Abstract
Background
Voiding dysfunction became a difficult problem for the elderly because of the underactive bladder (UAB). It was considered that the degeneration of detrusor muscle was the main etiology. In recent years, more articles focus on relationship between UAB and decreased muscle strength. Besides, handgrip strength (HGS) is an early indicator to detect frailty and muscle weakness in systemic reviews.
Method
Our study involved 2258 males from NHANES datasets (2011–2012, who were divided into quartiles by urine flow rate (UFR), which was measured by uroflowmetry. Multivariate regression models were performed to analyze the associations between UFR and HGS.
Results
The UFR had a positive correlation to the HGS by multivariate regression models in males (β coefficient: 1.348, 95% confidence interval (CI): 0.530, 2.166, p = 0.001). The male participants with the highest quartile of UFR have a greater HGS than those with lowest quartile of UFR (β coefficient: 4.546, 95% CI: 2.462, 6.630, p < 0.001). Higher UFR was associated with lower odds of low HGS (OR: 0.489, 95% CI: 0.350, 0.684, p < 0.001) in the fully-adjusted model.
Conclusions
Our research highlighted that the UFR had a strong associated with the HGS in the healthy group.
Introduction
Voiding dysfunction which is generally divided into obstructive symptom or underactive symptom is certainly one of the problems of high prevalence bothering the elderly in the aging society. In a large U.S. cohort study showed men with low urinary tract symptom (LUTS) had a worse health status [Citation1]. In the perspective of histology, men aged over 80-year-old almost develop benign prostatic hyperplasia (BPH). However, but the degree of urinary symptom caused by the BPH is highly variable. In addition to BPH, another common etiology of voiding symptom is an impaired bladder contractility and even an acontractile bladder [Citation2]. According to the statistics, approximately one-third of the patients’ diagnosis of BPH still had the urinary problem even after BPH surgery. It was suggested that the cause of voiding symptom is multifactorial, especially underactive bladder (UAB) played the important rule in urinary dysfunction.
In recent years, more articles focused on the relationship between UAB contraction and the age-related loss of muscle mass and muscle strength [Citation3,Citation4]. The UAB caused by neurogenic, myogenic, aging, and drug side effects was also known as detrusor smooth muscle underactivity. Besides, reduced handgrip strength (HGS) was an early indicator of sarcopenia or dynapenia in the systemic review [Citation5]. To the best of our knowledge, reduced muscle strength in aging would be accompanied with increased comorbidity and mortality of the elderly [Citation6–9]. Many articles took the HGS as a standard for assessing muscle strength due to feasibility and convenience [Citation10–12] in all population. Urinary flow rate (UFR) is a non-invasive screening tool to evaluate and quantify voiding, which is influenced by detrusor contraction strength and bladder outlet resistance. In our study, we provide the association between UFR and HGS in the healthy group.
Methods
Study design and participants selection
During the period 2011–2012, a cross-sectional research including 2258 participants who involved the program at the National Health and Nutrition Examination Survey (NHANES) was performed. The subjects with missing data such as biochemical examinations, body composition measurement, and urine collection were also excluded. These eligible participants were divided into four groups based on the urine flow rate (UFR) quartiles: Q1 = UFR < 0.449, Q2 = 0.449 < UFR < 0.736, Q3 = 0.736 < UFR < 1.238, Q4 = UFR > 1.238. The NCHS Institutional Review Board (IRB) had permitted the NHANES research protocol. The whole informed consents from every eligible participant were obtained after explaining the whole processes. All experimental methods were performed in accordance with the relevant guidelines and regulations of the CDC.
Measurement of urine flow rate
The UFR was earned with uroflowmetry and unit is (mL/min). Urine excretion rate (mg/min) was a measurement of the quantity of urine produced in a specified period of time. The urine excretion rate (mg/min) is the product of the UFR (mL/min) and the urine analyte concentration (mg/mL). Before coming to the mobile examination center (MEC), the subjects had to record their last urination time. Next, they would record the voiding time and volume of the urine sample and count the UFR three times. Collection of specimens was divided into different containers to guaranteeing enough data for various analyses. The composite UFR (mL/min) was measured as dividing total urine volume collected by the total time covered by all collected voids [Citation13].
Description of laboratory methodology
Before coming to the MEC, the subjects had to record their last urination time. Next, they would record the voiding time and volume of the urine sample and count the UFR three times. Collection of specimens was divided into different containers to guaranteeing enough data for various analyses. Every participant had three data of maximum UFRs by each urinary, but the final data was collected depending on the total number of spot urines in the MEC. The following diagram presented the protocol for collecting urine samples and recording time duration for each voiding.
Measurement of handgrip strength
The participants squeezed the dynamometer with maximal force maintaining with the hand vertical to the ground for 5 s. We measured the best of three values for each hand to determine the muscle strength. We defined low muscle strength as the participants with the lowest strength of handgrip tertiles in our research.
Measurement of the covariates
All people were drawn to the blood sample according to the standard procedure. All samples would be processed, preserved and transported to the Collaborative Laboratory Services for examination. Aspartate aminotransferase (AST) was measured by using the enzymatic rate method in the DxC800 Chemistry Analyzers. Albumin concentration was exanimated by a bichromatic digital endpoint method. Chemical analyses of serum total cholesterol level were performed by the timed-endpoint method. The variety in absorbance at 520 nm and the TC level are in direct proportion. The level of the serum creatinine was evaluated based on the Jaffe rate method.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) software program, version 18.0 was adopted for the statistical analysis in our research. We defined the threshold for statistical significance as the p-value of ≤0.05. Besides, we presented four extended-model methods for variables adjustment. Unadjusted model 1 and estimated multivariable-adjusted models 2–4 were investigated. Model 2 was adjusted for age and race. Model 3 was further adjusted for BMI and biochemical biomarkers; Model 4 was adjusted for past medical histories, including arthritis, congestive heart failure, coronary heart disease, angina, heart attack, and smoking. Last, logistic regression was examined for the correlation between UFR and the presence of low muscle strength.
Result
Characteristics of the study participants of urine flow rate association with handgrip strength
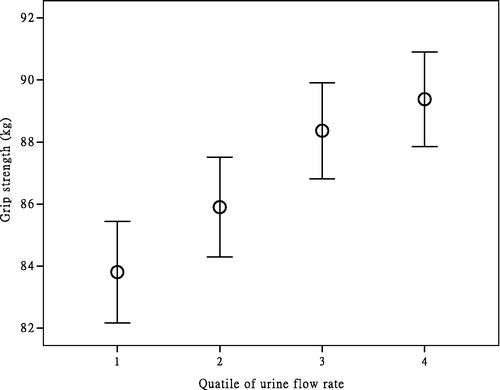
All 2258 individuals who participated in the program in NHANES from 2011 to 2012 were stratified by biochemical and demographic data according to UFR quartiles shown in . The people with greater HGS or young age had tended to higher UFR compared to the participants in the lower quartiles (p < 0.005). On the aspect of biochemistry data, higher albumin or lower creatinine had a significant tendency to higher urine flow. On the contrary, the subject’s history of arthritis had worse urine flow (p < 0.001). Other data such as BMI, Waist circumference, SBP, AST, serum TC, serum FG, race and cardiogenic disease however, had no obvious relationship to the UFR (p > 0.025). The distribution of the quartiles of UFR and HGS is shown in .
Table 1. Characteristics of study participants.
Association between urine flow rate and hand grip strength
The regression models listed in showed that HGS had a direct correlation to the UFR in the unadjusted models (β coefficient: 2.310, 95% CI: 1.357, 3.263, p < 0.001). Noticeably, the result remained significant in total adjusted model. (model 2: p < 0.001, model 3: p < 0.001, model 4: p < 0.001).
Table 2. Regression coefficients of urine flow rate for hand grip strength.
Association between the quartiles of urine flow rate and hand grip strength
The relationship between the HGS and UFR quartiles was shown in . For male participants, the β coefficients of a total adjusted model for each quartile were 2.935 (CI 0.781–5.089, p = 0.008), 3.876 (CI 1.763–5.989, p < 0.001) and 4.546 (CI 2.462–6.630, p < 0.001), respectively. Collectively, the male participants with the highest quartile of UFR have a greater HGS than those with the lowest quartile of UFR (β coefficient: 4.546, CI: 2.462, 6.630, p < 0.001).
Table 3. Association between the quartiles of urine flow rate and hand grip strength.
Odd ratios of the presence of low muscle strength categorized by the quartiles of the UFR
The quartile-based multivariate logistic regression models were investigated to clarify the association between low muscle strength and UFR (). In model 4, the odds ratios of the low muscle strength for each quartiles of the UFR were 0.668 (0.486–0.919, p = 0.013), 0.535 (0.387–0.741, p < 0.001), 0.489 (0.350–0.684, p < 0.001), respectively. Taken together, males with higher quartiles of UFR tended to have a lower risk of low muscle strength.
Table 4. Association between the quartiles of urine flow rate and low muscle strength.
Discussion
In our cross-sectional study, we explored the relationship between UFR and HGS. Notably, our study provided evidences that the UFR had a strongly positive association with HGS in all adjusted models. Greater UFR quartiles presented a prominent correlation with greater strength of handgrip in all models. We observed the dose-dependent relationship between the rate of urine flow and strength of the handgrip. Furthermore, the higher rate of urine flow was significantly associated with the presence of low muscle strength.
In recent years, some articles showed the relationship among handgrip, testosterone and LUTSs. Several kinds of research showed that grip strength had a significant association with testosterone [Citation14,Citation15]. In a population-based cohort study published in 2015, Sean Martin reported that HGS and testosterone levels had a negative correlation with LUTSs [Citation16]. In the new article by Anne M. Suskind, indicated that frailty, aging might be linked to LUTSs such as the UAB [Citation17]. Karim and his colleagues indicated that testosterone therapy had a prominent improvement in people with urinary problems or frailty [Citation18,Citation19]. The previous study between arthritis and UFR is rare. Lee [Citation20] showed that people with rheumatic arthritis combined with Sjögren’s syndrome had greater severity of LUTSs due to chronic inflammation which affected all body organs. Emerging evidence revealed that low glomerular filtration rate had a positive correlation with high HGS [Citation21]. The consequence of the article above was consistent with our study that a low UFR was a biomarker to detect lower HGS in the specific group. Especially, our article is the first one to explore the effect of biochemical values on the UFR. It is also the first large-scale study to analyze the correlation between UFR and HGS in all healthy populations. Although the subject has no complaints of dysuria, the UFR has decreased potentially with the decrease of grip strength. In the end, it is a relatively new topic to compare the relationship between low muscle strength and UFR.
To the best of our knowledge, the low UFR was regarded as a kind of bladder voiding disorder describing UAB due to detrusor underactivity [Citation22]. Detrusor underactivity defined as a lower contract strength leading to weak stream or prolonged bladder empty time in accordance with the International Continence Society [Citation23]. Previous studies indicated that poor voiding function such as poor urinary stream has resulted from both myogenic and neurogenic mechanisms [Citation24]. In S. A. GILPIN’s research involving people from age 20 to 79-year-old, he observed that the nerves with acetylcholinesterase in detrusor muscle had a negative correlation with age. Therefore, the stimulation of the detrusor muscle would be decreased resulting in the decline of detrusor contractility. Moreover, a similar finding discovered by the electron microscope that the numbers of axon profiles and smooth muscle cells revealed an obvious linear reduction with age [Citation25]. Elbadawi [Citation26] found that some microscopic changes such as the detrusor muscle fibrosis with loss and the axonal degeneration observed in people with UAB. Furthermore, the new research by Ito et al. [Citation27] presented that the patients with UAB expressed reduced activity in the M3 muscarinic receptor. According to many articles shown above, it is certainly that aging played an important role in the decline of muscle strength such as detrusor muscle leading to the UAB. In recent years, scientists had found the complex aging process to cause loss of muscle mass and strength [Citation28]. Aging was thought to be related to reduced numbers of muscle fibers and motor units resulting in the decline of muscle mass [Citation29–31].
Besides, the decline of muscle fiber may be related to apoptosis which neuron degeneration and even loss would be revealed [Citation32]. It is suggested that not only detrusor smooth muscle but skeletal muscle had a similar neuromuscular change in aging. The study [Citation33] showed the people with muscle wasting and weakness had decreased functioning motor units and slowly impulse conduction in distal regions of axons of their extensor digitorum brevis muscles. Relative search [Citation34,Citation35] showed the older people had reduced skeletal muscle fibers and contractility than the younger group. Recently, many articles took the strength of handgrip as a predictor of the strength of muscle due to its suitable and convenience [Citation36,Citation37]. Therefore, we believed that aging-related low muscle strength is extensive, including smooth muscle and skeletal muscle. The inference was reasonable according to the result of our study that lower UFR had a significant association with lower HGS.
There were several limitations in our article. First, it was not a longitude study but a cross-sectional study so that we acquired limited causal inference between UFR and HGS. Secondary, the assessment of the detrusor pressure by cystometry was not available in the dataset. Although the assessment of the maximum UFR was currently the most common method to measure obstructive urination, the average UFR might be a useful indicator to detect detrusor muscle weakness while focusing on the aging process of healthy population. Next, the generalization of the finding to the female population may be precluded because of only male participants in our study. Last, recall bias would exist because we acquired the participant’s past medical history by questionnaire.
Conclusions and implications
Our research showed that the greater UFR was significantly correlated with greater HGS in the healthy population. Besides, the weak urine stream had a strong association with low muscle strength in the specific group. Furthermore, it is inferred that the decline of muscle strength was affected not only the skeletal muscle but the smooth muscle such as detrusor muscle even in the healthy individuals. In other words, we found that the simultaneous decline in UFR and muscle strength occurs in the healthy population. This consequence will be helpful for us to identify the etiology of urinary dysfunction. Compared the UFR with HGS will be a clue to identify detrusor muscle underactivity. Although the healthy subjects did not have urinary symptoms, the UFR also potentially decreased synchronously as decreased HGS. Moreover, the degeneration of muscle power is not simply occurred in the elder. Voiding symptom is a disease involving multiple factors of dysregulation. In addition to the evaluation of the UFR, there will be more biological indicators to assess the etiology of the urinary problem to get the appropriate and precise treatment for patients in the future.
Author contributions
Hao-Tse Chiu contributed to the design of the study, was responsible for the management and retrieval of data, contributed to initial data analysis and interpretation, and drafted the initial manuscript. Hao-Tse Chiu, Tung-Wei Kao, Tao-Chun Peng, Yuan-Yuei Chen and Wei-Liang Chen decided upon the data collection methods. Hao-Tse, Chiu and Wei-Liang Chen were also responsible for the data analysis decisions. Wei-Liang Chen conceptualized and designed the study, supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors meet the ICMJE criteria for authorship.
Disclosure statement
The authors reported no conflicts of interest in this work.
References
- Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the health professionals follow-up study. Urology. 2002;59:245–250.
- Kaplan SA, Ikeguchi EF, Santarosa RP, et al. Etiology of voiding dysfunction in men less than 50 years of age. Urology. 1996;47:836–839.
- Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013;15:11–22.
- Goh VH, Hart WG. Associations of physical exercise as a lifestyle habit with lean and fat body mass and handgrip strength and age in Asian men. Aging Male. 2014;17:131–135.
- Zammit AR, Robitaille A, Piccinin AM, et al. Associations between aging-related changes in grip strength and cognitive function in older adults: a systematic review. J Gerontol A. 2018;74:519–527.
- Newman AB, Kupelian V, Visser M, et al.; on Behalf of the Health, Aging and Body Composition Study Investigators. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A. 2006;61:72–77.
- Chung HS, Shin MH, Park K. Association between hand-grip strength and erectile dysfunction in older men. Aging Male. 2018;21:225–230.
- Ucak S, Sivritepe R, Kara O, et al. Association between sarcopenia and erectile dysfunction in males with type II diabetes mellitus. Aging Male. 2019;22:20–27.
- Ceresini G, Ceda GP, Lauretani F, et al. Mild thyroid hormone excess is associated with a decreased physical function in elderly men. Aging Male. 2011;14:213–219.
- Trosclair D, Bellar D, Judge LW, et al. Hand-grip strength as a predictor of muscular strength and endurance. J Strength Cond Res. 2011;25:S99.
- Wind AE, Takken T, Helders PJM, et al. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr. 2010;169:281–287.
- Bahat G, Tufan A, Kilic C, et al. Cut-off points for height, weight and body mass index adjusted bioimpedance analysis measurements of muscle mass with use of different threshold definitions. Aging Male. 2018:1–6.
- Hays SM, Aylward LL, Blount BC. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect. 2015;123:293–300.
- Chiu HT, Shih MT, Chen WL. Examining the association between grip strength and testosterone. Aging Male. 2019:1–8.
- Chin KY, Soelaiman IN, Naina Mohamed I, et al. Testosterone is associated with age-related changes in bone health status, muscle strength and body composition in men. Aging Male. 2012;15:240–245.
- Martin S, Vincent A, Taylor AW, et al. Lower urinary tract symptoms, depression, anxiety and systemic inflammatory factors in men: a population-based cohort study. PLoS One. 2015;10:e0137903.
- Suskind AM. Frailty and lower urinary tract symptoms. Curr Urol Rep. 2017;18:67.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199:257–265.
- Strollo F, Strollo G, More M, et al. Low-intermediate dose testosterone replacement therapy by different pharmaceutical preparations improves frailty score in elderly hypogonadal hyperglycaemic patients. Aging Male. 2013;16:33–37.
- Lee KL, Chen MY, Yeh JH, et al. Lower urinary tract symptoms in female patients with rheumatoid arthritis. Scand J Rheumatol. 2006;35:96–101.
- Tufan A, Tufan F, Akpinar TS, et al. Low glomerular filtration rate as an associated risk factor for sarcopenic muscle strength: Is creatinine or cystatin C-based estimation more relevant? Aging Male. 2017;20:110–114.
- Aizawa N, Igawa Y. Pathophysiology of the underactive bladder. Investig Clin Urol. 2017;58:S82–S89.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178.
- Taylor JA, 3rd, Kuchel GA. Detrusor underactivity: clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc. 2006;54:1920–1932.
- Gilpin SA, Gilpin CJ, Dixon JS, et al. The effect of age on the autonomic innervation of the urinary bladder. Br J Urol. 1986;58:378–381.
- Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. III. Detrusor overactivity, The. J Urol. 1993;150:1668–1680.
- Ito H, Kamei J, Aizawa N, et al. Preventive Effects of long-term caloric restriction on aging related in vivo bladder dysfunction and molecular biological changes in the bladder and dorsal root ganglia in rats. J Urol. 2016;196:1575–1583.
- Bauer JM, Kaiser MJ, Sieber CC. Sarcopenia in nursing home residents. J Am Med Dir Assoc. 2008;9:545–551.
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol (Bethesda, Md.: 1985). 2003;95:1717–1727.
- Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol (Bethesda, Md.: 1985). 2001;91:1341–1349.
- Thompson LV. Age-related muscle dysfunction. Exp Gerontol. 2009;44:106–111.
- Ozkaya GY, Aydin H, Toraman FN, et al. Effect of strength and endurance training on cognition in older people. J Sports Sci Med. 2005;4:300–313.
- Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles, Journal of neurology. Neurosur Psychiatr. 1973;36:174–182.
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294.
- D’Antona G, Pellegrino MA, Adami R, et al. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511.
- Wind AE, Takken T, Helders PJ, et al. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr. 2010;169:281–287.
- Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care. 2015;18:465–470.