Abstract
Depressive symptoms are throughout our life, especially in the older population, the sex hormones reduction link to a high risk of depression. In this study, we investigated whether bilateral orchiectomy (ORX) modifies mice behaviors and antidepressant drugs effects through tail suspension test (TST). We evaluated behavioral changes at 1 week, 2 weeks, 1 month, and up to 2 months after ORX. The behavior responses to doxepin, fluoxetine, and venlafaxine at 1 week, 2 weeks, 1 month, and 2 months after ORX were evaluated. No apparent difference was detected among the durations of immobility of the control group, sham operation group, and ORX group in the TST at 1 week and 2 weeks after ORX. But the immobility time of ORX group was obvious longer than that of both control group and sham operation group at 1 month and 2 months after ORX. Only the antidepressant effect of venlafaxine was observed at 1 week and 2 weeks after ORX, while the antidepressant response to fluoxetine decreased 1 month and 2 months after ORX. The response to antidepressant drugs was strongly modified in ORX mice. Our results suggest that not all antidepressant drugs are suitable for depression with androgen deficiency.
Mice with low androgen were more prone to depression-like behaviors.
The response to antidepressants changed under the condition of low androgen in mice.
Not all antidepressant drugs are appropriate for patients with low androgen.
Highlights
Introduction
Aging is a part of the life process, which brings various changes in structure and function to the body in both male or female. In men, during and after andropause, as serum androgen level decreases, individuals may develop disorders in body functions. Recent studies have shown that, in addition to sexual function, androgen also involves in multi-system functions such as locomotor movement [Citation1], circulation [Citation2], bone metabolism [Citation3], glucose and lipid metabolism [Citation4] and so on. The decrease of androgen may link to functional disorders in older men.
Depression is also a disorder with high prevalence in older men. Depression is a kind of mood disorder characterized by persistence of sadness, loss of interest or pleasure, feelings of guilt or low self-worth, and changes in weight or appetite [Citation5]. It has a high incidence over a lifetime, especially in climacterium [Citation6]. During the period of climacterium, a specific “window” in our life, we may experience various types of changes such as anxiety, insomnia, vasomotor symptoms, hot flushes, and temperature fluctuations. This condition is resulted from the continuous decrease of sex hormones production [Citation7,Citation8] both in male and female. This hint that the lowering of sex hormones may be a factor which changes the susceptibility of the body to this disease. Until today, lots of depression treatment drugs have been developed, commonly used anti-depression ones include tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs) and serotonin noradrenaline reuptake inhibitors (SNRIs), and these drugs have shown various degrees of therapeutic effect on depression-like behavior in both experimental and clinical studies [Citation9–11]. However, it is not completely clear whether the drugs are effective equally in the treatment of depression with andropause. Previous studies have shown that low estrogen status could affect the antidepressant effects of some medications [Citation12,Citation13], however, whether the low androgen could change the body’s response to antidepressant remains unclear. The purpose of this study was to see whether male hormone deprivation influence the body’s response to antidepressants. We simulated the andropause status in mice by bilateral orchiectomy, and observed the changes of the susceptibility to depression and the effect of three typical antidepressants, doxepin, fluoxetine and venlafaxine by the tail suspension test (TST) with this animal model.
Materials and methods
Animals
Two-month-old ICR male mice (25–30g) were housed in a humidity and temperature-controlled room for a minimum of 3 days before ORX with a 12-h light/dark cycle and had access to food and water ad libitum. All animals experiment procedures comply with international guideline for the ethical use of experimental animals.
Bilateral ORX
The study used a model of bilateral orchiectomy (ORX) to simulate andropause status in male mice. The bilateral testicles were resected following the general method narrated in literature [Citation14]. Mice were anesthetized by intraperitoneal injection of 10% chloral hydrate (40 mg/kg) before the operation. A vertical scrotal skin incision is made using a sterile scalpel, then the testicular content is gently exposed and isolated the testicular fat, testicular were resected bilaterally after vasectomy and testicular artery ligation. The sham group underwent the same procedure but without resection of the testicular.
Tail suspension test
The TST is a model of behavioral despair which is considered as an experimental tool able to predict antidepressant effects of drugs. The animals subjected to the short-term, inescapable stress of being suspended by their tail will develop an immobile posture. The procedure followed in this study was previously described in literatures [Citation15,Citation16]. The mouse was individually suspended by its tail (approximately 1.5 cm from the tip of the tail) using adhesive scotch tape in a box (30 × 30 × 30 cm) with the head 5 cm to the bottom of the box. Behavioral tests were always performed between 10 AM and 4 PM with minimal background noise, immobility time was recorded during a 6-min period, and all the tests were recorded by a video camera.
Drug treatment
In order to investigate the responses of antidepressant drugs in ORX mice, drug treatment was started from the day after bilateral ORX. We used intragastric administration of doxepin (38.57 mg/kg), fluoxetine (10.28 mg/kg), venlafaxine (28.92 mg/kg) and the treatment lasted for 2 months. The mice of vehicle groups were given an equal volume of sterile water. TST was used to measure the immobility time of mice at the specific timepoint (1 week, 2 weeks, 1month, and 2 months) after the first administration.
Behavioral evaluation
Generally, the TST from start to finish is six minutes in length and we counted the immobility time of last four minutes (3–6 min). We uploaded the video files from the camera to a computer and the length of immobility was quantified under blind conditions. Mice were considered immobile when they moved only slightly or took no action [Citation16].
Statistical analyses
All the data were presented as the mean ± SD, in order to compare the differences between groups, t-test and one-way analysis (One-way ANOVA) of variance were used to determine the results of the variance between groups. Comparisons were considered statistically significant for p < 0.05. All statistical analyses were made using the standard software package SPSS 22.0.
Results
Effect of ORX on depression-like behavior in the TST
There was no significant difference among the control group, sham group and ORX group in the TST at 1 week after bilateral ORX (). And the similar results were observed from the experiment at 2 weeks after the operation (). The duration of immobility between the control group and sham group remain no obvious difference at 1 month, as we predicted, however, the immobility time of ORX group was longer than both of the control group and sham group (p < 0.05, ). More pronounced differences were observed at 2 months after bilateral ORX, the average of durations of immobility of ORX group was longer than other two groups dramatically. It implies that chronic androgen deficiency may be a key factor in the occurrence of depression in a particular condition, such as TST ().
Figure 1. Behavioral changes in the TST in mice after bilateral ORX. (A and B) The immobility time of the ORX group was approximate with both control group and sham group in the TST at 1 week and 2 weeks after bilateral ORX. (C and D): The immobility time of the ORX group was longer than the other 2 groups in the TST 1 month and 2 months after the operation. *expresses p values < 0.05 as compared to control animals, #expresses p values < 0.05 as compared to sham animals. NS: no statistically significant (n = 8 animals in each group).
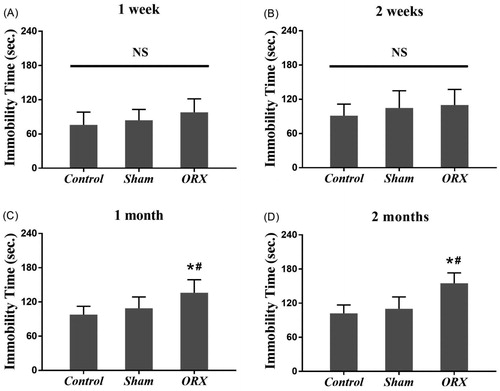
Figure 2. Changes in the response to antidepressants in the TST after ORX. (A and B) Only the venlafaxine group showed a significant reduction in the duration of immobility in ORX animals compared with the sham group in the TST at 1 week and 2 weeks after ORX. (C and D) There was no apparent difference on the immobility time between sham mice and ORX mice of doxepin group and venlafaxine group. A significant increase of immobility time in ORX mice, however, was observed in fluoxetine group compared with sham mice at 1 month and 2 months after operation. *expresses p values < 0.05 as compared to the control animals (n = 8 animals in each group).
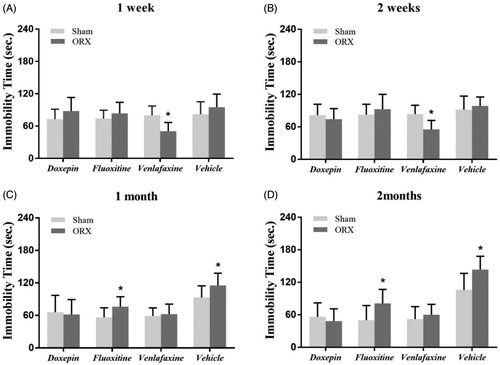
Changes in the response to antidepressant drugs after ORX
In order to explore whether ORX influence the response to antidepressant drugs, we studied if ORX modifies antidepressant treatment effects by TST. As shown in , there was no difference in the duration of immobility in both of the doxepin group and the fluoxetine group compared with the sham group, however, the duration of immobility of the mice which took venlafaxine decreased compared with sham group (p < 0.05), the similar phenomenon was found in experimental result at two weeks after ORX ().
At 1 month after operation, the duration of immobility of ORX mice in doxepin group remained no significant difference compared with sham group. The decrease, however, in the immobility time caused by venlafaxine at 1 week and 2 weeks in ORX mice was disappeared, there was no apparent difference of the immobility time between the sham group and ORX group mice which took venlafaxine. Furthermore, a changed response by ORX was discovered in the fluoxetine group: the duration of immobility was increased obviously compared with sham group 1 month and 2 months after the operation (p < 0.05). These suggest that ORX altered the individual response when administered with the drugs. Although there was no significant difference in the vehicle groups at 1 week and 2 weeks after the operation, the immobility time of ORX group was longer than the sham group and blank control group in the experiment at 1 month (p < 0.05), the similar result was observed from experiments at 2 months after ORX .
Discussion
The decline of the performance of the gonads during and after andropause leads to reduction of androgen, which affects the quality of life and health in older male. The changes of testosterone, a male hormone, in its production and metabolism may link to variations of the body, including locomotor functions [Citation1], sexual functions [Citation17,Citation18], cardiovascular functions [Citation2,Citation19], endocrine functions [Citation19], lipometabolism [Citation3,Citation4], and glucose metabolism [Citation20], and even affects seating behavior in elderly [Citation21]. Several epidemiological studies by Aging Male’s Symptom scale (AMS) have confirmed that serum testosterone level affects the physical and mental health in older males [Citation22–24]. Moreover, it is reported that testosterone replacement therapy (TRT) could help improve vascular function and glycemic control in obese hypogonadal males with type 2 diabetes mellitus [Citation25]. The findings of present study, the increase of susceptibility to depression and changes of response to antidepressants in the ORX mice, are also from the low testosterone.
This study explored the behavioral changes caused by ORX and the changes in the response to antidepressant drugs after ORX in mice. No significant difference was observed in the depressive behavior test among the three groups of mice including ORX group, control group and sham group within two weeks after operation, and this may be related to the stress response to the physical trauma resulting from the operation and related to the endogenous androgen which still exist in the mice. The immobility time of the ORX mice, though increased compared with control group and sham group after 1 month, and the difference of the duration of immobility were even more pronounced two months later. The results revealed that mice with long term androgen deficit were more prone to depression-like behaviors in a special stressful condition such as TST, which is consistent with previous reports that men who suffer from climacterium were more likely to have the appearance of depression [Citation26]. Recently, studies have shown that in older people, low testosterone associated with aging can increase the risk of a variety of diseases, such as hypertension, dyslipidemia, obesity, and diabetes. And low androgen results in decrease of antiinflammation ability of body [Citation27], the latter may contribute to the pathology of some aging related disease such as arteriosclerosis or Alzheimer’s disease. Here our findings indicate that low testosterone is also a negative factor on neuropsychiatric health.
The main finding of present study is the change of the response to antidepressant drugs under androgen deprived condition. Indeed, it was observed from the results that the effect of antidepressants has changed. Only the mice which administered with venlafaxine in ORX group expressed a significant efficacy in the TST within 2 weeks after the operation. The antidepressant effects of doxepin and fluoxetine, however, were barely observed at the same time point. In fact, these two drugs even had no effects on the sham ORX mice, which is probably related to the stress from the physical injuries shortly after the operation. Therefore, the study manifested that the antidepressant effect of venlafaxine was significantly better than that of doxepin and fluoxetine in the early phase of androgen deprivation. Venlafaxine is one of serotonin and norepinephrine reuptake inhibitors (SNRIs) and is frequently used in the treatment of clinical depression [Citation28]. Previous study showed that the effects of SNRI were independent of age in the treatment of depression [Citation29], in addition it has been reported that venlafaxine could induce a reduction in sensitivity of 5-HT1B autoreceptors in hippocampus and hypothalamus [Citation30]. The 5-HT1B receptor is broadly involved in body functions including satiety [Citation31], sleep [Citation32], memory and learning [Citation33], sexual behavior [Citation34], and emotion [Citation35]. This suggests that a sharp drop in testosterone level in the short-period may be responsible for the enhanced antidepressant effect of venlafaxine in early stage of androgen deficiency.
In the following experiments, although there was no apparent difference between the sham mice and ORX mice in the fluoxetine group within 2 weeks after operation, the immobility time was increased in ORX mice compared with sham operation mice after 1 month and 2 months. This reveals that the antidepressant effect of fluoxetine was on the decline during the two months, but the effects of doxepin and venlafaxine did not change significantly during the long-term observation with androgen deficiency. Fluoxetine, one of the selective serotonin reuptake inhibitors (SSRIs), because of the short cure period and the low incidence of adverse effects compared with tricyclic drugs and monoamine oxidase inhibitors [Citation36], since 1980s, a preference has developed for SSRIs. Previous study showed that fluoxetine may play an antidepressant role by increasing brain-derived neurotrophic factor (BDNF) levels, but the underlying mechanism still remains unclear [Citation37]. Recent researches revealed that male hormone increases adult neurogenesis within the dentate gyrus region of the hippocampus through an androgen-dependent pathway, latter including BDNF signaling [Citation38]. In this research, the decline of the antidepressant effect of fluoxetine may result from the drop in androgen levels during the antidepressant process. Previous clinical observation confirmed that the efficacy of fluvoxamine (SSRI) in young patients was better than that in elderly patients [Citation29]. The similar phenomenon was observed from the animal experiment in this study.
Doxepin is a tricyclic antidepressant, the result of the study showed that the effect of doxepin on the mice with long-term deprivation of androgen was significantly better than its effect for short term deprivation of estrogen. It suggests that doxepin performed well after a long-term estrogen deficiency. However, the clinical application of the drug is limited due to the low selectivity and serious side effects [Citation39].
In conclusion, even though the extrapolation of our experimental results to humans may not be fully suitable, the present data underline that not all antidepressant drugs are appropriate for patients with androgen deficiency, especially for those with andropause. This suggests that the response of patient’s body to the drugs may change due to low androgen, and this factor should be taken into account when clinical treatment for depression in older men. Furthermore, the present study indicates that venlafaxine will be a choice for depressive patients with androgen deficiency, and that supplementing appropriate dose of testosterone preparations with antidepressant may be more desirable, and the latter is consistent with the views of some recent clinical studies [Citation19,Citation21,Citation26]. Finally, according to the present study, antidepressant-related behaviors can be easily and reliably tested in an experimental model, which may help to develop new antidepressant drugs for male climacteric depression patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fink JE, Hackney AC, Matsumoto M, et al. Mobility and biomechanical functions in the aging male: testosterone and the locomotive syndrome. Aging Male. [cited 2018 Sep 29]; [8 p.]. DOI:10.1080/13685538.2018.1504914
- Gagliano-Jucá T, Basaria S, Gagliano-Jucá T, et al. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol. 2019;16(9):555–574.
- Lu Y, Li J, Cheng X, et al. testosterone level in aging male with different glucose tolerance state and its association with osteocalcin. Nat Rev Cardiol. 2019;22(1):68–73.
- Kwon H, Lee D-G, Kang HC, et al. The relationship between testosterone, metabolic syndrome, and mean carotid intima-media thickness in aging men. Aging Male. 2014;17(4):211–215.
- Peña CJ, Nestler EJ. Progress in epigenetics of depression. Prog Mol Biol Transl Sci. 2018;157:41–66.
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74(1):67–83.
- Khosravi S, Ardebili HE, Larijani B. Are andropause symptoms related to depression?. Aging Clin Exp Res. 2015;27(6):813–820.
- Birkhäuser M. Depression, menopause and estrogens: is there a correlation?. Maturitas. 2002;41(Suppl 1):S3–S8.
- Laffleur F, Zilio M, Shuwisitkul D. Modified biomolecule as potential vehicle for buccal delivery of doxepin. Ther Deliv. 2016;7(10):683–689.
- Manao K, Yuri H, Kayoko F, et al. Anti-depressant fluoxetine reveals its therapeutic effect via astrocytes. EBioMedicine. 2018;32:72–83.
- Nikfarjam M, Rakhshan R, Ghaderi H. Comparison of effect of Lavandula officinalis and venlafaxine in treating depression: a double blind clinical trial. J Clin Diagn Res. 2017;11(7):KC01–KC04.
- Hernandez-Hernandez OT, Martinez-Mota L, Herrera-Perez JJ, et al. Role of estradiol in the expression of genes involved in serotonin neurotransmission: implications for female depression. Curr Neuropharmacol. 2019;17(5):459–471.
- Pae CU, Mandelli L, Kim TS, et al. Effectiveness of antidepressant treatments in pre-menopausal versus post-menopausal women: a pilot study on differential effects of sex hormones on antidepressant effects. Biomed Pharmacother. 2009;63(3):228–235.
- Felix-Patrício B, Miranda AF, Medeiros JL, et al. The prostate after castration and hormone replacement in a rat model: structural and ultrastructural analysis. Int Braz J Urol. 2017;43(5):957–965.
- Steru L, Chermat R, Thierry B, et al. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985;85(3):367–370.
- Can A, Dao DT, Terrillion CE, et al. The tail suspension test. J Vis Exp. 2012;59:e3769.
- Ajo R, Segura A, Mira L, et al. The relationship of salivary testosterone and male sexual dysfunction in opioid-associated androgen deficiency (OPIAD). Aging Male. 2017;20(1):1–8.
- Kaya E, Sikka SC, Kadowitz PJ, et al. Aging and sexual health: getting to the problem. Aging Male. 2017;20(2):65–80.
- Carruthers M, Cathcart P, Feneley MR. Evolution of testosterone treatment over 25 years: symptom responses, endocrine profiles and cardiovascular changes. Aging Male. 2015;18(4):217–227.
- Nucci RAB, Teodoro ACS, Krause Neto W, et al. Effects of testosterone administration on liver structure and function in aging rats. Aging Male. 2017;20(2):134–137.
- Kummer KK, Pope HG, Hudson JI, et al. Aging male symptomatology and eating behavior. Aging Male. 2019;22(1):55–61.
- Taniguchi H, Kawa G, Kinoshita H, et al. Symptomatic change in Japanese hypogonadal patients several years after androgen replacement therapy. Aging Male. 2011;14(3):190–194.
- Horie S, Hisasue S-i, Nakao M, et al. Correlation between the Japanese Aging Male Questionnaire (JAMQ) and Aging Male’s Symptom (AMS) scale in Japanese male. Aging Male. 2014;17(1):35–41.
- Erenpreiss J, Fodina V, Pozarska R, et al. Prevalence of testosterone deficiency among aging men with and without morbidities. Aging Male. [cited 2019 Jun 1]; [5p.].DOI:10.1080/13685538.2019.1621832
- Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21(3):158–169.
- Miller KK, Wexler TL, Zha AM, et al. Androgen deficiency: association with increased anxiety and depression symptom severity in anorexia nervosa. J Clin Psychiatry. 2007;68(6):959–965.
- Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129–140.
- Thase M, Asami Y, Wajsbrot D, et al. A meta-analysis of the efficacy of venlafaxine extended release 75-225 mg/day for the treatment of major depressive disorder. Curr Med Res Opin. 2017;33(2):317–326.
- Naito S, Sato K, Yoshida K, et al. Gender differences in the clinical effects of fluvoxamine and milnacipran in Japanese major depressive patients. Psychiatry Clin Neurosci. 2007;61(4):421–427.
- Gur E, Dremencov E, Van De Kar LD, et al. Effects of chronically administered venlafaxine on 5-HT receptor activity in rat hippocampus and hypothalamus. Eur J Pharmacol. 2002;436(1-2):57–65.
- Voigt JP, Fink H. Serotonin controlling feeding and satiety. Behav Brain Res. 2015;277:14–31.
- Boutrel B, Franc B, Hen R, et al. Key role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. J Neurosci. 1999;19(8):3204–3212.
- Buhot MC, Martin S, Segu L. Role of serotonin in memory impairment. Ann Med. 2000;32(3):210–221.
- Giuliano F. 5-Hydroxytryptamine in premature ejaculation: opportunities for therapeutic intervention. Trends Neurosci. 2007;30(2):79–84.
- Tiger M, Varnäs K, Okubo Y, et al. The 5-HT(1B) receptor – a potential target for antidepressant treatment. Psychopharmacology (Berl). 2018;235(5):1317–1334.
- Carlini EA, Noto AR, Nappo SA, et al. Fluoxetina: indícios de uso inadequado. Jornal Brasileiro de Psiquiatria. 2009;58(2):97–100.
- Hodes GE, Hill-Smith TE, Lucki I. Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett. 2010;484(1):12–16.
- Spritzer MD, Roy EA. Testosterone and adult neurogenesis. Biomolecules. 2020;10(2):225.
- Wilson K, Mottram P. A comparison of side effects of selective serotonin reuptake inhibitors and tricyclic antidepressants in older depressed patients: a meta-analysis. Int J Geriatr Psychiatry. 2004;19(8):754–762.

