IC and BS) (100 mg) collected after cervical dislocation were minced with anatomical scissor and it was homogenized in radioimmunoprecipitation assay buffer, centrifuged at 11,000g for 15 min to pellet any insoluble material and the supernatant was collected in a clean centrifuge tube. Protein level of supernatant was determined by using the Lowry’s method [Citation37]. Protein samples (75 μg) were resolved by using 10% or 12% SDS-PAGE gel and electrotransferred to polyvinylidene difluoride filter membrane at 100 V for 50 min. To reduce non-specificity, the transferred membrane was blocked with 5% nonfat milk in phosphate-buffered saline-Tween 20 (PBS-T) for 5 h at room temperature. Thereafter, the membrane was incubated overnight at 4 °C with specific antibody rabbit polyclonal anti nNOS (Santa Cruz Biotechnology, Santa Cruz, CA), synaptophysin and α-tubulin antibody in 5% nonfat milk in PBS-T buffer. After washing three times with PBS-T buffer, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), 1:2000–10,000 in PBS-T buffer at room temperature for 60 min. The membrane was washed five times with PBS-T buffer. Chemiluminescent substrate was applied according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL). The light emission was detected by using Chemidoc cooled camera and the band intensities of nNOS and synaptophysin were normalized against the loading control α-tubulin using ‘Quantity One” Software (Bio-Rad, Hercules, CA) and expressed as optical density units relative to β-actin.
Statistical analysis
Test of significance between the mean values of control and experimental groups was determined by using student’s’ test. p Value <0.05 was considered as statistically significant [Citation38].
Results
Blood glucose level
Serum glucose levels were estimated by using the glucometer (Accu Chek active, Roche, Germany). The data revealed that serum glucose levels found to be significantly (p < 0.001) increased in STZ-injected animals when compared to control animals at 120 days ().
Hormonal analysis
The levels of serum hormones (T, FSH and LH) are shown in . Data indicate that the levels of serum T, FSH and LH in 120 days diabetic animals were significantly (***p < 0.001) reduced when compared to control.
Evaluation of penile reflexes
The reflex study was indicative of erectile capacity of the penis along with the action of supporting structure (perineal muscles). The data showed significant reduction in the E, QF, LF and TGR in the STZ-treated rat when compared to control ().
Electromyography
Electrophysiological study to analyze the functional status of IC and BS muscles showed marked change in both IC and BS of the STZ-treated animals compared to control. The electromyographic activities of the IC and BS muscles in control groups showed prominent EMG activity concomitant to penile reflex. Whereas in the case of diabetic rat there was diminish activity and indicating obvious drop in action potential ().
Morphology and histological study
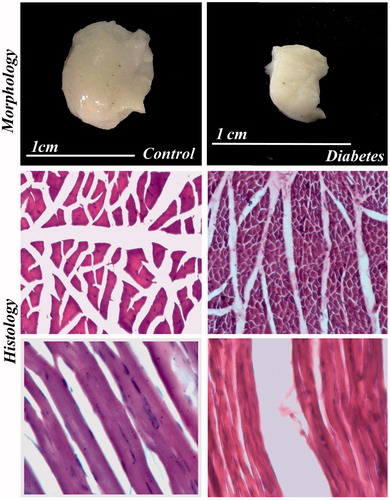
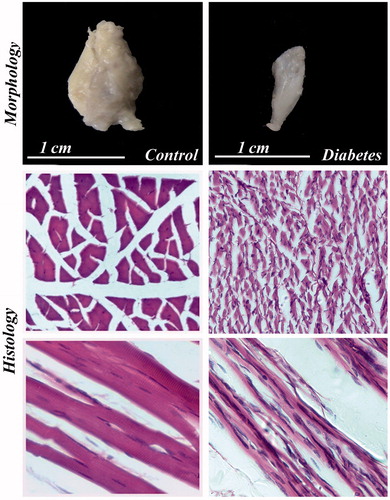
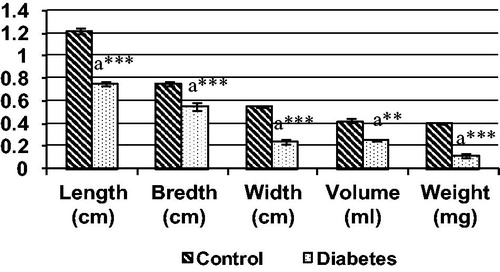
The morphological study showed that significant reduction in the length, breadth, volume and weight of both IC and BS in the long-term STZ-injected animals when compared to the control ( and , respectively). IC and BS showed wide degree of histological alterations in these muscles ( and , respectively). In diabetic animals, the atrophy of the muscle fiber was indicated by the crowding and angulations of the fibers on cross section. The longitudinal section of the muscles showed atrophy of the fiber and mild amount of fraying. The BS muscles also showed similar atrophy of the individual muscle fiber leading to increase crowding of the angulated muscle fiber and thinning of the muscle fiber and internalization of the nuclei on longitudinal section indicating degenerative changes when compared with control animals.
Figure 5. Various gross measurements of IC muscle in control and diabetic groups, each bar represents the mean ± SEM. aControl, **p < 0.01 and ***p < 0.001.
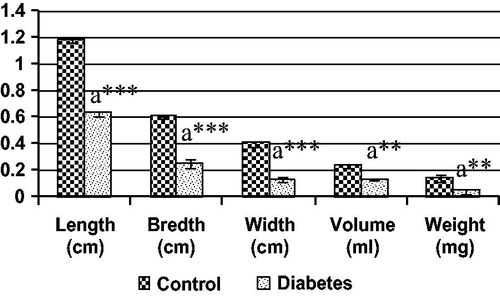
Figure 6. Various gross measurements of BS muscle in control and diabetic groups, each bar represents the mean ± SEM. aControl, **p < 0.01 and ***p < 0.001.
Morphometric and stereological investigations
The histological alterations were quantitatively analyzed by the histomorphometric study. Data of both IC and BS muscles showed significant reduction in the diameter and volume of the muscle fiber and significant increase in the numerical density of the muscle fiber in diabetic group when compared to control ().
Table 1. Histomorphometry of IC and BS muscle fibers in control and diabetic rats.
Immunohistochemistry
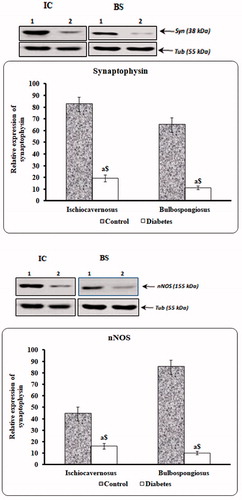
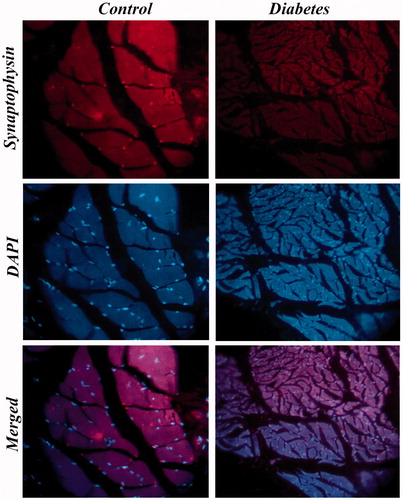
The status of neuromuscular junction was studied by the immunostaining of synaptophysin in IC and BS. The data revealed that immuno-localization of the synaptophysin in STZ-treated animals showed markedly decreased expression when compared to the control rats. The immunohistological images of IC and BS are shown in and , respectively.
Figure 9. Immunostaining of synaptophysin in IC muscle of control and diabetic rats. Expression of synaptophysin in IC muscle of diabetic rats was severely diminished when compared with control. 40×.
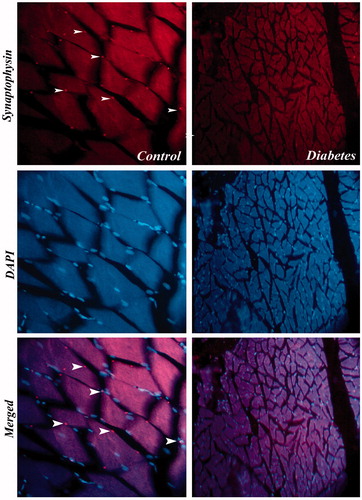
Figure 10. Immunostaining of synaptophysin in BS muscle of control and diabetic rats. Expression of synaptophysin in BS muscle of diabetic rats was severely diminished when compared with control. 40×.
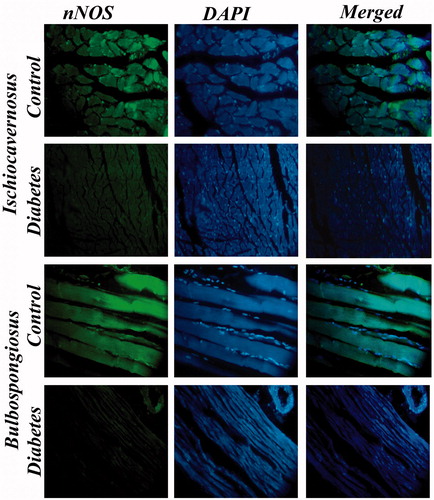
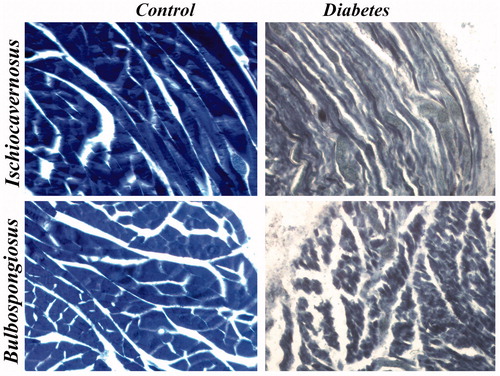
Immunostaining of nNOS showed reduced nNOS expression in IC and BS of diabetic rats when compared to control rats. The immunohistological images of IC and BS are shown in . Histochemical staining of NADPH diaphorase containing muscle fibers in IC and BS indicated sever depletion of enzyme in diabetic rat muscles when compared to control. The NADPH diaphorase staining of IC and BS is shown in .
Immunoblotting
Expression of synaptophysin and nNOS was estimated by immunoblotting. Levels of these proteins were found to be significantly reduced in long-term STZ-injected animal when compared to control ().
Discussion
ED induced by diabetes is one of the most common secondary complications seen in diabetic men. For the penile erection, a well-coordinated system of the vascular, endocrine and neuromuscular network is essential. The prevalence of reduction in the erection or sexual potency would be a feature of ED in men [Citation39–42]. One or more pathophysiological conditions associated with hormonal, vascular, muscular and neuronal contribute to ED, many studies indicate metabolic syndrome as a potential risk factor for ED [Citation43,Citation44].
Thence, the multifactorial nature of ED does present more challenging avenues to basic science researcher. Though the initiation and maintenance of penile erection depend on nerve control over vasculature. The erection of penis to optimum level is achieved by the striated muscle system, i.e. IC and BS muscles. These muscles form an essential component for sustenance of rigid erection. The role of IC is straightening, lengthening and flexion of the penis toward abdomen and role of BS is erection of glans, apart from controlling bulbar region of the urethra. Thus, these muscles exert a greater mechanical influence on the penile erection. Present study showed significant alterations in the structural integrity of perineal muscles in diabetic rat and contributed significantly to ED in these animals.
In the case of rat, the potency was evaluated by testing the frequency of penile reflexes such as E, QF, LF and TGR. These analyses signify the functional status and real-time testing of their structural integrity, i.e. nerve and muscle coordination [Citation25]. The test of the penile reflex revealed a reduction in E, QF, LF and TGR parameters when compared with the control, indicating structural disturbance and hypotrophy of the perineal musculature. Concomitantly, testosterone level was depleted in the diabetic rats when compare to control. The strong relative correlation between serum testosterone level and penile reflexes in the rat was observed in previous studies [Citation23,Citation25,Citation45,Citation46], indicating the crucial impact exerted by testosterone on these muscles. Reduction in the testosterone level might be due to the hyperglycemia-induced degenerative changes in the organs involved for hormone production [Citation16]. This clearly indicates that hyperglycemia has direct role in impairing the pituitary and testicular axis [Citation47] and decreased testosterone level [Citation48]. These facts indicate that in addition to the hyperglycemia-induced degenerative changes in the perineal muscles, androgen depletion also inflicts adverse effect in the muscle fibers [Citation30]. In general, testosterone depletion exemplifies loss of muscle mass. The erosion of skeletal muscle is predominantly occurring through mismatch between protein degradation and the inability of leucine to stimulate protein synthesis [Citation49,Citation50]. The structural and functional alterations in these muscles might lead to reduction in the penile reflex and lack of intromission in the sexual behavior in diabetic condition [Citation23,Citation24] especially due to lack of dorsiflexions (flips) of the glans penis. The surgical removal of IC muscle do showed nil dorsiflexions and ED [Citation31]. Thus, indicating that degeneration in perineal muscles plays a crucial role in ED.
Morphological, histological and histomorphometric analyses indicate severe alteration in long-term diabetic rats, in terms of muscle volume, micro-anatomical disturbance and reduction in fiber size, i.e. muscle cells shrank and skeletal muscle fibers space increased. Since both IC and BS muscles act to increase the corporal pressure [Citation29]. Impaired erectile function accounted for muscle degeneration in long-term hyperglycemia leading to erection dysfunction, by increasing the time taken to achieve full erection or very fast reversal to the flaccid state.
Our previous study indicated that reduced mating score, i.e. mounting and intermission latency in diabetic animals [Citation24], indicative that diabetic-induced maculopathy and peripheral neuropathy contribute to reduce activity of the muscles and forms an important cause for poor sexual behavior outcome. This may be caused by impaired Ca + homeostasis in the muscles [Citation51] and with a diminished expression of synaptophysin in IC and BS of diabetic rats indicate defective neuromuscular junction due to degeneration occurs in the junction level or pre-synaptic neuronal circuitry. Similarly, overall synaptophysin protein expression level in diabetic rat IC and BS muscles was significantly reduced around 120 days indicating that severity of the pathology.
The high level of circulatory glucose induces high oxidative stress and their secondary cascades in multifaceted mechanism induce muscle degeneration in diabetic rats. The influenced by hyperglycemia can greatly enhance the formation of peroxynitrite in tissue causing tetrahydrobiopterin (BH4) oxidization (a NOS III cofactor to dihydrobiopterin) [Citation52,Citation53]. With BH4 deficiency, the NOS III is in an uncoupled state, which means that the electrons flowing from NOS III reductase domain to the oxygenase domain are diverted to molecular oxygen rather than to L-arginine resulting in production of superoxide rather than NO [Citation54–56].
Generally, nNOS depletion is a feature of muscle dystrophy; NO supposed to involve in influencing muscle growth and development. Studies indicate that NOS activity in skeletal muscle cells increased dramatically and transiently at the time of muscle cell fusion and that NOS inhibition impaired fusion [Citation57]. Thus, it plays an important influence in muscle cell differentiation and overall regeneration through NO. nNOS deficiency could contribute to the regenerative defects and more severe changes in advanced stages of the disease with misregulation muscle cell growth [Citation58].
The presence of NADPH diaphorase histochemical staining showed significant reduction in enzyme level in IC and BS muscle fibers of diabetic rats when compared with control. Concomitant, reduced expression of nNOS in immunostaining indicates degenerative changes. The diminished staining for NADPH diaphorase and immuno-localization of nNOS indicates suppressed bioavailability of NADPH diaphorase and nNOS results in decreased NO availability; results were concomitant to the nNOS immunoblot. This can cause impairment of nitrergic relaxations, leading to poor smooth muscle relaxation in penis for effective erection and at the cellular level loss of nNOS localization in skeletal muscle fibers can lead to functional ischemia due to a decrease in contraction-induced vasodilation [Citation59].
The principal outcome of the hyperglycemia is considered to be oxidative stress and neuronal apoptosis in the major pelvic ganglion [Citation19] and impairing perineal muscle function and leading to erection defect in diabetic animals. Impaired muscle activity might be due to the neuronal alteration produced by androgen deficiency, which has physiological control over the perineal muscle [Citation25,Citation60]. Based on these findings, it is hypothesized that the reduced strength in diabetic animals is due to down-regulated synthesis of neurotrophin, resulting in diminished neurotrophic support to motor neurons and leading to insufficient re-innervation and atrophy [Citation61]. It seems that the ICP increases to a certain level and stimulates the intracavernosal pressure receptors to send impulses to the spinal cord. These impulses are possibly transmitted through pudendal nerve to the cavernous muscles, affecting their contraction with a resulting increase in the ICP [Citation29]. These facts indicate that any disturbance in the circuit integrity will hamper penile erection significantly.
It is obvious that the rise in ICP is affected through IC and BS contraction [Citation62,Citation63]. The cavernous muscles inserted into the corpus cavernosum most probably facilitate contraction of dorsal penile vessels and consequential lead to increase blood pooling in the cavernous tissue [Citation29]. The analysis of IC and BS muscles’ EMG showed intermittent activity indicating the intermittent contraction of cavernous muscle, a typical feature of striated muscles. The intermittent contraction of cavernous muscle along with penile corpora supposes to produce reflex jerk thereby increasing ICP for a rigid erection. However, the EMG of both IC and BS showed reduced activity in 120 days of diabetic rats. Indicate the poor evoked action potential response ensuing degenerative changes in these muscles in long-term diabetes. Resulting in reduced cavernosal pressure and ensuing ED. Moreover, intermittent contraction seems to offer a mechanism by which fresh oxygenated blood find entry in the cavernosal tissue [Citation29], with the loss or mismatch of intermittent contraction in IC and BS could reduce oxygenated blood entry during rigid erection and probably can cause ischemic or hypoxic degeneration in cavernous tissue in diabetic condition.
From the present study, it was concluded that the observed degenerative changes in IC and BS played a major role in ED. Despite forecasting that these diabetic-related alterations occur, their real impact on male erectile function and reproductive health are still far from being fully understood, highlighting that research in the field is crucial. Thus, the individual variations seen in erectile response to therapeutic drugs might be due to the degree of impairment of perineal musculature. These data also correlate with poor response outcome observed to drug like sildenafil citrate (PDF5 inhibitor) in similar condition, indicating want of further systematic investigations in this area. Moreover, while developing selective and safer treatment strategies for diabetic-induced ED in men, a due consideration should be given to the integrity of the perineal musculature. Thus, these observations serve as key factors for implication to treat diabetes-induced ED in mankind.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
DST-PURSE consumable grant through University of Madras awarded to Dr. S. Prakash, Professor of Anatomy, Department of Anatomy, University of Madras. [Award Lr. No: C5/DST-PURSE PhaseII/PIG/Consumable/Jun18/151 Dt:05.07.18.]
References
- Panach-Navarrete J, Martínez-Jabaloyas JM. The influence of comorbidities on the aging males' symptoms scale in patients with erectile dysfunction. Aging Male. 2017;20:146–152.
- Salama MN, Eid AA, Hatem A, et al. Prevalence of erectile dysfunction in Egyptian males with metabolic syndrome. Aging Male. 2018;19:1–7.
- Guay AT. Testosterone and erectile physiology. Aging Male. 2006;9:201–206.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199:257–265.
- Saad F, Caliber M, Doros G, et al. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male. 2019;20:1–12.
- Aykurt Karlıbel İ, Dülger S, Kasapoğlu Aksoy M, et al. Effect of cigarette smoking on sexual functions, psychological factors, and disease activity in male patients with ankylosing spondylitis. Aging Male. 2019;22:109–115.
- Basat S, Sivritepe R, Ortaboz D, et al. The relationship between vitamin D level and erectile dysfunction in patients with type 2 diabetes mellitus. Aging Male. 2018;21:111–115.
- Demirtaş Şahin T, Yazir Y, Utkan T, et al. TNF-α antagonism with etanercept enhances penile NOS expression, cavernosal reactivity, and testosterone levels in aged rats. Can J Physiol Pharmacol. 2018;96:200–207.
- De Berardis G, Pellegrini F, Franciosi M, et al. Clinical and psychological predictors of incidence of self-reported erectile dysfunction in patients with type 2 diabetes. J Urol. 2007;177:252–257.
- Corona G, Mannucci E, Petrone L, et al. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res. 2006;18:190–197.
- Maiorino MI, Bellastella G, Esposito K. Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndr Obes. 2014;7:95–105.
- Saenz de Tejada I, Goldstein I, Azadzoi K, et al. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989;320:1025–1030.
- Escrig A, Marin R, Abreu P, et al. Changes in mating behavior, erectile function and nitric oxide levels in penile corpora cavernosa in streptozotocin-diabetic rats. Biol Reprod. 2002;66:185–189.
- Braunstein GD. Impotence in diabetic men. Mt Sinai J Med. 1987;54:236–240.
- Dinulovic D, Radonjic G. Diabetes mellitus/male infertility (review). Arch Androl. 1990;25:277–293.
- Scarano WR, Messias AG, Oliva SU, et al. Sexual behaviour sperm quality and quantity after short-term streptozotocin induced hyperglycemia in rats. Int J Androl. 2006;26:482–488.
- Jesmin S, Sakuma I, Salah-eldin A, et al. Diminished penile expression of vascular endothelial growth factor and its receptors at the insulin-resistant stage of a type II diabetic rat model: a possible cause for erectile dysfunction in diabetes. J Mole Endo. 2003;31:401–418.
- Saenz de Tejada I, Goldstein I. Diabetic penile neuropathy. Urol Clin North Am. 1988;15:17–22.
- Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–2363.
- Schmeichel AM, Schmelzer JD, Low PA. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes. 2003;52:165–171.
- Zheng H, Bidasee KR, Mayhan WG, et al. Lack of central nitric oxide triggers erectile dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1158–R1164.
- Dorfman VB, Vega MC, Coirini H. Reduction of the spinal nucleus of the bulbocavernosous volume by experimental diabetes. Brain Res. 2004;1019:265–269.
- Suresh S, Prakash S. Effect of Mucuna pruriens (Linn.) on oxidative stress‐induced structural alteration of corpus cavernosum in streptozotocin‐induced diabetic rat. J Sex Med. 2011;8:1943–1956.
- Suresh S, Prakash S. Effect of Mucuna pruriens (Linn.) on sexual behavior and sperm parameters in streptozotocin‐induced diabetic male rat. J Sex Med. 2012;9:3066–3078.
- Prakash S, Ibrahim M, Karthik Ganesh M, et al. Therapeutic potential of Mucuna pruriens (Linn.) on ageing induced damage in dorsal nerve of the penis and its implication on erectile function: an experimental study using albino rats. Aging Male. 2018;15:1–14. doi:10.1080/13685538.2018.1439005
- Skandalakis JE, Colborn GL, Weidman TA, et al. Male genital system. In: Skandalakis JE, editor. Skandalakis’ surgical anatomy: the embryologic and anatomic basis of modern surgery. 1st ed. Athens, Greece: Paschalidis Medical Publications; 2004. p. 1381–1473.
- Prakash S, Muhammed I. The penile baculum in monkey (Macaca radiata), rat (Rattus norvegicus), and bat (Pipistrellus mimus) and its functional significance—a mini review. J Basic Appl Zool. 2018;79:38.
- Breza J, Aboseif SR, Orvis BR, et al. Detailed anatomy of penile neurovascular structures: surgical significance. J Urol. 1989;141:437–443.
- Shafik A, Sibai OEL, Shafik AA, et al. Cavernosus muscle contraction during erection: is it voluntary or reflex, given the striated nature of the muscles? J Androl. 2006;27:695–699.
- Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236.
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443.
- Lue TF, Tanagho EA. Hemodynamics of erection. In: Tanagho EA, Lue TF, McClure RD, editors. Contemporary management of impotence and infertility. 1st ed. Baltimore, MD: Williams & Wilkins; 1988. p. 28–38.
- CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003;35:257–274.
- Suresh S, Prithiviraj E, Prakash S. Dose- and time-dependent effects of ethanolic extract of Mucuna pruriens Linn. seed on sexual behaviour of normal male rats. J Ethnopharmcol. 2009;22:497–501.
- Holmes G, Chapple WD, Leipheimer RE, Sachs BD. Electromyographic analysis of male rat perineal muscles during copulation and reflexive erections. Physiol Behav. 1991;49:1235–1246.
- Prakash S, Prithiviraj E, Suresh S. Development of seminiferous tubules in prenatal, postnatal and adult testis of bonnet monkey (Macaca radiata.). Anat Hist Emb. 2008;37:19–23.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin’s phenol reagent. J Biol Chem. 1951;193:265–276.
- Zar JH. Biostatistical analysis. Engle Wood Cliffs, NJ: Prentice Hall Inc.; 1974.
- Rowland DL, Greenleaf WJ, Dorfman LJ, et al. Aging and sexual function in men. Arch Sex Behav. 1993;22:545–557.
- Bacon CG, Mittleman MA, Kawachi I, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–168.
- Dean R, Lue T. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–395.
- Romanelli F, Sansone A, Lenzi A. Erectile dysfunction in aging male. Acta Biomed. 2010;81:89–94.
- Traish AM1, Guay A, Feeley R, et al. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2008;30:10–22.
- Sanchez E, Pastuszak AW, Khera M. Erectile dysfunction, metabolic syndrome, and cardiovascular risks: facts and controversies. Transl Androl Urol. 2017;6:28–36.
- Murray FT, Orth J, Gunsalus G, et al. The pituitary – testicular axis in the streptozotocin diabetic male rat: evidence for gonadotroph, Sertoli cell and Leydig cell dysfunction. Int J Androl. 1981;4:265–280.
- Davidson JM, Stefanick ML, Sachs BD, et al. Role of androgen in sexual reflexes of the male rat. Physiol Behav. 1978;21:141–146.
- Baccetti B, La Marca A, Piomboni P, et al. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002;17:2673–2677.
- Beatrice AM, Dutta D, Kumar M, et al. Testosterone levels and type 2 diabetes in men: current knowledge and clinical implications. Diabetes Metab Syndr Obes. 2014;7:481–486.
- Griggs RC, Kingston W, Jozefowicz RF, et al. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. 1989;66:498–503.
- Jiao Q, Pruznak AM, Huber D, et al. Castration differentially alters basal and leucine-stimulated tissue protein synthesis in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2009;297:E1222–E1232.
- Biessels GJ, Van der Heide LP, Kamal A, et al. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441:1–14.
- Alp NJ, Mussa S, Khoo J, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735.
- Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25:81–88.
- Chen CA, Druhan LJ, Varadharaj S, et al. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047.
- Yeo TW, Lampah DA, Kenangalem E, et al. Impaired systemic tetrahydrobiopterin bioavailability and increased dihydrobiopterin in adult falciparum malaria: association with disease severity, impaired microvascular function and increased endothelial activation. PLoS Pathog. 2015;11:e1004667.
- Bendall JK, Douglas G, McNeill E, et al. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 2014;20:3040–3077.
- Lee KH, Baek MY, Moon KY, et al. Nitric oxide as a messenger molecule for myoblast fusion. J Biol Chem. 1994;269:14371–14374.
- Tidball JG, Wehling-Henricks M. Nitric oxide synthase deficiency and the pathophysiology of muscular dystrophy. J Physiol. 2014;592:4627–4638.
- Gonzalez JP, Crassous PA, Schneider JS, et al. Neuronal nitric oxide synthase localizes to utrophin expressing intercalated discs and stabilizes their structural integrity. Neuromuscul Disord. 2015;25:964–976.
- Dorfman VB, López-Costa JJ, Vega C, et al. Changes of NADPH-diaphorase reactivity in lumbar spinal cord of short-term streptozotocin induced diabetic rats. Brain Res. 2004;997:185–193.
- Andreassen CS, Jakobsen J, Flyvbjerg A, et al. Expression of neurotrophic factors in diabetic muscle-relation to neuropathy and muscle strength. Brain. 2009;132:2724–2733.
- Lavoisier P, Proulx J, Courtois F, et al. Relationship between perineal muscles contractions, penile tumescence and penile rigidity during nocturnal erections. J Urol. 1988;139:176–179.
- Fournier GR, Juenemann KP, Lue TF, et al. Mechanisms of venous occlusion during canine penile erection: an anatomic demonstration. J Urol. 1987;137:163–167.