Abstract
The relative proportional increase of the elderly population within many countries will become one of the most significant social transformations of the twenty-first century and, for the first time in history, persons aged 65 or above outnumbered children under five years of age globally. One in four persons living in Europe and Northern America will be aged 65 or over. One of the goals of ISSAM is to raise awareness of the special health needs of older men. Since a significant number of aging men will eventually become testosterone deficient, the Hypogonadism panel of ISSAM updates its guidelines.
Introduction
The relative proportional increase of the elderly population within many countries will become one of the most significant social transformations of the twenty-first century and, for the first time in history, persons aged 65 or above outnumbered children under five years of age globally. One in four persons living in Europe and Northern America will be aged 65 or over. One of the goals of ISSAM is to raise awareness of the special health needs of older men. Since a significant number of aging men will eventually become testosterone deficient, the Hypogonadism panel of ISSAM updates its guidelines periodically.
The International Society for the Study of the Aging Male (ISSAM) Hypogonadism panel consists of a multidisciplinary group of experts, including urologists, endocrinologists, andrologists and internists with various subspecialties. The first recommendations were published in 2002 [Citation1]. Due to the need for ongoing re-evaluation of the information presented in the recommendations they were revised in 2005 [Citation2], 2009 [Citation3], 2013 [Citation4] and 2015 [Citation5].
It must however be remembered that recommendations can never replace clinical expertise. Treatment decisions, selection of treatment protocols or choice of products for individual patients must take into account patients’ personal needs and wishes. Hypogonadism or Testosterone Deficiency (TD) in adult men as defined by low levels of serum testosterone accompanied by symptoms and/or signs as detailed below. These symptoms are compiled from observations and recognized in clinical entities such as Klinefelter’s syndrome, Kallmann’s syndrome, pituitary or testicular disorders, as well as in men with idiopathic, metabolic or iatrogenic conditions that result in testosterone deficiency. These recommendations do not encompass the full range of pathologies leading to hypogonadism (testosterone deficiency) but instead focus on the clinical spectrum of hypogonadism related to associated morbidities, metabolic disturbances and idiopathic disorders that contribute to the majority of cases occurring in adult and elderly men. New data have accumulated since the last revision of the recommendations were published that adds to a better understanding of the benefits and limitations of testosterone therapy (TTh) in elderly men (i.e. 65+) [Citation6]
Recommendation 1: definition
Testosterone deficiency (TD) in adult men is a clinical and biochemical syndrome associated with a low level of testosterone, which may adversely affect multiple organ functions and quality of life. Although the clinical significance of TD in adult men is becoming increasingly recognized, the extent of its prevalence in the general population is still a matter of uncertainty and perhaps underappreciated. A large number of men with TD who would be expected to benefit from testosterone treatment, continue to remain undiagnosed and untreated due to deficient basic knowledge and postgraduate medical training in sexual medicine [Citation7,Citation8].
Recommendation 2: clinical diagnosis
Check for TD in men with symptoms and signs of testosterone deficiency
The diagnosis of TD requires the presence of characteristic symptoms and signs in combination with decreased serum concentration of testosterone. Symptoms of TD may be categorized as sexual and non-sexual. The European Male Aging Study (EMAS) a population-based survey performed on more than 3,400 men aged 40–80 clearly showed that sexual symptoms, including erectile dysfunction (ED), diminished frequency of morning erections and decrease in sexual thoughts (low libido) were the most frequent symptoms in identifying patients with low T [Citation9]. Similar results were reported by other authors [Citation10–13]. Other sexual symptoms associated with TD include difficulties in achieving orgasm or reduced intensity of orgasm [Citation14].
Several other non-sexual symptoms such as fatigue, impaired concentration, depression and decreased sense of vitality and/or wellbeing have been associated with TD. However, the role of psychological and physical symptoms in identifying subjects with low testosterone (T) is more conflicting [Citation9]. Signs or risk factors for TD also include anemia, osteopenia and osteoporosis, low-energy fracture, myopathy and frailty, tender gynecomastia, abdominal obesity and metabolic syndrome [Citation15]. Principally, the clinician has to distinguish between TD of primary (testicular) and secondary (hypothalamic/pituitary) etiology and TD associated with other conditions and/or co-morbidities including drug-induced TD, potentially reversible and recently also named as functional hypogonadism [Citation16]. Hence, the classical etiologies with well-known congenital and acquired testicular or pituitary dysfunctions that require lifelong substitution (e.g. Kallmann syndrome, Klinefelter syndrome. anorchia due to trauma or orchiectomy, pituitary lesions/tumors) should be distinguished from forms of TD that might be reversible. The latter, potentially reversible forms of TD are most often found in co-existence with metabolic disorders such as obesity/type 2 diabetes mellitus (T2DM), inflammatory diseases (e.g. chronic obstructive pulmonary disease, chronic inflammatory bowel diseases, prolactinoma) or psychological problems such as depressive mood or stress [Citation16]. Screening questionnaires or structured interviews on male symptomatic TD, although sensitive, have low specificity [Citation17]. Morley et al. compared the most commonly used questionnaires in 148 men using bioavailable testosterone (BT) for the diagnosis of TD and found the sensitivity to be 97% for the ADAM (Androgen Deficiency in the Aging Male questionnaire), 83% for the AMS (Aging Male’s Symptoms scale) and 60% for the MMAS (Massachusetts Male Aging Study questionnaire). Specificity was 30% for the ADAM, 59% for the MMAS and 39% for the AMS [Citation18] (now validated in many languages [Citation19,Citation20]. Althogh other large face-to-face comparisons are lacking, more recently a large systematic review including 40 studies concluded that [Citation17], a specific structured interview, ANDROTEST, for detecting hypogonadism related symptoms and signs, showed both the most favourable positive and negative likelihood ratio for detecting low T [Citation17]. Despite having low specificity, the AMS and other male TDcase-history tools may be useful to assess the presence and severity of symptoms [Citation21] and for monitoring the clinical response to testosterone therapy [Citation22–25]. However, it should be recognized that the latter instruments can not be used to diagnose TD which requires the demonstration of reduced T circulating levels.
Physical examination of patients with suspected TD should include an assessment of the amount and distribution of body hair (including beard growth and pubic hair); the presence of acanthosis nigricans, associated with insulin resistance [Citation26–28], presence of gynecomastia; size and consistency of the testes; abnormalities in the scrotum and size, the appearance of the penis. Weight, height, body mass index (BMI) and waist circumference should also be measured since symptoms and signs potentially indicative of TD in men include height loss, reduced muscle bulk and strength and increased body fat, in particular, abdominal fat accumulation and BMI [Citation29–32]. The greater the number of symptoms in a man, the greater the probability that he truly has TD [Citation33]. However, the presence of even one symptom may raise suspicion of symptomatic TD. A high prevalence of symptomatic TD has been observed in populations of aging men, especially those with diabetes mellitus type 2, obesity [Citation34], benign prostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTS) [Citation35,Citation36].
The presence of symptoms alone does not constitute testosterone deficiency. Symptoms must be accompanied by decreased serum concentrations of total testosterone (TT) or free T level to support a diagnosis of symptomatic TD.
Various prospective studies have reported the occurrence of hypogonadal symptoms as side effects of androgen deprivation therapy, including hot flashes, decreased libido and ED [Citation37,Citation38]. Other complications of androgen-deprivation therapy include osteoporosis, with increased risk of fractures, and worsening of comorbidities such as diabetes mellitus, cardiovascular disease and metabolic syndrome, as well as physical, functional and cognitive impairment [Citation39–42].
Based on the above and below mentioned data, we recommend the investigation of TD in men with the following conditions (amost frequently associated with TD ) ().
Table 1. Disorders and conditions most frequently associated with TD.
Reasons for drug-induced TD are listed in .
Table 2. Medications that may be the reason for low testosterone level/decreased testosterone bioactivity.
Recommendation 3: pathogenesis
Check for concurrent diseases and drug consumption as a reason of impaired testosterone production
The metabolic disorders such as obesity, T2DM, inflammatory diseases and other co-morbidities mentioned above modify the hypothalamic–pituitary–gonadal axis by suppressing one or more of its components (decreased hypothalamic–pituitary/decreased Leydig cell function) but not in a constant fashion as in the permanent forms of TD where one of the components has irrevocably decreased function.
The age-related decrease in testosterone production is related to an impairment of hypothalamic–pituitary–gonadal axis [Citation46], but in contrast, may not be reversible. The current opinion is that TD and the respective co-morbidity reinforce each other.
Risk factors for TD may include chronic illnesses, including T2DM, hyperprolactinemia, chronic obstructive lung disease, rheumatoid arthritis, renal and HIV-related diseases, obesity, metabolic syndrome [Citation47], stress and hemochromatosis, vitamin D deficiency [Citation48–50]. Severe vitamin D deficiency may contribute to lower TT level [Citation51], which is also confirmed by a recent meta-analysis [Citation52] Such chronic diseases should be investigated and treated.
Though there is still a controversy in defining normal thyroid stimulating hormone (TSH) levels in the elderly [Citation53–56], thyroid gland function impairment should be considered in all patients with TD, as symptoms of hypothyroidism may overlap those of TD.
TT levels have been reported to be lower in depressed men compared with non-depressed men [Citation57]. TT is particularly low in men with severe, treatment-resistant depression [Citation58].
Poor sleep habits, as identified by shift work sleep disorder, may also contribute to the decrease of TT level [Citation59].
Drugs such as glucocorticoids, opioids, antipsychotics induce TD [Citation60–62]. Glucocorticoids are widely used as anti-inflammatory drugs. However, prolonged use of glucocorticoids results in undesirable side effects, including TD [Citation62]. It has also been reported that statins may reduce TT [Citation63]. In addition, several other drugs can interfere with T production acting either at central or peripheral levels and/or influencing T bioactivity ().
Aloisi and colleagues [Citation64] were the first to show that morphine induces a dramatic and long-lasting decrease in TT. This finding has now been corroborated by numerous subsequent studies [Citation65–67].
Recommendation 4: laboratory diagnosis
In patients at risk or suspected of TD, a thorough physical and biochemical work-up is recommended.
Liquid chromatography–tandem mass spectrometry (LCMS/MS) assays are considered the gold standard for TT measurement provide consistently higher accuracy, specificity and sensitivity than do most immunoassays [Citation68]. LCMS is not universally available, and immunoassays are still much more frequently used around the world, with reasonable accuracy in most cases. The key laboratory tests to confirm the diagnosis of TD are serum total and free testosterone. Theoretically, free testosterone (FT) concentrations may have a better correlation with clinical symptoms of testosterone deficiency [Citation69,Citation70]. LCMS/MS should remain the gold standard method even for free T evaluation [Citation68]. Free T assays based on analog displacement immunoassays are still widely available and used some positive results [Citation71], but their reliability has been seriously questioned by some authors [Citation72,Citation73]. The use of formulae or algorithms based on the binding characteristics of sex hormone binding globulin (SHBG) and albumin have been proposed as a method to calculated FT (http://www.issam.ch/freetesto.htm) [Citation68]. Among the latter, the Vermeulen method [Citation74] showed the most consistent results when compared to LCMS/MS. Regarding the best method for free T evaluation, no consensus among the experts composing this group has been reached.
Another promising method to define TD consists of salivary-free testosterone evaluation, but the lower testosterone limit is still disputable. Saliva contains only the non-SHBG bound fraction of T, which can freely diffuse across capillaries and salivary ducts [Citation75]. It should be noted that some conditions affect interpretation such as hydration state, moreover, saliva collection devices may alter salivary concentrations of sex steroids.
No consensus has been reached regarding the lower TT threshold defining TD, and there is no generally accepted lower limits of normal TT [Citation76]. This lack of consensus follows from the fact that no studies have shown a clear threshold for TT or free T that distinguishes men who will respond to treatment from those who will not. Data from EMAS studies proposed 11 nmol/L as a lower cut-off value for TT [Citation9]. TD may be considered if the TT level is below 12.1 nmol/L based on LCMS/MS measurements in three large cohorts comprising more than 10000 men of various ages [Citation76]. Similarly, data derived from meta-analyses suggest a lack of benefits of TTh in subjects with a total T > 12 nmol/L [Citation77–79].
The single greatest variable confounding the interpretation of total T is that SHBG concentrations vary enormously from one individual to another, among both younger and older men. Since SHBG concentrations greatly influence test results for hormones that bind to SHBG, recognition of this large interindividual variability should be considered in the clinical interpretation of these hormone results, particularly for T [Citation80]. Routine SHBG testing should be considered for men suspected of T deficiency.
Note should be made that transient decreases of serum T levels can occur, due to acute illnesses [Citation81], and this should be excluded by careful clinical evaluation and repeated hormone measurement.
Free T levels as low as 220–347 pmol/L (63.5–100 pg/mL) [Citation69,Citation71,Citation82] have been recommended as a lower limit of the normal range and together with the presence of one or more hypogonadal symptoms can provide supportive evidence for TTh.
It is preferred to obtain a serum sample for TT determination between 07.00 and 11.00 h [Citation83], although diurnal variation is substantially blunted in older men. In a cross-sectional study of 3006 men with the mean age 60.3 years presenting for prostate cancer screening, serum testosterone concentrations were unchanged from 6 am to 2 pm, and then decreased by only 13% between 2 pm and 6 pm [Citation84]. However, other authors have reported that the diurnal circadian rhythm of free testosterone and bioavailable testosterone may persist in men younger than 75 years [Citation85].
A large body of evidence has documented that circulating T levels are substantially decreased (from 14 to more than 30%) if not measured in fasting conditions [Citation86,Citation87]. Hence blood testing for T should be performed in a fasting state which allows to adequately measure also glucose and lipids to detect co-morbidities.
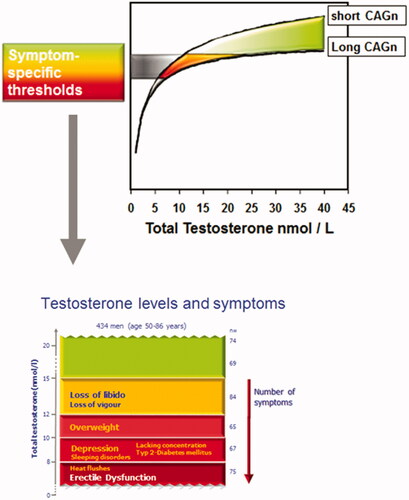
The number of cytosine–adenine–guanine triplet (CAG) repeats in androgen receptors differs in men and influences the androgen receptor activity [Citation88,Citation89,Citation90,Citation91] (). Hence testosterone sensitivity may vary in different individuals. It has also been argued that the magnitude of the decrease in serum T concentrations might be a better predictor of TD than the actual concentrations of TT and BT [Citation92].
The same applies to androgen receptor gene CAG repeat lengths >24 in the presence of symptoms and normal testosterone levels may be considered as a state of preclinical TD [Citation93].
The prevalence of hypogonadal symptoms increases with TT levels below 12.1 nmol/L (350 ng/dL) [Citation33]. However, Zitzmann et al. have shown that TD symptoms may also be seen with TT levels as high as 15 nmol/L. This study showed that the prevalence of loss of libido or vigor increased at testosterone concentrations below 15 nmol/L (p < 0.001), whereas depression and T2DM (also in non-obese men) were significantly more prevalent in men with TT concentrations below 10 nmol/L (p < 0.001). ED has been identified as a composite pathology of metabolic risk factors, smoking and depression, whereas only TT concentrations below 8 nmol/L contributed to that symptom (p = 0.003). Behre et al. [Citation21] demonstrated that 6 months of TTh improved body composition and quality of life in men aged 50–80 years with TT < 15 nmol/L and hypogonadal symptoms; these men showed further improvements in body composition and quality of life over the following 12 months of TTh. Lower TT levels have also been shown to be associated with sub-threshold symptoms of anxiety and depression [Citation94]. There is also a recent study reporting increased hypogonadal symptoms in younger men ≤40 years with TT below 400 ng/dL (13.9 nmol/L) [Citation95]. Free or bioavailable T should be considered when the TT concentration does not correspond with clinical presentation, since individual variation in SHBG concentrations may influence total testosterone results.
Measurements of serum luteinizing hormone (LH) will assist in differentiating between primary and secondary TD. All cases of elevated LH level and testosterone below normal or in the lower quartile range would indicate testicular failure and TTh should be considered [Citation96]. When elevated LH is present with normal TT without any signs of TD, a compensated TD should be considered, that is an early state of (overt) TD, in that case, dynamic monitoring is recommended [Citation97]. Measurement of serum prolactin level is indicated when TT is <5.2 nmol/L (150 ng/dL) [Citation98,Citation99] or when hypogonadotropic TD due to a pituitary tumor (like prolactinoma) is suspected [Citation100–102]. Similar considerations should be applied for pituitary MRI scans.
In general, it is currently speculated that variable phenotypes of androgen insensitivity exist, mainly owing to mutated androgen receptors. More subtle modulation of androgen effects is related to the CAG repeat polymorphism in exon 1 of the androgen receptor gene: transcription of androgen-dependent target genes is attenuated with the increasing length of triplets. As a clinical entity, the CAG repeat polymorphism can relate to variations of androgenicity in men in various tissues and psychological traits: the longer the CAG repeat polymorphism, the less prominent is the androgen effect when individuals with similar testosterone concentrations are compared. A strictly defined threshold to TD is likely to be replaced by a continuum spanned by genetics as well as symptom specificity. In addition, the effects of externally applied testosterone can be markedly influenced by the CAG repeats and respective pharmacogenetic implications are likely to influence indications as well as modalities of testosterone treatment of hypogonadal men. Investigation of CAG repeat polymorphism in exon 1 of the androgen receptor gene may be useful in testosterone treatment regimens adjustment.
Animal and human studies suggested a direct and indirect stimulatory effect of androgens on erythropoiesis, as well as the fact that TD is a well-established cause for anemia, especially in a condition, known as «unexplained anemia in the elderly». The current knowledge on this topic is summarized in 2019 in a systematic review by Al-Sharefi et al. [Citation103] and demonstrated an increase in hemoglobin level in the TTh trials with men >65 years old with the known and unknown reasons for anemia. Taking into account the role of testosterone in pathogenesis and treatment of anemia in the elderly we recommend the investigation of total blood count and ferritin in men with suspected age-related TD and concomitant treatment of diagnosed anemia [Citation6].
We recommend 12.1 nmol/L as a lower limit of normal for serum TT level. However, due to individual SHBG concentration variation, TTh may be reasonably offered to symptomatic men with testosterone concentrations higher than 12 nmol/L if free T concentrations are reduced.
Recommendation 5: assessment of treatment outcome and decisions on continued therapy
Improvement in hypogonadal signs and symptoms occur at different times for different organ systems
The only indication for TTh in men is confirmed TD. Improvement in hypogonadal signs and symptoms occur at different times for different organ systems [Citation104].
Reduction in fat mass and increased lean body mass and muscle strength occur within 12–16 weeks of starting TTh, data from available meta-analyses on observational studies indicate that the effects on body weight can require up to 2 years [Citation79].
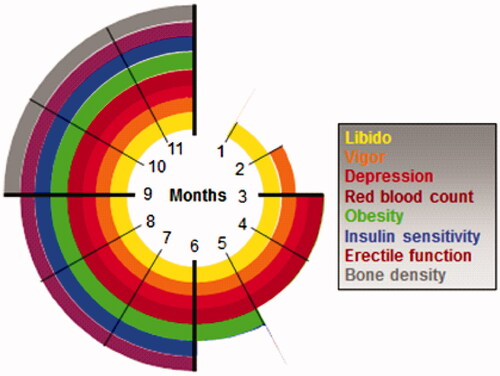
Significant improvement in libido is usually experienced within 3–6 weeks of commencing TTh whereas ED improvement can be observed after 12 weeks [Citation78]. Up to 12 months of TTh may be required before significant improvement in ejaculatory function is observed [Citation105]. Some publications show continued improvement of erectile function for up to 4-9 years [Citation106,Citation107]. Significant improvement in quality of life (QoL) usually occurs within 3–4 weeks of starting TTh; longer-term TTh is required to achieve maximum QoL benefit. Effects on depressive mood become detectable after 3–6 weeks of starting TTh, with maximum improvement occurring after 18–30 weeks. Improvements in bone are detectable after 6 months of TTh, while the full beneficial effect of TTh on bone mineral density may take 2–3 years [Citation108,Citation109] or even 6 years as suggested by Haider et al. [Citation110].
Effects of TTh on lipids appear after 4 weeks, with maximal effects being seen after 6–12 months of treatment. Insulin sensitivity may improve within a few days of starting TTh, but effects on glycaemic control become evident only after 3–12 months. Failure to improve clinical symptoms within a reasonable period should result in reevaluation of TTh with regard to dosage, compliance and level of serum T achieved. Further investigation should be undertaken to determine other causes of the symptoms.
Recommendation 6: body composition and mobility
TTh lead to a reduction of fat massas well as the consistent increase in lean mass
In hypogonadal men, TTh improves body composition (decrease of fat mass, increase of lean body mass). Meta-analyses of randomized trials in middle-aged and older men have demonstrated the beneficial effects of TTh in reducing fat mass [Citation79,Citation111,Citation112] with a significant increase in lean body mass and grip strength.
Rodrıguez-Tolrà et al. demonstrated clearly that TTh in men with TD decreased fat mass overall, and to the greatest extent in the android and gynecoid regions and caused improvements in body composition, increasing lean mass, primarily in arms and legs [Citation113].
Data mainly derived from observational studied showed that TTh is potentially effective treatment in aging obese men with TD [Citation27]. There is also some evidence that long-term T may result in substantial and sustained reductions in body weight, waist circumference and BMI in obese hypogonadal men [Citation114–116]. The successful achievement of weight loss, as well as the consistent increase in lean mass, lead to beneficial effects on diabetes mellitus type 2 [Citation117]. However, it should be recognized that observational studies present important limitations and that the data obtained in placebo-controlled RCTs did not completely confirm the positive role of TTh on body weight and metabolic profile [Citation118,Citation119].
Within the T-Trials, a greater magnitude of subjective improvement in physical ability compared to objective measurements was observed within the active group compared to placebo [Citation6].
Higher free testosterone concentration is positively associated with a lower risk of developing mobility limitation and progression of mobility limitations [Citation120].
In young male cancer survivors with TD TTh compared with placebo was associated with decreased trunk fat mass, decreased whole-body fat mass and increased lean body mass [Citation121].
Long-term observational data indicate weight and waist circumference improve progressively in overweight and obese men on TTh over a period of up to 11 years [Citation116].
Recommendation 7: bone density and fracture rate
TD is a risk factor for secondary osteoporosis
TTh significantly increases vertebral and hip bone density in hypogonadal men
Osteopenia, osteoporosis and fracture prevalence rates are increased in younger and older hypogonadal men [Citation122]. In a recent meta-analysis and in the Fracture Risk Assessment Tool (FRAX) algorithm TD was identified as a known disorder strongly associated with secondary osteoporosis [Citation123,Citation124]. Total testosterone measurement is suggested in all men evaluated for osteoporosis or considered for pharmacological treatment with bone-active agents [Citation125].
TTh significantly increased vertebral and hip bone density in hypogonadal men of all ages [Citation108,Citation111,Citation126–128].
In older men, low testosterone levels are associated with an increased risk of falls [Citation129]. TTh has beneficial effects on muscle mass and strength that may reduce the propensity to fall and therefore decrease fracture risk. Physical exercise, including stretching and equilibrium exercises, is mandatory in combination with TTh. In older men, dihydrotestosterone (DHT) was inversely associated with hip fracture risk and SHBG was positively associated with hip fracture risk [Citation130]. Causal effects of serum estradiol levels on fracture risk were robust in sensitivity analyses and remained unchanged in stratified analyses for age, body mass index, bone mineral density as estimated by quantitative ultrasound of the heel, smoking status, and physical activity [Citation131].
Assessment of bone density at 2-year intervals is recommended in aging, hypogonadal men with normal bone density. In men with lowered bone mineral density receiving TTh, stabilization or progress may be monitored with annual dual-energy X-ray absorptiometry (DXA). This should be performed using DXA as a gold standard method providing the largest amount of reliable data.
Meanwhile, there are no studies on TTh on fracture risk, TTh is not recommended as an approved osteoporosis treatment, though clinical experience shows that TTh may be efficient. We suggest TT measurement in all men with osteoporosis.
TTh should not be prescribed as a monotherapy in patients with TD and increased bone fracture risk.
Recommendation 8: testosterone and sexual function
TTh improves libido and erectile function in men with TD
The initial assessment of men with ED and/or diminished libido or impaired morning erections should include determination of TT and free T level. These symptoms, with or without testosterone deficiency, might be related to co-morbidities (i.e. T2DM, hyperprolactinemia, obesity, bladder outlet obstruction, peripheral vascular disease or medications).
Men with poor morning erections, ED and/or diminished libido and documented TD are candidates for TTh. Meta-analyses of randomized, placebo-controlled trials of TTh in men with sexual dysfunction and varying TT levels demonstrated benefits in some aspects of sexual desire, erectile function and performance [Citation77,Citation78,Citation132].
Long-term TTh in hypogonadal men resulted in significant improvements in urinary and sexual function, and quality of life [Citation133]. Several recently published placebo-controlled trials showed TTh improve sexual activity and desire in older men with TD [Citation134–136].
In sildenafil non-responders with T2DM, a combination of oral T undecanoate and sildenafil was associated with improvement in erections, a significant increase in International Index of Erectile Function-5 scale and increased sexual contacts [Citation137].
In hypogonadal men with an inadequate response to phosphodiesterase type-5 inhibitors, TTh has been shown to be of benefit. In an international, multicenter, prospective study (IPASS) with a sample of 1493 men, TTh showed a significant improvement in libido, erectile function and response to PDE-5 inhibitors (PDE5i) therapy [Citation138]. However, it should be recognized the other studies have failed to confirm the positive outcomes between TTh and PDE5i [Citation118]. Hence, more placebo-controlled studies are advisable to better clarify the latter issue.
In aging hypogonadal men presenting with one or more sexual dysfunction symptoms and low TT levels, a short (3–6 months) trial of TTh may be justified. Meanwhile, there is data that a 12- months period is necessary to see an improvement in sexual function in some men [Citation105]. If no improvement in sexual function is noted after an adequate trial of treatment, further investigation should be undertaken to determine other causes of ED.
An inadequate response to TTh requires a reassessment of the causal mechanisms responsible for the sexual dysfunction.
Recommendation 9: testosterone and obesity, metabolic syndrome and type 2 diabetes mellitus
We suggest measurement of serum T level in men with obesity and diabetes mellitus type 2, particularly in symptomatic subjects
Many of the components of the metabolic syndrome (MetS) such as obesity, hypertension, dyslipidemia, impaired glucose regulation and insulin resistance are also present in hypogonadal men [Citation139]. The MetS and T2DM are frequently associated with low TT levels and a majority of the patients with these conditions display symptoms of TD [Citation34,Citation140,Citation141]. In a large epidemiologic study of more than 1150 healthy middle-aged Japanese men, the probability of MetS was associated with lower levels of serum TT [Citation142]. The meta-analysis of the available cross-sectional data suggests that MetS can be considered an independent association of male TD [Citation143].
In addition to improving TD symptoms, TTh may have other benefits on metabolic status in hypogonadal men with diabetes and/or MetS, that include improvement of surrogate parameters of cardiometabolic risk [Citation144], such as significant reductions in fasting plasma glucose, homeostasis model assessment index (HOMA), triglycerides and waist circumference. It has been demonstrated that TTh may be safely utilized to ameliorate somatic and psychological frailty symptoms in association with improved anthropometric and glucometabolic parameters in aging, overweight men with TD and impaired fasting glucose [Citation145]. TTh improved significantly glycemic control (HbA1c), insulin levels and sensitivity, triglycerides, and waist circumference and C-reactive protein levels [Citation105,Citation143,Citation146–148]. In a recently published double-blind placebo-controlled randomized trial TTh showed significant improvement in glucose metabolism and reduced proportion of participants with type 2 diabetes beyond the effects of a lifestyle program [Citation149]. However, it should be recognized that that study enrolled a cohort of obese, borderline hypogonadal [TT < 14 nmol/L], subjects, the majority of them with impaired glucose tolerance and a minor fraction with T2DM [20%]. Hence, the available placebo-controlled studies and the number of enrolled patients are too limited to draw final conclusions on the metabolic effects of TTh. Hence, at present, TTh cannot be prescribed with the aim to improve metabolic profile in subjects with MetS T2DM or obesity.
Recommendation 10: testosterone, cardiovascular disease and all-cause mortality
There is no evidence showing increased cardiovascular (CV) mortality or morbidity with TTh
Several studies have reported a significant inverse association between serum T levels and markers of atherosclerosis. In addition, a negative correlation between TT levels, endothelial dysfunction and carotid intima–media thickness (IMT) independent of other cardiovascular risk factors have been also documented [Citation150–152]. Prospective observational studies in TTh-treated men with coronary artery disease showed that TTh improved endothelial function [Citation153]. Conversely, data from placebo-controlled RCT are more conflicting:2 RCT showed a reduction of carotid IMT with the effect being independent of BMI after 2 years of TTh (mean age 58 years) [Citation144,Citation154], whereas data derived from TTrials showed a significant increase in noncalcified coronary artery plaque volume but not in calcified coronary artery calcium score during 1 year of TTh in older men (mean age 72 years) [Citation6].
While the role of inflammation in cardiovascular disease is becoming apparent [Herring MJ, Oskui PM, Hale SL, Kloner RA], several studies confirm the association between low testosterone and low-grade systemic inflammation. Observational evidence suggests that several pro-inflammatory cytokines and TT are inversely associated in patients with coronary artery disease and T2DM [Citation155], while TTh in hypogonadal men with the MetS may reduce levels of inflammatory markers [Citation138,Citation156–158].
Data derived from retrospective observational studies published in the last 10 years have suggested possible increased CV mortality and morbidity in men under TTh [Citation159–162]. However, it should be recognized that the latter studies have been submitted to important criticisms which have limited their clinical significance [Citation163,Citation164]. ISSAM guidelines authors claim these results should be interpreted with caution.
Meanwhile, several meta-analyses show a neutral or beneficial effect of TTh on cardiovascular disease. Meta-analysis of 37 observational studies including 43,041 subjects with a mean age of 63.5 years and mean follow-up of 6 years showed low endogenous TT predicted overall and CV mortality, as well as CV morbidity when both unadjusted and fully adjusted models were considered [Citation165].
Recently published meta-analysis of pharmaco-epidemiological studies documented that TTh reduced overall mortality and cardiovascular morbidity. Meta-analysis of all placebo-controlled randomized clinical trials does not support a causal role between TTh and adverse cardiovascular events and even showed a protective role when studies enrolling obese (body mass index >30 kg/m2) patients were considered [Citation166].
A big systematic review including 10 randomized controlled trials showed TTh in patients with heart failure (HF) was not associated with an increase in mortality or HF hospitalization rate [Citation167]. Moreover, Oni et al. demonstrated in a large observational cohort of male veterans with previous myocardial infarction (MI), normalization of TT levels with TTh was associated with decreased all-cause mortality compared with those with non-normalized TT levels and the untreated group. Furthermore, in this high-risk population, TTh was not associated with an increased risk of recurrent MI [Citation168].
Several epidemiological studies as well as a recently published meta-analysis showed no association between TTh and risk of venous thromboembolism in men [Citation169–172].
TD may influence not only the quality of life in men, but also the life span. There are strong observational data indicating that low endogenous testosterone levels are associated with increased risk of all-cause and cardiovascular disease-related mortality [Citation165,Citation173–175], meanwhile, TTh may improve survival in hypogonadal men [Citation107,Citation176–178].
Lower serum testosterone is independently associated with higher all-cause and cancer-related, but not CV-related, mortality in middle-aged to older men as shown in a recent case analysis of 149,436 men with 10,053 deaths (1,925 CVD and 4,927 cancer-related) [Citation179].
In conclusion, available data showed that when appropriately diagnosed and managed TTh in subjects with TD is not associated with increased CV mortality and morbidity. Possible preliminary positive results on CV outcomes should be confirmed through larger placebo-controlled RCT.
Recommendation 11: depression and cognitive function
TTh is associated with a mild reduction of depressive symptoms
Some meta-analyses showed a significant positive effect of TTh to be effective and efficacious in reducing depressive symptoms in men, assessed by the Hamilton Rating Scale for Depression when compared with placebo [Citation180,Citation181]. TTh has been shown to reduce depression symptoms in hypogonadal men, including middle-aged men with MetS [Citation182] and those using antidepressants [Citation183,Citation184]. However, data derived from TTrials showed that the final effect of TTh on depressive symptoms was small in magnitude [Citation6]. In line addition, the largest meta-analysis of available studies, considering up to 1900 hypogonadal (baseline total testosterone <12 nmol/L or fT <225 pmol/L) men showed that the positive effect of TTh was particularly evident only in patients with milder symptoms [Citation181].
Though the effect of TTh on cognitive function in men with TD remains controversial [Citation185,Citation186], the recent meta-analysis supports the potential for TTh as a preventative measure against cognitive decline [Citation187] and it can be considered after exclusion of other causes of cognitive impairment [Citation188,Citation189].
In a meta-analysis of seven prospective cohort studies, low plasma TT level was significantly associated with an increased risk of Alzheimer's disease in elderly men [Citation190].
Within the T-Trials, there was no improvement in cognitive function and no difference between placebo controls and active treatment. In line with the latter data the most recent meta-analysis including 17 studies enrolling 1,438 patients with a mean age of 70.4 years and a mean follow-up of 45.6 weeks failed to detect a positive effect of TTh on several cognitive outcome measures [Citation191].
Recommendation 12: benign prostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTS)
There is no evidence that TTh either increases the risk of BPH or contributes to the worsening of LUTS
Approximately one in five men with BPH has low TT. There is a well-established relationship between LUTS/BPH and increased BMI and low TT [Citation192].
At present, there is no evidence that TTh either increases the risk of BPH or contributes to the worsening of LUTS [Citation104,Citation193]. Several studies, including long-term TTh data, reported improvement in LUTS in hypogonadal men with mild BPH [Citation133,Citation194–196]. Data derived from TTrials showed no difference in IPSS (International Prostate Symptom Score) score in T treated group compared with placebo [Citation6].
Recommendation 13: prostate cancer (PCa)
There is no evidence of increased PCa risk in men on TTh
Recent evidence fails to support the longstanding fear that T therapy will increase prostate cancer risk or cause rapid growth of occult cancer. Indeed, a recent meta-analysis including 608 testosterone-deficient PCa survivors, of whom 109 were high-risk, showed TTh may not increase biochemical recurrence risk [Citation197], another meta-analysis did not observe a higher rate of biochemical recurrence risk after TTh for nonmetastatic PCa [Citation198]. Furthermore, in a number of studies TTh was prescribed in hypogonadal men with untreated prostate cancer undergoing active surveillance with no signs of cancer progression [Citation199–201] or nonsignificant compared to the controls [Citation202].
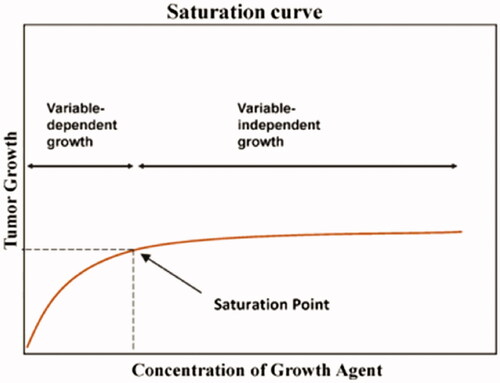
The relationship between testosterone and prostate cancer appears to follow a saturation curve, present in many biological systems, in which growth corresponds with a concentration of a key nutrient until a concentration is reached in which an excess of the nutrient is achieved [Citation203] (). Clinical data indicate the saturation point for serum T is approximately 250 ng/dL (8.68 nmol/L) [Citation183,Citation204,Citation205].
Figure 2. Saturation curve, demonstrating the relationship between testosterone and prostate cancer.
There is no evidence that TTh will convert sub-clinical prostatic lesions to clinically detectable PCa.
A recently published review of observational studies did not find any significant association between TT level and PCa risk [Citation206]. Analysis of pooled worldwide data from 18 prospective studies (more than 3000 cases and 6000 controls) found no significant association between serum testosterone concentrations and prostate cancer risk [Citation207]. A meta-analysis showed no significant association between TTh and the incidence of PCa or the need for prostate biopsy when compared with the placebo/non-intervention group [Citation208,Citation209].
In a multicenter, prospective study (IPASS) with a sample of 1493 men, the prevalence of such adverse effects like increase of the hematocrit and increase of the prostate-specific antigen (PSA) was 51%, no cases of prostate cancer were observed [Citation138]. In observational, prospective registry studies in 1023 patients receiving long-term TTh with a median follow-up of 5–6 years, the incidence of prostate cancer remained well below the incidence reported in screening studies in the general population [Citation210]. These data were confirmed by a large study with a median follow-up of 5.3 years including 10,311 men on TTh and 28,029 controls [Citation159]. The study showed the rate of a new diagnosis of PCa was lower for men who received TTh compared with men who did not, the longer the duration of TTh, the lower the risk of developing PCa [Citation159].
Two meta-analyses showed no increased risk of prostate cancer development or progression on TTh [Citation211,Citation212], and the placebo arm of the REDUCE trial showed no increased risk of cancer associated with serum T or DHT levels in43000 men who underwent study biopsies of the prostate at year 2 and year 4.
Nonetheless, in the absence of large-scale, long-term controlled studies, it is impossible to definitively assert the safety of TTh with regard to PCa. Therefore, prior to starting TTh, a patient’s risk of PCa must be assessed using, at a minimum measurement of serum prostate-specific antigen (PSA). Pretreatment assessment should include PCa risk predictors such as age, family history of PCa and ethnicity/race. If suspicion of PCa exists, it may be reasonable to perform a prostate biopsy if warranted by clinical presentation. Testosterone therapy may be initiated in these men if a prostate biopsy is negative.
After initiation of TTh, patients should be monitored for prostate disease with measurement of serum PSA at 3–6 months, 12 months and at least annually thereafter. In a subject with an increased risk of PCa urologist supervision is required.
An initial increase of prostate-specific antigen (PSA) and prostate volume with TTh is frequently seen over the first 2–6 months because the prostate is an androgen-dependent organ. The increase in PSA will be greatest in men with marked TD and least (or absent) in men with milder degrees of TD [Citation204]. The PSA level at 6 months after initiation of TTh should be used as the new baseline. Should a patient’s PCa risk be sufficiently high based on suspicious findings on digital rectal examination (DRE)/prostate ultrasound or PSA >4.0 ng/ml, transrectal ultrasound-guided biopsies of the prostate are recommended and the patient should be referred to a urologist for further clinical examination.
Referral to a urologist for prostate evaluation and possible biopsy during TTh should be made with the development of a new palpable prostate abnormality on DRE or with a worrisome rise in PSA. Recommendations regarding what constitutes a concerning rise in PSA include an increase of 1.0 ng/ml over baseline PSA [Citation213] or a PSA velocity greater than 0.35 ng/ml per year [Citation214].
Recommendation 14: treatment and delivery systems
T delivery systems should be prescribed according to patient preference and clinical conditions
Preparations of aromatizable T should be used for TTh
Preparations of native testosterone or its esters (aromatizable T) should be used for TTh. Currently available intramuscular, subdermal, transdermal, oral and buccal T preparations are safe and effective.
However, long-acting injectable T undecanoate and T gels have demonstrated the best safety profile when compared to older shorter-acting T esters such as propionate or enanthate. Similarly, older oral T preparations are not longer recommended since their absorption is unpredictable and tightly related to the type of meals [Citation215].
In the case of primary TD, testosterone preparations are the only option of TTh. In the case of secondary TD, testosterone monotherapy should be used if fertility is not required. Conversely, when fatherhood is desired, the combination of hCG with menopausal/recombinant FSH should be considered [Citation216–218].
The treating physician should have sufficient knowledge and adequate understanding of the pharmacokinetics as well as of the advantages and drawbacks of each TTh preparation.
There is some evidence that selective estrogen receptor modulators (SERMs) such as clomiphene citrate and tamoxifen citrate can be used in TD treatment in men. The main action of SERMs is the inhibition of the negative feedback of estrogen at the hypothalamus and pituitary level, which results in the release of LH and FSH, which in turn increases testosterone biosynthesis and “stimulates” spermatogenesis in responsive gonads. [Citation219]
Aromatase Inhibitors (AI) such as anastrazol and letrozole lower estrogen levels by blocking the aromatase enzyme, which converts testosterone to estradiol.
However, the available data on the use of SERMS and AI for the treatment of TD are limited and of poor quality. Estrogens are vitally important players in many physiologic functions in men including bone metabolism, cardiovascular health, spermatogenesis, cognition and sexual function [Citation220,Citation221]. In addition, the use of these preparations for TD is off-lable. Hence, we recommended against the use of these drugs in men with TD.
Some authors recommend that TTh be discontinued if hematocrit is >54%, which may be reasonable while baseline hematocrit level >50% is a relative contraindication for starting testosterone therapy. However, these recommendations are based on assumptions – the clinical significance of a hematocrit >54% is unknown. The meta-analysis by Fernandez-Balsells [Citation208] showed that, despite a higher incidence of elevated hematocrit, no clinical adverse effects were reported. Results of earlier studies (MEDLINE database search from 1966 to 2004) showed that, despite TTh-treated men being nearly four times as likely to have hematocrit >50% compared with placebo-treated men (OR = 3.69, 95% CI, 1.82–7.51), the frequency of cardiovascular events, sleep apnea or death was not significantly different between the two groups. Hematocrit elevations were reported in 43.8% of patients administered intramuscular T enanthate injections and in 15.4% of patients administered transdermal T treatment [Citation222]. The lack of increase in cardiovascular events with elevated hematocrit may be due to the fact that T acts as a vasodilator and has anti-atherosclerotic effects [Citation223]. In addition, testosterone is able to decrease plasma concentrations of pro-coagulatory substances such as fibrinogen and PAI-1 as well as Factor XII [Citation224] Isolated hematocrit elevations can be the result of insufficient fluid intake on a hot day. Only repeated measures of hematocrit >54% should be followed by concomitant administration of aspirin, bleeding, therapeutic phlebotomy and/or discontinuation of TTh until hematocrit declines below 54%. After normalization of hematocrit levels, TTh can be continued with a reduced dosage.
Periodic hematological assessment is, however, indicated, i.e. before TTh, then 3–4 months and 12 months in the first year of treatment, and annually thereafter. Although it is not yet clear what upper limit of hematocrit level is clinically desirable, dose adjustments may be necessary to keep hematocrit below 52–54%.
It is recommended to clinically apply the various time-dependent effects of TTh. Each target symptom or tissue has a specific timeframe of expected response to normalization of TT level ().
Inadequate data are available to determine the optimal target serum T level for men with TD. For the present time, the treatment goal with TTh is to maintain serum T levels in the normal range. Sustained supra-physiological serum T levels should be avoided. No evidence exists for or against the need to maintain the physiological circadian rhythm of serum T levels.
Men with significant erythrocytosis (hematocrit >52%), severe untreated obstructive sleep apnoe or untreated severe congestive heart failure should not be started on treatment with TTh without prior resolution of the co-morbid condition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Morales A, Lunenfeld B. International society for the study of the aging male. Aging male. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. Aging Male. 2002;5(2):74–86.
- Nieschlag E, Swerdloff R, Behre HM, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2005;8(2):56–58.
- Wang C, Nieschlag E, Swerdloff RS, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2009;12(1):5–12.
- Lunenfeld B, Mskhalaya G, Kalinchenko S, et al. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men – a suggested update. Aging Male. 2013;16(4):143–150.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18(1):5–15.
- Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39(3):369–386.
- Al-Sharefi A, Quinton R. Current national and international guidelines for the management of male hypogonadism: helping clinicians to navigate variation in diagnostic criteria and treatment recommendations. Endocrinol Metab. 2020;35(3):526–540.
- Trinick TR, Feneley MR, Welford H, et al. International web survey shows high prevalence of symptomatic testosterone deficiency in men. Aging Male. 2011;14(1):10–15.
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in Middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135.
- Rastrelli G, Corona G, Tarocchi M, et al. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest. 2016;39(4):473–484.
- Petak SM, Nankin HR ,Spark RF, et al. American association of clinical endocrinologists. American association of clinical endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients–2002 update. Endocr Pract. 2002; 8(6):440–456.
- Lejeune H, Huyghe É, Droupy S. Diminution du désir sexuel et déficit en testostérone chez l'homme [hypoactive sexual desire and testosterone deficiency in men]. Prog Urol. 2013;23(9):621–628.
- Lunenfeld B, Arver S, Moncada I, et al. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male. 2012;15(4):187–197.
- Corona G, Mannucci E, Ricca V, et al. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl. 2009;32(6):720–728.
- Salonia A, Rastrelli G, Hackett G, et al. Paediatric and adult-onset male hypogonadism. Nat Rev Dis Primers. 2019;5(1):38.
- Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102(3):1067–1075.
- Millar AC, Lau ANC, Tomlinson G, et al. Predicting low testosterone in aging men: a systematic review. CMAJ. 2016;188(13):E321–E330.
- Morley JE, Perry HM, Kevorkian RT, et al. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas. 2006;53(4):424–429.
- Chen W, Liu ZY, Wang LH, et al. Are the aging male’s symptoms (AMS) scale and the androgen deficiency in the aging male (ADAM) questionnaire suitable for the screening of late-onset hypogonadism in aging Chinese men? Aging Male. 2013;16(3):92–96.
- Rabah DM, Altaweel W, Arafa MA. Clinical assessment and validation of an Arabic aging male symptoms questionnaire in patients with androgen deficiency. Aging Male. 2011;14(1):33–36.
- Behre HM, Tammela TL, Arver S, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male. 2012;15(4):198–207.
- Zengerling F, Schrader AJ, Cronauer MV, et al. The “aging males’ symptoms” scale (AMS): predictive value for lowered circulating androgens. Aging Male. 2012;15(4):253–257.
- Lee CP, Jiang JR, Chen Y, et al. The “aging males’ symptoms” (AMS) scale assesses depression and anxiety. Aging Male. 2013;16(3):97–101.
- Moore C, Huebler D, Zimmermann T, et al. The aging males’ symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol. 2004;46(1):80–87.
- Nakamura M, Fujimura T, Nagata M, et al. Association between lower urinary tract symptoms and sexual dysfunction assessed using the core lower urinary tract symptom score and international index of erectile function-5 questionnaires. Aging Male. 2012;15(2):111–114.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulinresistance: pathophysiology and management. Am J Clin Dermatol. 2004;5(3):199–203.
- Saad F, Aversa A, Isidori AM, et al. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev. 2012;8(2):131–143.
- Corona G, Rastrelli G, Vignozzi L, et al. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25(2):337–353.
- Wu FC, Tajar A, Pye SR, et al. European male aging study group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J Clin Endocrinol Metab. 2008;93(7):2737–2745.
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241–4247.
- Schneider G, Nienhaus K, Gromoll J, et al. Depressive symptoms in men aged 50 years and older and their relationship to genetic androgen receptor polymorphism and sex hormone levels in three different samples. Am J Geriatr Psychiatry. 2011;19(3):274–283.
- Rastrelli G, Corona G, Maggi M. Both comorbidity burden and low testosterone can explain symptoms and signs of testosterone deficiency in men consulting for sexual dysfunction. Asian J Androl. 2020;22(3):265–273.
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91(11):4335–4343.
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2008;60(7):762–769.
- Kaplan SA, O’Neill E, Lowe R, et al. Prevalence of low testosterone in aging men with benign prostatic hyperplasia: data from the proscar long-term efficacy and safety study (PLESS). Aging Male. 2013;16(2):48–51., Jun Epub 2013 Mar 12. PMID: 23480623.
- Schatzl G, Brössner C, Schmid S, et al. Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. The prostate study group of the Austrian society of urology. Urology. 2000;55(3):397–402.
- Higano C. Androgen deprivation therapy: monitoring and managing the complications. Hematol Oncol Clin North Am. 2006;20(4):909–923.
- Choong K, Basaria S. Emerging cardiometabolic complications of androgen deprivation therapy. Aging Male. 2010;13(1):1–9.
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244.
- Joly F, Alibhai SM, Galica J, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176(6):2443–2447.
- Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109(4):802–810.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Lee JH, Lee SW. Testosterone and chronic prostatitis/chronic pelvic pain syndrome: a propensity score-matched analysis. J Sex Med. 2016;13(7):1047–1055.
- Kato Y, Shigehara K, Kawaguchi S, et al. Efficacy of testosterone replacement therapy on pain in hypogonadal men with chronic pain syndrome: a subanalysis of a prospective randomised controlled study in Japan (EARTH study). Andrologia. 2020;52(9):e13768.
- White HD, Brown LA, Gyurik RJ, et al. Treatment of pain in fibromyalgia patients with testosterone gel: pharmacokinetics and clinical response. Int Immunopharmacol. 2015;27(2):249–256.
- Saad F, Gooren LJ. Late onset hypogonadism of men is not equivalent to the menopause. Maturitas. 2014;79(1):52–57.
- Cattabiani C, Basaria S, Ceda GP, et al. Relationship between testosterone deficiency and cardiovascular risk and mortality in adult men. J Endocrinol Invest. 2012;35(1):104–120.
- Lee DM, Tajar A, Pye SR, et al. Association of hypogonadism with vitamin D status: the European male ageing study. Eur J Endocrinol. 2012;166(1):77–85.
- Pilz S, Frisch S, Koertke H, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43(03):223–225.
- Wehr E, Pilz S, Boehm BO, et al. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol. 2010;73(2):243–248. Epub 2009 Dec 29. PMID: 20050857.
- Rafiq R, van Schoor NM, Sohl E, et al. Associations of vitamin D status and vitamin D-related polymorphisms with sex hormones in older men. J Steroid Biochem Mol Biol. 2016;164:11–17.
- D’Andrea S, Martorella A, Coccia F, et al. Relationship of vitamin D status with testosterone levels: a systematic review and Meta-analysis. Endocrine. 2021;72(1):49–61.
- Bremner AP, Feddema P, Leedman PJ, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554–1562.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499.
- Brochmann H, Bjøro T, Gaarder PI, et al. Prevalence of thyroid dysfunction in elderly subjects. A randomized study in a Norwegian rural community (Naerøy). Acta Endocrinol. 1988;117(1):7–12.
- Boucai L, Hollowell JG, Surks MI. An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid. 2011;21(1):5–11.
- Fischer S, Ehlert U, Amiel Castro R. Hormones of the hypothalamic-pituitary-gonadal (HPG) axis in male depressive disorders - A systematic review and Meta-analysis. Front Neuroendocrinol. 2019;55:100792.
- Dobs AS, Few WL, 3rd, Blackman MR, et al. Serum hormones in men with human immunodeficiency virus-associated wasting. J Clin Endocrinol Metab. 1996;81(11):4108–4112.
- Balasubramanian A, Kohn TP, Santiago JE, et al. Increased risk of hypogonadal symptoms in shift workers with shift work sleep disorder. Urology. 2020;138:52–59.
- Knegtering H, van der Moolen AE, Castelein S, et al. What are the effects of antipsychotics on sexual dysfunctions and endocrine functioning? Psychoneuroendocrinology. 2003;28 Suppl 2(Suppl 2):109–123.
- Molitch ME. Drugs and prolactin. Pituitary. 2008;11(2):209–218.
- Martens HF, Sheets PK, Tenover JS, et al. Decreased testosterone levels in men with rheumatoid arthritis: effect of low dose prednisone therapy. J Rheumatol. 1994;21(8):1427–1431. PMID: 7983641.
- Schooling CM, Au Yeung SL, Freeman G, et al. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
- Aloisi AM, Aurilio C, Bachiocco V, et al. Endocrine consequences of opioid therapy. Psychoneuroendocrinology. 2009;34 Suppl 1:S162–S8.
- Reddy RG, Aung T, Karavitaki N, et al. Opioid induced hypogonadism. BMJ. 2010;341:c4462.
- Basaria S, Travison TG, Alford D, et al. Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial. Pain. 2015;156(2):280–288.
- Coluzzi F, Billeci D, Maggi M, et al. Testosterone deficiency in non-cancer opioid-treated patients. J Endocrinol Invest. 2018;41(12):1377–1388.
- Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95(10):4542–4548.
- Antonio L, Wu FC, O’Neill TW, et al. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab. 2016;101(7):2647–2657.
- Rastrelli G, O’Neill TW, Ahern T, et al. Symptomatic androgen deficiency develops only when both total and free testosterone decline in obese men who may have incident biochemical secondary hypogonadism: prospective results from the EMAS. Clin Endocrinol. 2018;89(4):459–469.
- Kacker R, Hornstein A, Morgentaler A. Free testosterone by direct and calculated measurement versus equilibrium dialysis in a clinical population. Aging Male. 2013;16(4):164–168.
- Morgentaler A. Commentary: guideline for male testosterone therapy: a clinician’s perspective. J Clin Endocrinol Metab. 2007;92(2):416–417.
- Giagulli VA, Castellana M, Lisco G, et al. Critical evaluation of different available guidelines for late-onset hypogonadism. Andrology. 2020;8(6):1628–1641.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672.
- Keevil BG, Adaway J. Assessment of free testosterone concentration. J Steroid Biochem Mol Biol. 2019;190:207–211.
- Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439.
- Corona G, Isidori AM, Buvat J, et al. Testosterone supplementation and sexual function: a Meta-analysis study. J Sex Med. 2014;11(6):1577–1592.
- Corona G, Rastrelli G, Morgentaler A, et al. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur Urol. 2017;72(6):1000–1011.
- Corona G, Giagulli VA, Maseroli E, et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016;39(9):967–981.
- Krakowsky Y, Conners W, Morgentaler A. Serum concentrations of sex hormone-binding globulin vary widely in younger and older men: clinical data from a men’s health practice. Eur Urol Focus. 2019;5(2):273–279.
- Isidori AM, Lenzi A. Risk factors for androgen decline in older males: lifestyle, chronic diseases and drugs. J Endocrinol Invest. 2005;28(3 Suppl):14–22.
- Morgentaler A, Khera M, Maggi M, et al. Commentary: who is a candidate for testosterone therapy? A synthesis of international expert opinions. J Sex Med. 2014;11(7):1636–1645.
- Diver MJ, Imtiaz KE, Ahmad AM, et al. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol. 2003;58(6):710–717.
- Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100(3):509–513.
- Guay A, Miller MG, McWhirter CL. Does early morning versus late morning draw time influence apparent testosterone concentration in men aged ≥45 years? Data from the hypogonadism in males study. Int J Impot Res. 2008;20(2):162–167.
- Caronia LM, Dwyer AA, Hayden D, et al. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin Endocrinol. 2013;78(2):291–296.
- Gagliano-Jucá T, Li Z, Pencina KM, et al. Oral glucose load and mixed meal feeding lowers testosterone levels in healthy eugonadal men. Endocrine. 2019;63(1):149–156.
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22(15):3181–3186.
- Rajender S, Singh L, Thangaraj K. Phenotypic heterogeneity of mutations in androgen receptor gene. Asian J Androl. 2007;9(2):147–179.
- Zitzmann M. The role of the CAG repeat androgen receptor polymorphism in andrology. Front Horm Res. 2009;37:52–61.
- Zitzmann M. Pharmacogenetics of testosterone replacement therapy. Pharmacogenomics. 2009;10(8):1341–1349.
- Holm AC, Fredrikson MG, Theodorsson E, et al. Change in testosterone concentrations over time is a better predictor than the actual concentrations for symptoms of late onset hypogonadism. Aging Male. 2011;14(4):249–256.
- Canale D, Caglieresi C, Moschini C, et al. Androgen receptor polymorphism (CAG repeats) and androgenicity. Clin Endocrinol. 2005;63(3):356–361.
- Berglund LH, Prytz HS, Perski A, et al. Testosterone levels and psychological health status in men from a general population: the Tromsø study. Aging Male. 2011;14(1):37–41.
- Scovell JM, Ramasamy R, Wilken N, et al. Hypogonadal symptoms in young men are associated with a serum total testosterone threshold of 400 ng/dL. BJU Int. 2015;116(1):142–146.
- Tajar A, McBeth J, Lee DM, et al. Elevated levels of gonadotrophins but not sex steroids are associated with musculoskeletal pain in Middle-aged and older European men. Pain. 2011;152(7):1495–1501.
- Corona G, Rastrelli G, Dicuio M, et al. Subclinical male hypogonadism. Minerva Endocrinol. 2020. doi:https://doi.org/10.23736/S0391-1977.20.03208-3
- Citron JT, Ettinger B, Rubinoff H, et al. Prevalence of hypothalamic-pituitary imaging abnormalities in impotent men with secondary hypogonadism. J Urol. 1996;155(2):529–533.
- Bunch TJ, Abraham D, Wang S, et al. Pituitary radiographic abnormalities and clinical correlates of hypogonadism in elderly males presenting with erectile dysfunction. DAGM. 2002;5(1):38–46.
- Dalvi M, Walker BR, Strachan MW, et al. The prevalence of structural pituitary abnormalities by MRI scanning in men presenting with isolated hypogonadotrophic hypogonadism. Clin Endocrinol. 2016;84(6):858–861.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in Middle-aged and older men: estimates from the Massachusetts male aging study. J Clin Endocrinol Metab. 2004;89(12):5920–5926.
- Vermeulen A. Hormonal cut-offs of partial androgen deficiency: a survey of androgen assays. J Endocrinol Invest. 2005;28(3 Suppl):28–31.
- Al-Sharefi A, Mohammed A, Abdalaziz A, et al. Androgens and anemia: current trends and future prospects. Front Endocrinol (Lausanne)). 2019;10:754.
- Saad F, Aversa A, Isidori AM, et al. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165(5):675–685.
- Hackett G, Cole N, Bhartia M, et al. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int J Clin Pract. 2014;68(2):203–215.
- Saad F, Caliber M, Doros G, et al. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male. 2020;23(1):81–92.
- Hackett G, Cole N, Mulay A, et al. Long-term testosterone therapy in type 2 diabetes is associated with decreasing waist circumference and improving erectile Function. World J Mens Health. 2020;38(1):68–77.
- Snyder PJ, Peachey H, Berlin JA, et al. Of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670–2677.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of long-acting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male. 2012;15(2):96–102.
- Haider A, Meergans U, Traish A, et al. Progressive improvement of T-Scores in men with osteoporosis and subnormal serum testosterone levels upon treatment with testosterone over six years. Int J Endocrinol. 2014;2014:1–9.
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in Middle-aged men: a Meta-analysis. Clin Endocrinol. 2005;63(3):280–293.
- Bhasin S, Calof OM, Storer TW, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2(3):146–159.
- Rodriguez-Tolrà J, Torremadé Barreda J, del Rio L, et al. Effects of testosterone treatment on body composition in males with testosterone deficiency syndrome. Aging Male. 2013;16(4):184–190.
- Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes. 2013;3(3-4):73–83.
- Francomano D, Ilacqua A, Bruzziches R, et al. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology. 2014;83(1):167–173.
- Saad F, Doros G, Haider KS, et al. Differential effects of 11 years of long-term injectable testosterone undecanoate therapy on anthropometric and metabolic parameters in hypogonadal men with normal weight, overweight and obesity in comparison with untreated controls: real-world data from a controlled registry study. Int J Obes. 2020;44(6):1264–1278.
- Haider A, Yassin A, Doros G, et al. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:1–15.
- Corona G, Torres LO, Maggi M. Testosterone therapy: what We have learned from trials. J Sex Med. 2020;17(3):447–460.
- Grossmann M, Ng Tang Fui M, Cheung AS. Late-onset hypogonadism: metabolic impact. Andrology. 2020;8(6):1519–1529.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham offspring study. J Clin Endocrinol Metab. 2010;95(6):2790–2799.
- Walsh JS, Marshall H, Smith IL, et al. Testosterone replacement in young male cancer survivors: a 6-month double-blind randomised placebo-controlled trial. PLOS Med. 2019;16(11):e1002960.
- Kacker R, Conners W, Zade J, et al. Bone mineral density and response to treatment in men younger than 50 years with testosterone deficiency and sexual dysfunction or infertility. J Urol. 2014;191(4):1072–1076.
- Drake MT, Murad MH, Mauck KF, et al. Clinical review. Risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1861–1870.
- Kanis JA, Oden A, Johansson H, et al. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–743.
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–1822.
- Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177(4):471–479.
- Aminorroaya A, Kelleher S, Conway AJ, et al. Adequacy of androgen replacement influences bone density response to testosterone in androgen-deficient men. Eur J Endocrinol. 2005;152(6):881–886.
- Pizzocaro A, Vena W, Condorelli R, Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest. 2020;43(12):1675–1687.
- Orwoll E, Lambert LC, Marshall LM, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166(19):2124–2131.
- Rosenberg EA, Bůžková P, Fink HA, et al. Testosterone, dihydrotestosterone, bone density, and hip fracture risk among older men: the cardiovascular health study. Metabolism. 2021;114:154399.
- Nethander M, Vandenput L, Eriksson AL, et al. Evidence of a causal effect of estradiol on fracture risk in Men. J Clin Endocrinol Metab. 2019;104(2):433–442.
- Algeffari M, Jayasena CN, MacKeith P, et al. Testosterone therapy for sexual dysfunction in men with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2018;35(2):195–202.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199(1):257–265.
- Cunningham GR, Stephens-Shields AJ, Rosen RC, et al. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab. 2016;101(8):3096–3104.
- Brock G, Heiselman D, Maggi M, et al. Effect of testosterone solution 2% on testosterone concentration, sex drive and energy in hypogonadal men: results of a placebo controlled study. J Urol. 2016;195(3):699–705.
- Hackett G, Cole N, Saghir A, et al. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 2016;118(5):804–813.
- Kalinchenko SY, Kozlov GI, Gontcharov NP, et al. Oral testosterone undecanoate reverses erectile dysfunction associated with diabetes mellitus in patients failing on sildenafil citrate therapy alone. Aging Male. 2003;6(2):94–99.
- Zitzmann M, Mattern A, Hanisch J, et al. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J Sex Med. 2013;10(2):579–588.
- Tan WS, Ng CJ, Khoo EM, et al. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic asian men study (the subang men's health study). Aging Male. 2011;14(4):231–236. Dec PMID: 22115177.
- Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30(4):911–917.
- Tajar A, Huhtaniemi IT, O’Neill TW, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the european male aging study (EMAS). J Clin Endocrinol Metab. 2012;97(5):1508–1516.
- Tsujimura A, Miyagawa Y, Takezawa K, et al. Is low testosterone concentration a risk factor for metabolic syndrome in healthy middle-aged men? Urology. 2013;82(4):814–819.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8(1):272–283.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in Middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7(10):3495–3503.
- Strollo F, Strollo G, Morè M, et al. Low-intermediate dose testosterone replacement therapy by different pharmaceutical preparations improves frailty score in elderly hypogonadal hyperglycaemic patients. Aging Male. 2013;16(2):33–37.
- Heufelder AE, Saad F, Bunck MC, et al. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30(6):726–733.
- Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21(3):158–169.
- Li SY, Zhao YL, Yang YF, et al. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: a Meta-Analysis. Int J Endocrinol. 2020;2020:1–12.
- Wittert G, Bracken K, Robledo KP, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9(1):32–45.
- Muller M, van den Beld AW, Bots ML, et al. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109(17):2074–2079.
- Groti Antonič K, Antonič B, Žuran I, et al. Testosterone treatment longer than 1 year shows more effects on functional hypogonadism and related metabolic, vascular, diabetic and obesity parameters (results of the 2-year clinical trial). Aging Male. 2020;23(5):1442–1413.
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30(11):1029–1034.
- Kang SM, Jang Y, Kim Ji Chung N, et al. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol. 2002;89(7):862–864.
- Zitzmann M, Vorona E, Wenk M, et al. Testosterone administration decreases carotid artery intima media thickness as indicator of vascular damage in middle-aged overweight men. J Androl. 2008;29:54–55.
- Nettleship JE, Pugh PJ, Channer KS, et al. Inverse relationship between serum levels of interleukin-1beta and testosterone in men with stable coronary artery disease. Horm Metab Res. 2007;39(5):366–371.
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled moscow study. Clin Endocrinol. 2010;73(5):602–612.
- Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45.
- Vodo S, Bechi N, Petroni A, et al. Testosterone-induced effects on lipids and inflammation. Mediators Inflamm. 2013;2013:183041
- Wallis CJ, Lo K, Lee Y, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4(6):498–506.
- Tan RS, Cook KR, Reilly WG. Myocardial infarction and stroke risk in young healthy men treated with injectable testosterone. Int J Endocrinol. 2015;2015:1–8
- Loo SY, Azoulay L, Nie R, et al. Cardiovascular and cerebrovascular safety of testosterone replacement therapy among aging men with low testosterone levels: a cohort Study. Am J Med. 2019;132(9):1069–1077.e4.
- Sesti F, Pofi R, Minnetti M, et al. Late-onset hypogonadism: reductio ad absurdum of the cardiovascular risk-benefit of testosterone replacement therapy. Andrology. 2020;8(6):1614–1627.
- Traish AM, Hackett G, Miner M, et al. Cardiovascular and cerebrovascular safety of testosterone therapy. Am J Med. 2019;132(10):e748.
- Rastrelli G, Dicuio M, Reismann Y, et al. Cardiovascular impact of testosterone therapy for hypogonadism. Expert Rev Cardiovasc Ther. 2018;16(9):617–625.
- Corona G, Rastrelli G, Di Pasquale G, et al. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15(9):1260–1271.
- Corona G, Rastrelli G, Di Pasquale G, et al. Testosterone and cardiovascular risk: meta-analysis of interventional Studies. J Sex Med. 2018;15(6):820–838.
- Park S, Gale SE, Watson K. The role of testosterone in patients with heart failure: a systematic review. Cardiol Rev. 2021;29(3):156–161.
- Oni OA, Dehkordi SHH, Jazayeri MA, et al. Relation of testosterone normalization to mortality and myocardial infarction in men with previous myocardial infarction. Am J Cardiol. 2019;124(8):1171–1178.
- Baillargeon J, Urban RJ, Morgentaler A, et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015;90(8):1038–1045.
- Houghton DE, Alsawas M, Barrioneuvo P, et al. Testosterone therapy and venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2018;172:94–103.
- Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016;355:i5968.
- Corona G, Dicuio M, Rastrelli G, et al. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is ‘new’? J Investig Med. 2017;65(6):964–973.
- Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007–3019.
- Hyde Z, Norman PE, Flicker L, et al. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the health in menstudy. J Clin Endocrinol Metab. 2012;97(1):179–189.
- Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050–2058.
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725–733.
- Yeap BB. Testosterone therapy and mortality in US veterans. Asian J Androl. 2012;14(5):667–668.
- Wu FC. Caveat emptor: does testosterone treatment reduce mortality in men? J Clin Endocrinol Metab. 2012;97(6):1884–1886.
- Yeap BB, Marriott RJ, Antonio L, et al. Serum testosterone is inversely, and sex hormone-binding globulin directly, associated with all-cause mortality in men. J Clin Endocrinol Metab. 2021;106(2):e625–e637.
- Zarrouf FA, Artz S, Griffith J, et al. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15(4):289–305.
- Walther A, Breidenstein J, Miller R. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and Meta-analysis. JAMA Psychiatry. 2019;76(1):31–40.
- Giltay EJ, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7(7):2572–2582.
- Khera M, Bhattacharya RK, Blick G, et al. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the testim registry in the US (TRiUS). Aging Male. 2012;15(1):14–21.
- Zitzmann M. Testosterone, mood, behaviour and quality of life. Andrology. 2020;8(6):1598–1605.
- Morley JE, Kaiser F, Raum WJ, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A. 1997;94(14):7537–7542.
- Maggio M, Dall’Aglio E, Lauretani F, et al. The hormonal pathway to cognitive impairment in older men. J Nutr Health Aging. 2012;16(1):40–54.
- Tan S, Sohrabi HR, Weinborn M, et al. Effects of testosterone supplementation on separate cognitive domains in cognitively healthy older men: a meta-analysis of current randomized clinical trials. Am J Geriatr Psychiatry. 2019;27(11):1232–1246
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10(2):77–82.
- Etgen T, Sander D, Bickel H, et al. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch Arztebl Int. 2011;108(44):743–750.
- Lv W, Du N, Liu Y, et al. Low testosterone level and risk of alzheimer’s disease in the elderly men: a systematic review and meta-Analysis. Mol Neurobiol. 2016;53(4):2679–2684.
- Corona G, Guaraldi F, Rastrelli G, et al. Testosterone deficiency and risk of cognitive disorders in aging males. World J Mens Health. 2021;39(1):9–18.
- Kim JW, Oh MM, Yoon CY, et al. Nocturnal polyuria and decreased serum testosterone: is there an association in men with lower urinary tract symptoms? Int J Urol. 2014;21(5):518–523.
- Pearl JA, Berhanu D, François N, et al. Testosterone supplementation does not worsen lower urinary tract symptoms. J Urol. 2013;190(5):1828–1833.
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011;14(1):53–58.
- Yassin DJ, El Douaihy Y, Yassin AA, et al. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol. 2014;32(4):1049–1054.
- Kalinchenko S, Vishnevskiy EL, Koval AN, et al. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male. 2008;11(2):57–61.
- Teeling F, Raison N, Shabbir M, et al. Testosterone therapy for high-risk prostate cancer survivors: a systematic review and meta-analysis. Urology. 2019;126:16–23.
- Kardoust Parizi M, Abufaraj M, Fajkovic H, et al. Oncological safety of testosterone replacement therapy in prostate cancer survivors after definitive local therapy: a systematic literature review and meta-analysis. Urol Oncol. 2019;37(10):637–646.
- Morgentaler A, Lipshultz LI, Bennett R, et al. Testosterone therapy in men with untreated prostate cancer. J Urol. 2011;185(4):1256–1260.
- Giganti F, Moore CM. Magnetic resonance imaging in active surveillance-a modern approach. Transl Androl Urol. 2018;7(1):116–131.
- Ory J, Flannigan R, Lundeen C, et al. Testosterone therapy in patients with treated and untreated prostate cancer: impact on oncologic outcomes. J Urol. 2016;196(4):1082–1089.
- Kacker R, Hult M, San Francisco IF, et al. Can testosterone therapy be offered to men on active surveillance for prostate cancer? Preliminary results. Asian J Androl. 2016;18(1):16–20.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310–320.
- Morgentaler A, Benesh JA, Denes BS, et al. Factors influencing prostate-specific antigen response among men treated with testosterone therapy for 6 months. J Sex Med. 2014;11(11):2818–2825.
- Rastrelli G, Corona G, Vignozzi L, et al. Serum PSA as a predictor of testosterone deficiency. J Sex Med. 2013;10(10):2518–2528.
- Lopez DS, Advani S, Tsilidis KK, et al. Endogenous and exogenous testosterone and prostate cancer: decreased-, increased- or null-risk? Transl Androl Urol. 2017;6(3):566–579.
- Roddam AW, Allen NE, Appleby P, et al. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100(3):170–183.
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(6):2560–2575.
- Boyle P, Koechlin A, Bota M, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: a meta-analysis. BJU Int. 2016;118(5):731–741.
- Haider A, Zitzmann M, Doros G, et al. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193(1):80–86.
- Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in Middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–1457.
- Cui Y, Zong H, Yan H, et al. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014;17(2):132–143.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350(5):482–492.
- Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267(16):2215–2220.
- Rastrelli G, Maggi M, Corona G. Pharmacological management of late-onset hypogonadism. Expert Rev Clin Pharmacol. 2018;11(4):439–458.
- Chen YW, Niu YH, Xu H, et al. Testosterone undecanoate supplementation together with human chorionic gonadotropin does not impair spermatogenesis in males with isolated hypogonadotropic hypogonadism: a retrospective study. Asian J Androl. 2019;21(4):413–418.
- Hsieh TC, Pastuszak AW, Hwang K, et al. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189(2):647–650.
- Rastrelli G, Corona G, Mannucci E, et al. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014;2(6):794–808.
- Rambhatla A, Mills JN, Rajfer J. The role of estrogen modulators in male hypogonadism and infertility. Rev Urol. 2016;18(2):66–72.
- Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022.
- Finkelstein JS, Lee H, Leder BZ, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126(3):1114–1125.
- Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84(10):3469–3478.
- Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318–327.
- Zitzmann M, Junker R, Kamischke A, et al. Contraceptive steroids influence the hemostatic activation state in healthy men. J Androl. 2002;23(4):503–511.