Abstract
Background
Pathogenesis and endothelial function in subclinical hypogonadism (SCH) remain unclear. Undercarboxylated osteocalcin (ucOC) participates in atherosclerosis and reproduction. We explored the underlying mechanisms and interplay of endothelial dysfunction, unOC and reproductive hormones in SCH and primary late-onset hypogonadism (LOH).
Methods
In the SCH, LOH, and healthy eugonadal male groups, we measured serum unOC, calculated luteinizing hormone/testosterone (LH/T), LH.T product, and estradiol/T (E/T) as indicators of impaired Leydig cells, androgen sensitivity index (ASI), and aromatase activity, respectively (LH set-point regulators), and assessed flow-mediated dilation of the brachial artery (FMD%), carotid-intima media thickness (CIMT), and aortic stiffness (AS).
Results
↑LH/T, ↑ASI, ↓aromatase activity, normal T, follicle-stimulating hormone (FSH) and sex hormone-binding globulin (SHBG) levels, ↑unOC, and enhanced atherosclerotic markers (↓FMD%, ↑CIMT, ↑AS) are characteristics of SCH. Testosterone was positively correlated with FMD% in SCH. The independent predictors were: SHBG and LH for FMD% and CIMT, respectively, and LH/T, ucOC, FSH, estradiol, and E/T ratio for AS in the LOH group; and LH for FMD% & AS and LH and LH/T for CIMT in all study subjects.
Conclusions
SCH is a distinct clinical entity characterized by impaired androgen sensitivity and aromatase activity, compensatory elevated unOC, endothelial dysfunction, and anti-atherogenic role of testosterone.
Introduction
According to the European Male Aging Study (EMAS), late-onset hypogonadism (LOH) is a clinical and biochemical syndrome characterized by a gradual decrease in serum testosterone (T) levels as an aspect of age-related reproductive and sexual decay across a life span. Its diagnosis depends on the presence of at least three sexual symptoms and either repeated (at least twice) total T levels <8 nmol/l or serum total T levels of 8–11 nmol/l and free T levels <220 pmol/l. Subclinical hypogonadism (SCH), or compensated hypogonadism, has been recognized as a clinical entity that affecting 9.5% of men. Similar to other subclinical endocrine disorders, its diagnosis depends on normal T and elevated luteinizing hormone (LH) levels [Citation1]. The hypothalamic–pituitary–testicular (HPT) axis is highly regulated. The gonadotropin-releasing hormone pulse generator controls gonadotropin release, namely LH and follicle stimulating hormone (FSH) from the anterior pituitary gland. They bind to testicular receptors on Leydig cells for T production and Sertoli cells for spermatogenesis, respectively. Aromatase enzymes convert T to estradiol (E) in the gonads and extra-gonadal target tissues. Both T and E mediate negative feedback on the HPT axis by binding to their receptors and regulating the HPT set point for LH [Citation2]. Impaired Leydig cell function, altered androgen receptor sensitivity, and aromatization capacity can all affect the LH set point. They can be estimated using the LH/T ratio, androgen sensitivity index (ASI), and E/T ratio [Citation3–5].
Compared to overt hypogonadism, SCH is significantly associated with physical symptoms, an identically increased cardiovascular risk, and a 10-fold increased risk of cardiovascular mortality [Citation6,Citation7]. Moreover, independent associations of elevated LH levels with decreased muscle strength and increased cardiovascular disease regardless of T level have been reported [Citation8,Citation9].
Osteocalcin (OC) is a polypeptide protein specifically expressed in osteoblasts and is the most abundant non-collagenous bone protein. The active form, undercarboxylated OC (ucOC), connects the bone–pancreas–reproductive axis and binds to its receptor in target tissues (pancreas, testes, and muscles) to regulate glucose metabolism, T synthesis, and muscle mass, respectively [Citation10]. In a meta-analysis, both OC and ucOC showed conflicting relationships with different atherosclerotic markers [Citation11]. Moreover, in vitro and in vivo studies have shown either a protective role or no role of ucOC in atherogenesis [Citation12,Citation13].
Until now, little data exist about the underlying mechanisms of SCH and debates whether it’s a paraphysiological condition as a barometer of poor health in aged men, a distinct clinical entity, or a precursor of overt hypogonadism [Citation2,Citation6,Citation7,Citation14]. In our study, we aimed to understand the underlying mechanisms and pathogenesis of SCH in an attempt to define this clinical condition, and compared it with late-onset hypogonadism. In the first assessment, we assessed the LH/T ratio, ASI, and E/T ratio as markers of impaired Leydig cell response, androgen insensitivity, and aromatase activity parameters, respectively, as regulators of the HPT set point for LH. Second, we addressed the association between ucOC and reproductive hormones, regulators of the HPT set point, and endothelial function parameters in an attempt to assess the relationship between ucOC and the previously mentioned underlying mechanisms of SCH and atherosclerotic markers in an effort to explore its action as a bridge between the bone to the gonadal state and atherosclerosis in SCH. Third, we attempted to determine whether SCH could be considered a cardiovascular risk factor by assessing its association with cardiovascular risk factors and cardiovascular function using the morphological and hemodynamic functional parameters of the endothelium. Additionally, we attempted to identify the role of reproductive hormones and studied HPT regulatory factors as predictors of endothelial function.
Methods
Study population
This cross-case–control study was conducted at the endocrinology and diabetic unit, Internal Medicine department, Minia University Hospital from March 2018 to July 2019. Men with primary LOH (n = 60) and SCH (n = 76) aged > 50 years were recruited for the study, in addition to age-matched apparently healthy eugonadal men (HE, n = 50), who served as the control group. Patient groups were selected from the general population who were asked for andropausal symptoms according to the Androgen Deficiency in Aging Males (ADAMS) Questionnaire [Citation15]. Participants with one or more positive sexual symptoms underwent further laboratory investigation for diagnosis. The diagnosis of SCH depends on the presence of normal total testosterone, normal calculated free testosterone, and elevated LH levels (T ≥ 10.5 nmol/l, calculated free testosterone > 220 pmol/l, and LH ≥ 9.4 IU/l). The diagnosis of primary LOH depends on three sexual symptoms (reduced sexual thoughts, weakened morning erections, and erectile dysfunction), and either repeated (at least twice) total T level <8 nmol/l or serum total T of 8–11 nmol/l and free T < 220 pmol/l [Citation1]. Free testosterone level was calculated using the Vermeulen formula [16]. Those with hypogonadotropic hypogonadism were excluded and the term "LOH" was the primary type in our study.
The study protocol was approved by the local institutional ethics committee and was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and the international conference of harmonization guidelines for good clinical practice. All participants provided written informed consent before their inclusion in the study.
All enrolled men underwent thorough history-taking (including marital status, fertility, smoking, and the ADAM questionnaire), clinical examination, and anthropometric measurements.
The exclusion criteria were as follows: age <50 years, hypogonadism since childhood or hypogonadotropic hypogonadism, alcohol consumption, any endocrine or systemic disorders, obesity greater than 30 kg/m2, malignancy, medical or hormonal therapy within 3 months of the study, and surgical castration or varicocele.
Biochemical and hormonal assay
On two separate occasions, fasting (8:00–10:00 AM), venous samples were drawn to assess serum total testosterone and LH levels to establish the diagnosis. Routine biochemical investigations, including hemogram, blood glucose level, renal and liver function, and lipid profile, were performed according to standardized laboratory methods. Serum blood samples were stored at −20 °C for the measurement of sex hormone-binding globulin (SHBG), estradiol, FSH, high-sensitive C-reactive protein (hsCRP), and undercarboxylated osteocalcin levels. Serum testosterone, estradiol, LH, and FSH levels were measured using enzyme-linked immunosorbent assay (ELISA) (Chemux Bioscience, South San Francisco, CA). hs-CRP levels were assessed by ELISA using commercial kits (Elabscience, Houston, TX). The free androgen index (FAI) was calculated using the Vermeulen formula [Citation16]. The ASI is the product of the LH and T values. An elevated ASI has been suggested as an indication of androgen insensitivity because the impaired feedback regulatory mechanism of the HPT axis leads to an elevation in LH and T. The estradiol-to-testosterone (E/T) ratio was calculated to assess aromatase activity [Citation3,Citation4,Citation17]. The ASI could be biased by high LH levels in primary LOH as the ASI value could be due more to Leydig dysfunction than to true sensitivity to testosterone. Therefore, the analysis was again corrected for testosterone levels via multiplying LH by the corrected testosterone level (patient testosterone/normal testosterone). Serum undercarboxylated osteocalcin (ucOC) levels were measured using a commercial ELISA kit supplied by Takara Bio Inc., Tokyo, Japan).
Assessment of endothelial function
High-resolution color-duplex ultrasonography (Aplio 500, superficial prob, TOSHIBA, Tokyo, Japan) using an 8–12-MHz linear array for
The measurement of the common carotid artery intimal media thickness (CIMT) following the recommendations of the Manheim CIMT Consensus [Citation18].
Calculated flow-mediated dilation (FMD) % of the brachial artery: (post occlusion diameter – baseline diameter) × 100/baseline diameter according to the method described by Celermajer et al. [Citation19].
Using an M-mode transthoracic echocardiograph (ACUSON SC2000 PRIME ultrasound system, Siemens AG, Munich, Germany) and
according to the American Society of Echocardiography/American Heart Association (ASE/AHA) guidelines for the aortic root, the aortic stiffness index (AS) = 100 × [natural [systolic blood pressure {SBP}/diastolic blood pressure {DBP}] [(aortic maximum systolic diameter minus aortic end diastolic diameter)/aortic end diastolic diameter] [Citation20].
Testicular ultrasound and duplex scan: scans were performed to exclude testicular abnormalities such as varicocele.
Statistical analyses
All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) test was used to compare the three groups, followed by post-hoc Tukey analysis between the two groups for parametric quantitative data. Qualitative data was expressed as percentages and compared using the Chi-square test. Non-parametric quantitative data were expressed as the median (25–75% interquartile range) and compared between the three groups using the Kruskal–Wallis test, followed by the Mann–Whitney test between the two groups. On the other hand, Spearman's correlations were used for bivariate analysis to determine relationships between reproductive hormone levels, hypothalamo-pituitary testicular axis regulatory factors (LH/T, LH.T, and E/T ratio) and undercarboxylated osteocalcin, on the one hand, and studied cardiovascular risk factors and endothelial function parameters on the other hand. To assess the role of reproductive hormones, hypothalamic-pituitary testicular axis regulatory factors, and undercarboxylated osteocalcin as independent predictors of endothelial function parameters, significantly correlated variables among these previously mentioned variables with endothelial parameters were analyzed via multiple linear regression analysis using an enter method after the exclusion of collinear variables. Statistical significance was set at a p-value < 0.05.
Results
1-Clinical and laboratory data
The three groups were comparable in terms of age, smoking status, blood pressure, and body mass index (BMI). Compared to the LOH and HE groups, the SCH group had significantly higher LH and corrected ASI levels and a lower E/T ratio. In addition, the SCH group had significantly higher TT, FAI, and estradiol levels and lower serum FSH, SHBG, and LH/T levels than the LOH group. The SCH and LOH groups were comparable in terms of atherogenic lipid profiles, ucOC, and hs-CRP levels. The SCH group had a significantly higher atherogenic lipid profile, unOC, and hsCRP levels and significantly lower estradiol levels than the HE group, and both groups had comparable TT, FAI, FSH, and SHBG levels. The LOH group had significantly higher fasting blood sugar levels than the other groups ( and ).
Figure 1. Undercarboxylated osteocalcin levels in the studied group. Box plot of undercarboxylated osteocalcin among healthy eugonadal, subclinical hypognadism and late onset hypogonadism groups. Each box indicates the 25th and 75th percentiles, the median is represented by the horizontal line within the box, the extreme measured values are indicated by whiskers. Dots and stars represent the outliers.
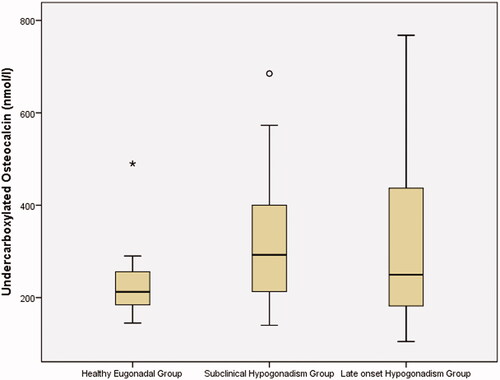
Table 1. Clinical, laboratory, and endothelial function parameters of the groups studied.
Endothelial function parameters
The LOH and SCH groups were comparable in terms of FMD %, CIMT, and AS, while both groups had significantly lower FMD %, higher CIMT, and higher AS than the HE group (p < 0.001 for all) ().
Correlation study
We studied the correlation between reproductive hormone levels and hypothalamo-pituitary testicular axis regulatory factors (LT/T, LT.T, and E/T ratio) and undercarboxylated osteocalcin on the one hand, and studied cardiovascular risk factors and endothelial function parameters on the other hand. A correlation study was reported on all study subjects and the SCH and LOH groups.
Correlation of studied hormonal parameters with cardiovascular risk factors
In all subjects: LH was related to dyslipidemia, being positively correlated with total cholesterol, triglyceride, and hsCRP and negatively correlated with HDL-c (p < 0.001 for all except p= 0.01 for HDL-c). Total testosterone was negatively correlated with DBP, fasting blood sugar, total cholesterol, and HDL-c and positively correlated with HDL-c (p < 0.001, p=
0.003, 0.04, and 0.03, respectively), whereas FAI was negatively correlated with DBP, FBS, 2 h postprandial blood sugar, total cholesterol, triglyceride, and hsCRP (p < 0.001, p = 0.003, 0.009, 0.01, 0.03, and 0.006, respectively). LH/T as a marker of impaired Leydig cell function was positively correlated with many cardiovascular risk factors such as DBP, FBS, cholesterol, triglyceride, and hsCRP and negatively correlated with HDL-c (p = 0.005, p < 0.001, p < 0.001, p < 0.001, p < 0.001, and p = 0.001, respectively). Correlation with FBS revealed that FSH and E/T ratio were positively correlated while the LH.T product was negatively correlated (p = 0.005, 0.002, and 0.005 respectively). Estradiol was positively correlated with BMI (p = 0.02) ().
In the SCH group, LH, LH/T, and LH.T were positively correlated with age (p < 0.001, p = 0.04, and 0.006, respectively). Estradiol and E/T ratio were negatively correlated, whereas SHBG was positively correlated with LDL-c (p = 0.03, 0.01, and 0.01, respectively). E/T was positively correlated with HDL-c (p = 04) and negatively correlated with hsCRP (p = 0.03) ().
In the LOH group: LH, FSH, estradiol, LH/T, and E/T ratio were positively correlated with age (p = 04, 0.02, 0.02, 0.01, and 0.01, respectively). LH levels were positively correlated with LDL and hsCRP levels (p = 0.001 and 0.01, respectively). Testosterone levels were negatively correlated with BMI, SBP, DBP, FBS, postprandial blood sugar, and triglyceride levels (p = 0.02, p < 0.001, p = 0.04, 0.02, 0.005, and 0.005, respectively). Estradiol levels were positively correlated with BMI (p = 0.005). SHBG levels were inversely related to postprandial blood sugar and triglyceride levels (p = 0.009 and 0.03, respectively), whereas FAI was found to be negatively correlated with SBP, postprandial blood sugar, triglyceride, and LDL levels (p = 0.001, 0.002, 0.03, and 0.004, respectively). LH/T was positively correlated with many cardiovascular risk factors such as BMI, SBP, DBP, FBS, and LDL-cholesterol (p = 0.04, 0.001, 0.045, 0.008, and p < 0.001, respectively). SBP and DBP were negatively correlated, while triglycerides, HDL, and hsCRP were positively correlated (p < 0.001, p = 0.007, 0.01, 0.03, and 0.001, respectively) to LH xTproduct. E/T was positively correlated with BMI and SBP (p = 0.001 and 0.006, respectively) ().
Table 2. Correlation between reproductive hormone levels, hypothalamo-pituitary testicular axis regulatory factors (LH/T, LH × T, and E/T ratio) and undercarboxylated osteocalcin on the one hand and studied cardiovascular risk factors and endothelial function parameters on the other hand, in all study subjects.
Table 3. Correlation between reproductive hormone levels, hypothalamo-pituitary testicular axis regulatory factors (LH/T, LH × T, and E/T ratio) and undercarboxylated osteocalcin on the one hand and studied cardiovascular risk factors and endothelial function parameters on the other hand in the subclinical hypogonadism group.
Table 4. Correlation between reproductive hormone levels, hypothalamo-pituitary testicular axis regulatory factors (LH/T, LH × T, and E/T ratio) and undercarboxylated osteocalcin on the one hand and studied cardiovascular risk factors and endothelial function parameters on the other hand in the late-onset hypogonadism group.
The B-correlation between ucOC and hormonal parameters, as well as endothelial function
In all subjects (), ucOC was positively correlated with LH, LH/T, estradiol, and SHBG and negatively correlated with FAI (p < 0.001, p= 0.003, 0.02,p <0.001, and p= 0.02, respectively). It was positively correlated with cardiovascular risk factors, including DBP, total cholesterol, and hsCRP (p = 0.04, p <0.001, and p <0.001 respectively). Regarding endothelial function parameters, ucOC was negatively correlated with FMD% and positively correlated with CIMT (p = 0.01 and 0.02, respectively).
In the SCH group (), ucOC was positively correlated with SHBG, SBP, DBP, and total cholesterol (p = 0.001, 0.004, 0.002, and 0.01, respectively). However, it was not associated with any endothelial function parameters.
In the LOH group (), ucOC was positively correlated with LH, FSH, estradiol, SHBG, E/T, and HDL and negatively correlated with FAI (p = 0.03, 0.04, p < 0.001, p < 0.001, p < 0.001, p = 0.02, and 0.002, respectively): ucOC was positively correlated to AS (p = 0.02).
C-Relation of hormonal parameters with endothelial function
In all study subjects (), FMD% was negatively correlated with LH and LH/T and positively correlated with TT and FAI (p < 0.001, p < 0.001, p = 0.01, and p = 0.01 respectively). CIMT was positively correlated with LH, FSH, and the LH/T ratio and negatively correlated with TT and FAI (p < 0.001, p = 0.008, p < 0.001, p = 0.03, and 0.02, respectively). AS was positively correlated with LH and LH/T and negatively correlated with estradiol and E/T (p < 0.001, <0.001, <0.001, and 0.02, respectively).
In the SCH group, TT and FAI were positively correlated with FMD% (; p = 0.01 and 0.007, respectively).
In the LOH group (), FMD% was negatively correlated with SHBG and positively correlated with FAI (p = 0.001 and 0.02, respectively). CIMT was positively correlated with LH and LH.T (p = 0.001 and 0.002, respectively). As was positively correlated with LH, FSH, and LH/T and negatively correlated with estradiol and E/T (p < 0.001, 0.008, <0.001, 0.01, and 0.001, respectively).
Multiple linear regression
Significantly correlated independent variables (reproductive hormones, HPT axis regulatory factors, and unOC) were entered into a multivariate linear regression analysis to determine the independent predictors of the target endothelial function parameter. This analysis was performed in all study groups and in the LOH group but not in the SCH group as testosterone was the only variable correlated with FMD%. Therefore, the multivariate analysis was not applicable to the SCH group.
Among all subjects (), LH was an independent predictor of FMD% and AS (p < 0.001 for both) (adjusted R2= 0.24 and 0.23, respectively, p < 0.001 for both). CIMT was independently predicted by LH and LH/T (p 0.001, 0.02) (adjusted R2 = 0.32, p 0.001).
In the SCH group, total testosterone and free androgen index were positively correlated with FMD% and no further analysis was performed as both variables were collinear.
In the LOH group (), sex hormone binding globulin level was an independent predictor of FMD% (p = 0.003). LH level was an independent contributor to CIMT (p = 0.03). Furthermore, LH/T, ucOC, FSH, estradiol, and E/T ratio were independent contributors to AS (p < 0.001, 0.004, 0.01, 0.02, and 0.02, respectively).
Table 5. Multiple linear regression analysis to assess the role of reproductive hormones, studied hypothalamo-pituitary regulatory factors and undercarboxylated osteocalcin as independent predictors of endothelial function parameters in all study participants.
Table 6. Multiple linear regression analysis to assess role of reproductive hormones, studied hypothalamo-pituitary regulatory factors and undercarboxylated osteocalcin as independent predictors of endothelial function parameters in the late-onset hypogonadism group.
Statistically signficant values are shown in bold.
Discussion
To date, the underlying mechanisms of subclinical hypogonadism remain unclear, including whether it is a precursor to hypogonadism or a marker of poor general health. We suggest that SCH is a clinical entity that differs greatly from LOH in its pathophysiology. However, both conditions may represent cardiovascular risk factors as they are identically associated with dyslipidemia, a pro-inflammatory state, hemodynamic and morphological endothelial dysfunction, and elevated ucOC levels. Multivariate analysis in all subjects demonstrated that LH was an independent contributor to hemodynamic changes in the peripheral muscular and central elastic arteries, whereas LH level and the LH/T ratio were independent predictors of CIMT. Among patients with SCH, total testosterone and FAI levels were positively correlated with FMD%. Among the patients with LOH, SHBG and LH were independent contributors to FMD% and CIMT, respectively. Meanwhile, LH/T, ucOC, FSH, estradiol, and E/T contributed to aortic stiffness. ucOC may be a link that connects bone to inflammation, dyslipidemia, and reproductive and endothelial functions in all subjects and among patients with LOH but not in SCH. These issues have not been previously discussed in the literature.
We suggest that SCH is a diverse clinical state and not a typical feature of hypogonadism. The main underlying mechanisms are impaired androgen sensitivity and lower aromatase activity with the equivocal minimal contributing role of compensated impaired Leydig cell function and normal spermatogenesis as evidenced by high ASI, decreased E/T, and mildly elevated LH/T ratio with high normal testosterone, and normal FSH levels, respectively. Similar to Corona et al. (2014), we reported elevated LH levels with high-normal testosterone levels in patients with SCH even though they were not significantly higher than those in healthy eugonadal subjects. In light of the current knowledge and our results, we suggest that SCH is not linked to Leydig cell dysfunction but is attributed to a hormone-resistant state at the pituitary gland level. There is a lack of feedback inhibition of androgen and estradiol in the pituitary gland, which is caused by insensitive pituitary androgen receptors and impaired aromatization, respectively. This pituitary gland insensitivity leads to the resetting of new HPT set points to higher LH levels. Therefore, we can conclude that higher LH levels in SCH may be attributed to pituitary receptor resistance to androgens and lower aromatase enzyme activity. Previous studies have reported that androgen receptor polymorphisms alter the LH/T ratio rather than being the hallmark of primary Leydig cell dysfunction and that both androgen receptor and aromatase gene mutation polymorphisms are associated with impaired feedback inhibition with relatively elevated testosterone levels and different individual HPT set points with elevated LH levels. In the context of these data, we may attribute a mildly higher LH/T ratio in SCH than in healthy euogonadism to pituitary receptor resistance and impaired aromatization rather than impaired Leydig cell function. Therefore, the role of impaired Leydig cell function in SCH requires further investigation. In our study, the SCH group showed normal SHBG and FSH levels. The latter is predominantly regulated by inhibin, which is produced by testicular cells. This supports the normal testicular function in SCH [Citation2,Citation21,Citation22].
Corona et al. (2014) suggested that SCH is not a novel clinical state but rather a response of the HPT axis to somatic illness. It is characterized by more psychiatric symptoms and fewer sexual symptoms than overt hypogonadism. A recent follow-up study suggested that SCH is a reversible process, especially at a young age, and is not a typical androgen deficiency but rather reflects worsening health. A vicious circle exists in which factors that accelerate functional aging and promote CVD such as diabetes, chronic pain, and physical inactivity predispose individuals to SCH occurrence, which in turn promotes CVD development and further functional decline (decreased hemoglobin and impaired cognitive functions) [Citation14]. Therefore, this may represent a viscous circle of SCH state and cardiovascular risk factors. In contrast, others have suggested that SCH is a precursor to overt hypogonadism with a compensatory increase in LH levels to stimulate the Leydig cell reserve to maintain a normal T level similar to other subclinical endocrine disorders [Citation6].
In our study, LOH was associated with markedly impaired uncompensated Leydig cell function (markedly reduced TT and FAI and the highest ratio of LH/T); and enhanced androgen sensitivity even with corrected ASI for testosterone and aromatase activity (low ASI and high E/T ratio, respectively). It is also associated with impaired spermatogenesis and elevated FSH and SHBG levels. This is consistent with the previously described pathophysiological mechanisms of LOH [Citation23,Citation24]. Therefore, we conclude that SCH is a distinct clinical entity that differs from overt hypogonadism in its underlying mechanisms and testicular functions.
Undercarboxylated osteocalcin (ucOC) is osteocalcin with deficient carboxylation at one or more sites produced by osteoblasts and represents the active metabolic form and the majority of circulating OC. In addition, it stimulates insulin secretion and sensitivity. Evidence from human studies and murine models (not in rats or mouse) suggests that ucOC may stimulate testosterone production by Leydig cells in a manner similar to LH via binding to its receptor, the G-protein coupled receptor (GPRC6A). The binding of unOC occurs in a bone–testicular axis independent of the HPT axis, or unOC acts as an enhancer of testosterone synthesis upon gonadotropin stimulation. Testosterone stimulates osteoblast proliferation and differentiation. Therefore, testosterone and unOC have a bidirectional relationship [Citation10,Citation25].
We are pioneering in reporting elevated ucOC level in men with primary LOH and SCH, its positive correlation with LH, LH/T, estradiol, and SHBG, and a negative correlation with FAI in all subjects. unOC was positively correlated with SHBG among patients with SCH, and finally, unOC had a significant positive correlation with LH, FSH, estradiol, SHBG, and E/T and a negative correlation with FAI among patients with LOH. Previous studies have described elevated OC in hypogonadal states in male LHb−/− mice, idiopathic hypogonadotropic hypogonadism in men, and among elderly men with testosterone deficiency [Citation25–27]. In this context, we hypothesized that elevated unOC levels in patients with late-onset hypogonadism may be a compensatory mechanism for the underlying impaired Leydig cell function and enhanced aromatization in an attempt to adjust testosterone levels, as unOC levels had a significant positive correlation with LH, estradiol, and E/T and were negatively correlated with androgen levels, respectively. Moreover, we hypothesized that elevated ucOC levels in men with SCH could be attributed to androgen insensitivity. However, one study reported low OC in low testosterone males presenting to the clinic for evaluation of hypogonadism but not mentioned as a primary or secondary type [Citation28].
The positive relationship between ucOC and SHBG in all subjects and among patients with SCH and LOH can be explained by the similar molecular moieties between SHBG and ucOC and their competition to bind to the common receptor GPRC6 with a displacement of the SHBG by ucOC when co-incubated with high concentrations [Citation29]. Inconsistent results exist regarding the relationship between testosterone (total and free) and OC or unOC in clinical and therapeutic male studies, and a positive correlation was observed mainly in secondary hypogonadism among obese, type 2 diabetic, and hyperthyroid subjects, or among men presenting to the clinic for the evaluation of hypogonadism, while no association was detected among eugonadal men or in young adult males from infertile couples [Citation27,Citation28,Citation30–33]. Different underlying pathophysiological mechanisms and methodologies explain these disparities. Our study described an insignificant correlation between FAI and unOC in healthy subjects and in patients with SCH, and a significant negative correlation between unOC and FAI in men with LOH. These findings were consistent with those of Nah et al. [Citation27]. Notably, ucOC was positively correlated with SHBG but not with testosterone and inversely correlated with estradiol in a large cohort of older eugonadal men [Citation34].
However, increasing either endogenous or exogenous testosterone intake did not affect OC levels in the animal study. In contrast, increased osteocalcin levels have been reported in hypogonadal men after testosterone replacement therapy. However, this study included a small number of participants, was non-randomized, and lacked a placebo-controlled group [Citation28,Citation35,Citation36].
We observed a positive association between estradiol and ucOC levels in all subjects and among patients with LOH but not among patients with SCH. In experimental studies, osteocalcin administration did not affect estradiol production in Leydig cells or aromatase gene expression [Citation26]. However, administration of aromatase inhibitors in elderly men leads to a decrease in estradiol levels and an increase in testosterone levels with a concomitant decrease in OC levels in the eugonadal group but not in the hypogonadal groups. These studies measured OC levels, but not the active form, unOC levels. The effects of exogenous aromatase inhibitors may not mimic those of endogenous estradiol or E/T. Importantly, extra-glandular aromatization of circulating androgen precursors is a major source of estrogen in men. Therefore, we hypothesized that estradiol regulates ucOC levels, particularly in men with LOH [Citation37–39].
In our study, we demonstrated that LH and LH/T levels increased with age among patients with SCH and LOH, and there was a positive association between age and FSH and estradiol levels and E/T ratio in the LOH group and with LT.T product in the SCH group. These age-related associations were matched with 5350 randomly chosen men from a general population study [Citation40]. As elevated FSH and estradiol levels and the E/T ratio in LOH and elevated LT.T in SCH were associated with significant positive relationships between these parameters and age in the related group, we may conclude that LOH and SCH are age-related conditions.
In our study, LH was associated with atherogenic lipid profile and inflammation (hsCRP) in all study subjects and in patients with LOH. In addition, LH/T was associated with traditional cardiovascular risk factors such as BMI, blood pressure, blood sugar, atherogenic lipid profile, and inflammation in all study subjects and among patients with LOH. As expected, total testosterone and free testosterone were associated with better metabolic and lipid profiles: lower BMI, blood pressure, blood glucose, cholesterol, triglyceride, and LDL levels and higher HDL levels among all study subjects and among patients with LOH. In accordance with our results, Kelly and Jones reported that low testosterone also has an adverse effect on several cardiovascular risk factors, including insulin resistance, diabetes mellitus, atherogenic lipid profile, central obesity, and endothelial dysfunction, and testosterone intake in hypogonadal men corrects these unfavorable effects [Citation41]. The effects of T on plasma lipids have been the focus of attention in many large cross-sectional and longitudinal studies. Consistent with our results, lower endogenous T levels are associated with a pro-atherogenic lipid profile as most of these studies reported an inverse relationship between T levels and both plasma triglycerides and total cholesterol, and a positive correlation with HDL-C [Citation42]. In testosterone double-blind trials, increased serum testosterone concentrations after receiving testosterone gel 1% in hypogonadal men aged 65 years or more for 1 year were associated with modest reduction in total cholesterol, HDL-c, and LDL-c levels. Similar effects have been observed after injectable testosterone [Citation43,Citation44]. Testosterone may affect serum TG levels by modifying the expression of genes involved in lipoprotein assembly and secretion. Testosterone may control serum total cholesterol and LDL levels by altering the expression of the LDL receptor and proprotein convertase subtilisin/kexin type 9 (PCSK9) [Citation45]. Contrarily, meta-analyses of testosterone trials that incorporated variable entry criteria for participants and doses and methods of testosterone intake have reported inconsistent effects on cholesterol [Citation46,Citation47]. An analysis of older men from the Framingham Heart Study reported no association between plasma lipid and T concentrations [Citation48].
A small recent study by Adorni et al. also demonstrated that both primary and secondary hypogonadism in men are associated with a normal range of lipoprotein profiles but with concomitant dysregulation of lipoprotein functions that favor atherogenesis. Hypogonadal men exhibited a noticeable reduction in HDL-cholesterol efflux capacity (CEC), as well as increased serum cholesterol loading capacity (CLC). CEC is a measure of the HDL anti-atherogenic activity. CLC as a marker of cholesterol influx and accumulation in arterial macrophages represents another index of atherogenicity. Impaired HDL-CEC is mediated via compromised ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) pathways. Moreover, testosterone levels may alter efflux/loading capacities as it had positive correlations with both total HDL CEC, and ABCG1 HDL CEC and inverse association with serum CLC [Citation49].
So, we propose the following explanation for the link between LH and impaired testicular function as well as cardiovascular risk factors: (i) androgen deficiency has been linked to an increased risk of cardiovascular disease, but LH and LH/T more accurately reflect current testosterone levels; and/or (ii) LH or LH/T has a direct independent effect on cardiovascular risk, with elevated levels being detrimental. It is unclear whether these effects involve the direct role of LH or an indirect underlying low testosterone level. In our study, the E/T ratio was positively correlated with HDL and negatively correlated with LDL and hsCRP in SCH patients. These findings were matched with the elevated LDL, enhanced inflammation, and lower HDL levels among men with aromatase enzyme deficiency [Citation50]. As a result, decreased aromatase activity in SCH could be the cause of dyslipidemia and inflammation.
Our study is the first to use different standard measures to identify established markers of subclinical atherosclerosis in SCHs, hemodynamic and morphological endothelial dysfunction: impaired FMD % of the brachial artery, increased CIMT, and AS. These imaging parameters have the potential to predict the risk of future cardiovascular events [Citation51]. Interestingly, these findings were supported by the previous two studies. SCH has the same increased cardiovascular risk and a nearly 10-fold increased risk of cardiovascular mortality as overt hypogonadism in one study, while others discovered an intriguing link between common cardiovascular risk factors and SCH in the presence of viscous circle [Citation7,Citation14].
Our findings suggested a strong link between elevated LH and LH/T ratio and atherosclerosis as LH was an independent predictor of impaired endothelial function and morphology (FMD%, CIMT, AS) in all subjects, and LH/T was a predictor of increased CIMT. However, these relationships were not the same as those found in the SCH and LOH groups. Only free testosterone and total testosterone were positively associated with FMD percent in SCH subjects. SHBG and LH were independent contributors to FMD% and CIMT in LOH-patients, respectively. Meanwhile, LH/T, ucOC, FSH, estradiol, and E/T were contributors to aortic stiffness. In accordance with our findings, LH "with a normal T" was linked to CIMT among andropausal middle-aged men; elderly men with elevated LH or primary hypogonadism rather than secondary hypogonadism had more ischemic heart disease events [Citation9,Citation52]. The LH/T ratio has been linked to CVD mortality in elderly men [Citation53].
The presence of extra-gonadal LH receptor expression in the vascular and smooth muscle cells, as well as the link between increased expression of these receptors and endothelial proliferation could theoretically shed light on LH's possible atherosclerotic role in our study. This was similar to the atherosclerotic role of thyroid stimulating hormone in subclinical hypothyroidism via binding to endothelial expressed TSH receptors [Citation54–57].
In our study, total testosterone and free testosterone were positively correlated with FMD% in all study participants and in the SCH group and they were negatively correlated with CIMT in all study groups. Testosterone is a vasoactive hormone that primarily acts as a vasodilator in a variety of vascular beds and has athero-protective properties through a variety of mechanisms. The inhibition of voltage-operated l-calcium channel channels and/or the activation of potassium channels on smooth muscle cells, which increases nitric oxide (NO) production, is thought to occur primarily through endothelium-independent testosterone mechanisms, whether via binding to androgen receptor, estrogen receptor after aromatization to estradiol, or interaction with various intracellular signaling pathways. However, this aspect remains unknown. Similar to our results, several human studies reported an association between testosterone levels and CIMT and FMD% in population-based men's studies [Citation41,Citation57].
In addition, estradiol levels and E/T ratios were negatively correlated with AS in all subjects and, additionally, were independent contributors to AS in the LOH group in multivariate analysis. These findings suggest that estradiol and aromatase enzyme activity may improve endothelial function and protect against arterial stiffness and atherosclerosis. Consistent with our findings, estradiol receptors and aromatase are expressed in the endothelium and vascular smooth muscle cells (VSMCs), and estradiol enhances vasodilation in men through several mechanisms. In addition, endogenous estradiol in men exerts an anti-atherosclerotic effect by limiting the proliferation and migration of endothelial cells and VSMCs. However, estradiol exposure increases atherosclerosis in the coronary arteries harvested from men. In accordance with our results, aromatase inhibition also decreased FMD% in men [Citation58]. Higher estradiol levels were associated with lower CIMT among men with diabetes, but others have reported a lack of association or a positive association [Citation59–61]. Of note, estradiol levels in adult men do not exactly indicate tissue activity owing to extra-gonadal aromatization activity and partial estradiol elimination in situ.
In our study, FSH was significantly correlated with CIMT but was not a contributor to it in all subjects. It was significantly correlated with AS and was an independent contributor to it among patients with LOH. High levels of FSH among patients with LOH may be an independent contributor to AS in this group. FSH receptors are expressed on the endothelium with enhancement by stimulating vascular endothelial growth factor expression [Citation62]. In prostate cancer studies, an increase in FSH levels during therapy is associated with increased cardiovascular risk. Specifically, the administration of androgen deprivation therapy agonist, which leads to elevated FSH levels, showed increased cardiovascular risk compared to androgen deprivation antagonist therapy, which does not affect FSH levels [Citation63].
In our study, SHBG was negatively correlated and was an independent predictor of FMD% in LOH. Although low SHBG levels are associated with insulin resistance [Citation64], two recent large cohort studies of over 150,000 men [Citation65,Citation66] found positive associations between circulating SHBG levels and CVD risk and mortality in middle-aged and older men. However, it is still unknown whether changes in the circulating plasma levels of SHBG, the local expression of SHBG and androgen-binding proteins in tissues, or the secondary effects of SHBG level fluctuations in free testosterone are responsible for these effects. Previous population studies in men reported no association or an insignificant inverse association between SHBG and FMD% [Citation67]. Higher SHBG among patients with LOH may explain the negative independent association.
Overall, there is conflicting evidence regarding the effect of ucOC on vascular function: in vivo and in vitro studies have reported both an anti-atherosclerotic effect with improved endothelial function and an unrecognized impact on endothelial function and hemostasis [Citation12,Citation13]. In all study subjects, the ucOC level was positively correlated with CIMT and negatively correlated with FMD% in the bivariate analysis. However, these findings were not confirmed in the multivariate analysis. However, ucOC was positively correlated to AS and was an independent contributor to it in the LOH group. In addition, ucOC was positively correlated with cholesterol and BP in all study subjects and in patients with SCH, and was positively correlated with hsCRP and HDL-c in all study subjects and among patients with LOH, respectively. In this context, ucOC is positively associated with CIMT in offspring with a positive family of metabolic syndrome. However, it is negatively associated with intimal thickness in patients with chronic kidney disease [Citation68–69]. In two studies, there was no association between ucOC and blood pressure or arterial stiffness. In one study, 162 community-dwelling men (mean age, 48 years old) were recruited, and a very small sample size of middle-aged and elderly men was included in the other study. However, due to the small sample size in the latter study, researchers [Citation70,Citation71] cannot draw definitive conclusions. The conflicting results with our study may be related to different ages and hormonal states, as both are important factors in determining ucOC levels [Citation72]. A meta-analysis of global human studies showed that no clear relationship could be established between measured total OC or ucOC and the extent of calcification or atherosclerosis as positive, negative, or no relationships were reported among these mostly cross-sectional observational studies that are limited in their clarification as a cause–effect relationship [Citation11]. One longitudinal study showed that baseline total OC was positively correlated with the baseline carotid plaque score, but delta change in OC was negatively correlated with final score changes. They hypothesized that atherosclerotic plaques initially enhance OC secretion as a protective mechanism to suppress atherosclerosis or calcification progression [Citation73]. However, all studies measuring OC positive cells or histological staining of OC showed a positive relationship with calcification, which may be due to its role in calcification rather than atherosclerosis by mediating abnormal vascular repair via activation of the osteogenic genes with further mineralization [Citation11,Citation74]. Ten hypogonadotropic hypogonadal male patients displayed lower circulating endothelial progenitor cell (EPC) levels and a higher EPC-OC + subpopulation [Citation75]. Overall, whether ucOC is a mediator or a marker of CVD processes requires further investigation.
Perspectives and significance
We concluded that SCH is a distinct clinical entity and not an androgen-deficient state. It is a state of pituitary androgen insensitivity, impaired aromatase activity, an equivocal minimal role of impaired Leydig cells, and normal SHBG and FSH levels. Late-onset hypogonadism is a state of impaired Leydig cells and Sertoli cells, enhances both aromatase and androgen sensitivity with increased SHBG. Similar to late-onset hypogonadism, SCH is associated with impaired hemodynamic and morphological endothelial function in the peripheral and central elastic arteries. In addition, late-onset hypogonadism and SCH conditions are associated with elevated ucOC as a compensatory mechanism for impaired Leydig cells or androgen insensitivity, respectively. They are also associated with an atherogenic lipid profile, and inflammation. ucOC is correlated with reproductive hormones, lipids, inflammation, endothelial function, and atherosclerosis.
In all study subjects, luteinizing hormone was an independent predictor of FMD% and AS, while both LH and LH/T were contributors to CIMT. In SCH, only a positive correlation between FMD% and testosterone was observed. In the LOH group, SHBG and LH were contributors to FMD% and CIMT, respectively, whereas LH/T, ucOC, FSH, estradiol, and E/T were contributors to aortic stiffness.
Limitations of our study: this was a cross-sectional study design. Therefore, a causal relationship could not be established. We did not include secondary hypogonadism in this study. Further studies are recommended to address the relationship between SCH and coronary artery disease, stroke, and peripheral arterial disease.
Consent for publication
All the authors involved in this study gave their consent for this article to be published in the Aging Male journal.
Consent to participate
Written informed consent was obtained from all participants.
Ethics approval and consent to participate
All procedures performed in our clinical study including human participants were in accordance with the ethical standards of the institutional and/or national research committee and the Helsinki Declaration. All participants provided informed consent for inclusion in this study.
Author contributions
Dr Matta provided the ideas and design of the study and wrote the protocol. She shared in conducting the literature review and recruited the participants, performed statistical analyses, and wrote the draft of the manuscript. Mr Abdelrahman who shared in conducting the literature review recruited the participants and collected the data. Dr Farrage performed the echocardiography and shared duplex studies. Dr Saedii performed laboratory work in this study. The authors approved the final draft of this manuscript. The authors have read and approved the final manuscript.
Acknowledgments
To later professor of internal medicine, Minia University, Egypt: Eglal Mohamed Shawky (deceased), and to Dr Ehab Ali Abdelgwad Ahmed, MD, Associate Professor, Department of Radiology, Faculty of Medicine, Minia University, Minia, Egypt. He shared in radiological investigations of the study.
Disclosure statement
The authors declare no conflicts of interest.
Data availability statement
The datasets generated and analyzed during the present study are not publicly accessible due to concerns of participant confidentiality but are offered by the corresponding author on realistic request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Tajar A, Forti G, O'Neill TW, the EMAS Group, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing study. J Clin Endocrinol Metab. 2010;95(4):1810–1818.
- Corradi PF, Corradi RB, Greene LW. Physiology of the hypothalamic pituitary gonadal axis in the male. Urol Clin North Am. 2016;25(1):151–162.
- Hiort O, Holterhus PM, Horter T, et al. Significance of mutations in the androgen receptor gene in males with idiopathic infertility. J Clin Endocrinol Metab. 2000;85(8):2810–2815.
- de Ronde W, van der Schouw YT, Muller M, et al. Associations of sex-hormone-binding globulin (SHBG) with non-SHBG-bound levels of testosterone and estradiol in independently living men. J Clin Endocrinol Metab. 2005;90(1):157–162.
- Giannetta E, Gianfrilli D, Barbagallo F, et al. Subclinical male hypogonadism. Best Pract Res Clin Endocrinol Metab. 2012;26(4):539–550.
- Tajar A, O'Connell MD, Mitnitski AB, European Male Aging Study Group, et al. Frailty in relation to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: results from the European male aging study. J Am Geriatr Soc. 2011;59(5):814–821.
- Corona G, Maseroli E, Rastrelli G, et al. Characteristics of compensated hypogonadism in patients with sexual dysfunction. J Sex Med. 2014;11(7):1823–1834.
- van den Beld AW, Huhtaniemi IT, Pettersson KSL, et al. Luteinizing hormone and different genetic variants, as indicators of frailty in healthy elderly men. J Clin Endocrinol Metab. 1999;84(4):1334–1339.
- Hyde Z, Norman PE, Flicker L, et al. Elevated LH predicts ischaemic heart disease events in older men: the health in men study. Eur J Endocrinol. 2011;164(4):569–577.
- Komori T. Functions of osteocalcin in bone, pancreas, testis, and muscle. IJMS. 2020;21(20):7513.
- Millar SA, Patel H, Anderson SI, et al. Osteocalcin, vascular calcification, and atherosclerosis: a systematic review and meta-analysis. Front Endocrinol. 2017;8:183.
- Tacey A, Qaradakhi T, Brennan-Speranza T, et al. Potential role for osteocalcin in the development of atherosclerosis and blood vessel disease. Nutrients. 2018;10(10):1426.
- Tacey A, Millar S, Qaradakhi T, et al. Undercarboxylated osteocalcin has no adverse effect on endothelial function in rabbit aorta or human vascular cells. J Cell Physiol. 2021;236(4):2840–2849.
- Eendebak RJAH, Ahern T, Swiecicka A, EMAS Group, et al. Elevated luteinizing hormone despite normal testosterone levels in older men-natural history, risk factors and clinical features. Clin Endocrinol (Oxf). 2018;88(3):479–490.
- Chen W, Liu ZY, Wang LH, et al. Are the aging male's symptoms (AMS) scale and the androgen deficiency in the aging male (ADAM) questionnaire suitable for the screening of late-onset hypogonadism in aging Chinese men? Aging Male. 2013;16(3):92–96.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672.
- Aiman J, Griffin JE, Gazak JM, et al. Androgen insensitivity as a cause of infertility in otherwise normal men. N Engl J Med. 1979;300(5):223–227.
- Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006). an update on behalf of the advisory board of the 3rd and 4th watching the risk symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23(1):75–80.
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115.
- Stefanadis C, Dernellis J, Tsiamis E, et al. Aortic stiffness as a risk factor for recurrent acute coronary events in patients with ischaemic heart disease. Eur Heart J. 2000;21(5):390–396.
- Rochira V, Carani C. Aromatase deficiency in men: a clinical perspective. Nat Rev Endocrinol. 2009;5(10):559–568.
- Crabbe P, Bogaert V, De Bacquer D, et al. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab. 2007;92(9):3604–3610.
- Mulligan T, Iranmanesh A, Johnson ML, et al. Aging alters feed-forward and feedback linkages between LH and testosterone in healthy men. Am J Physiol. 1997;273(4):R1407–R1413.
- McBride JA, Carson CC, Coward RM. Testosterone deficiency in the aging male. Ther Adv Urol. 2016;8(1):47–60.
- Yang YY, Zheng SC, Wang WC, et al. Osteocalcin levels in male idiopathic hypogonadotropic hypogonadism: relationship with the testosterone secretion and metabolic profiles. Front Endocrinol. 2019;10:687.
- Karsenty G, Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol Cell Endocrinol. 2014;382(1):521–526.
- Nah K, Jung S, Kim BT. Uncarboxyolated osteocalcin inversely correlated with total testosterone level in elderly man. Korean J Fam Pract. 2017;7(5):781–785.
- Posielski N, Krieger J, Madison W, et al. Osteocalcin as a novel regulator of testosterone production: a bench to bedside study in rats and humans. J Urol. 2019;201(4S):e855 MP58–08.
- De Toni L, Guidolin D, De Filippis V, et al. Osteocalcin and sex hormone binding globulin compete on a specific binding site of GPRC6A. Endocrinology. 2016;157(11):4473–4486.
- Kanazawa I, Tanaka K, Ogawa N, et al. Undercarboxylated osteocalcin is positively associated with free testosterone in male patients with type 2 diabetes mellitus. Osteoporos Int. 2013;24(3):1115–1119.
- Samavat J, Facchiano E, Cantini G, et al. Osteocalcin increase after bariatric surgery predicts androgen recovery in hypogonadal obese males. Int J Obes. 2014;38(3):357–363.
- Zhong N, Xu B, Cui R, et al. Positive correlation between serum osteocalcin and testosterone in male hyperthyroidism patients with high bone turnover. Exp Clin Endocrinol Diabetes. 2016;124(07):452–456.
- Schwetz V, Gumpold R, Graupp M, et al. Osteocalcin is not a strong determinant of serum testosterone and sperm count in men from infertile couples. Andrology. 2013;1(4):590–594.
- Yeap BB, Alfonso H, Chubb SA, et al. Higher serum undercarboxylated osteocalcin and other bone turnover markers are associated with reduced diabetes risk and lower estradiol concentrations in older men. J Clin Endocrinol Metab. 2015;100(1):63–71.
- Morley JE, Perry HM III, Kaiser FE, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41(2):149–152.
- Ghanim H, Dhindsa S, Green K, et al. Increase in osteocalcin following testosterone therapy in men with type 2 diabetes and subnormal free testosterone. J Endocr Soc. 2019;3(8):1617–1630.
- Taxel P, Kennedy DG, Fall PM, et al. The effect of aromatase inhibition on sex steroids, gonadotropins, and markers of bone turnover in older men. J Clin Endocrinol Metab. 2001;86(6):2869–2874.
- Leder BZ, Finkelstein JS. Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporos Int. 2005;16(12):1487–1494.
- Merlotti D, Gennari L, Stolakis K, et al. Aromatase activity and bone loss in men. J Osteoporos. 2011;2011:230671.
- Holmboe SA, Vradi E, Jensen TK, et al. The association of reproductive hormone levels and all-cause, cancer, and cardiovascular disease mortality in Men. J Clin Endocrinol Metab. 2015;100(12):4472–4480.
- Kelly DM, Jones TH. Testosterone and cardiovascular risk in men. Front Horm Res. 2014;43:1–20.
- Thirumalai A, Rubinow KB, Page ST. An update on testosterone, HDL and cardiovascular risk in men. Clin Lipidol. 2015;10(3):251–258.
- Mohler ER, Ellenberg SS, Lewis CE, et al. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metab. 2018;103(2):681–688. [published correction appears in J Clin Endocrinol Metab. 2020;105(1)].
- Whitsel EA, Boyko EJ, Matsumoto AM, et al. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111(4):261–269.
- Cai Z, Xi H, Pan Y, et al. Effect of testosterone deficiency on cholesterol metabolism in pigs fed a high-fat and high-cholesterol diet. Lipids Health Dis. 2015;14:18–20.
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in Middle-aged men: a meta-analysis. Clin Endocrinol. 2005;63(3):280–293.
- Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):29–39.
- Haring R, Xanthakis V, Coviello A, et al. Clinical correlates of sex steroids and gonadotropins in men over the late adulthood: the Framingham heart study. Int J Androl. 2012;35(6):775–782.
- Adorni MP, Zimetti F, Cangiano B, et al. High-density lipoprotein function is reduced in patients affected by genetic or idiopathic hypogonadism. J Clin Endocrinol Metab. 2019;104(8):3097–3107.
- Jones ME, Boon WC, Proietto J, et al. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17(2):55–64.
- Papageorgiou N, Briasoulis A, Androulakis E, et al. Imaging subclinical atherosclerosis: where do we stand? Curr Cardiol Rev. 2017;13(1):47–55.
- Huhtaniemi I, Mäkinen JI, Perheentupa A, et al. Late-onset hypogonadism in men. Experience from the Turku male ageing study (TuMAS). Hormones. 2008;7(1):36–45. PMID: 18359743
- Hsu B, Cumming RG, Naganathan V, et al. Temporal changes in androgens and estrogens are associated with all-cause and cause-specific mortality in older men. J Clin Endocrinol Metab. 2016;101(5):2201–2210.
- Toth P, Li X, Rao CV, et al. Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J Clin Endocrinol Metab. 1994;79(1):307–315.
- Berndt S, Perrier d'Hauterive S, Blacher S, et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. Faseb J. 2006;20(14):2630–2263.
- Lu M, Yang CB, Gao L, et al. Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (review). Exp Ther Med. 2015;9(1):3–10.
- Empen K, Lorbeer R, Dörr M, et al. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol. 2012;32(2):481–486.
- Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol. 2018;315(6):H1569–H1588.
- Fukui M, Kitagawa Y, Kamiuchi K, et al. Association between serum estradiol concentrations and carotid atherosclerosis in men with type 2 diabetes mellitus. Metabolism. 2008;57(2):285–289.
- Brand JS, den Ouden ME, Schuurmans MJ, et al. Endogenous sex hormones and subclinical atherosclerosis in middle-aged and older men. Int J Cardiol. 2013;168(1):574–576.
- Chan YX, Knuiman MW, Hung J, et al. Testosterone, dihydrotestosterone and estradiol are differentially associated with carotid intima-media thickness and the presence of carotid plaque in men with and without coronary artery disease. Endocr J. 2015;62(9):777–786.
- Stelmaszewska J, Chrusciel M, Doroszko M, et al. Revisiting the expression and function of follicle-stimulation hormone receptor in human umbilical vein endothelial cells. Sci Rep. 2016;6:37095.
- Melloni C, Roe MT. Androgen deprivation therapy and cardiovascular disease. Urol Oncol. 2020;38(2):45–52.
- Wallace IR, McKinley MC, Bell PM, et al. Sex hormone binding globulin and insulin resistance. Clin Endocrinol. 2013;78(3):321–329.
- Yeap BB, Marriott RJ, Antonio L, et al. Serum testosterone is inversely and sex hormone-binding globulin is directly associated with all-cause mortality in men. JCEM. 2021;106(2):e625–e637.
- Gyawali P, Martin SA, Heilbronn LK, et al. Cross-sectional and longitudinal determinants of serum sex hormone binding globulin (SHBG) in a cohort of community-dwelling men. PLoS One. 2018;13(7):e0200078.
- Mathews L, Subramanya V, Zhao D, et al. Endogenous sex hormones and endothelial function in postmenopausal women and men: the multi-ethnic study of atherosclerosis. J Womens Health. 2019;28(7):900–909.
- Prats-Puig A, Osiniri I, Soriano-Rodriguez P, et al. Undercarboxylated osteocalcin relates to cardiovascular risk markers in offspring of families with metabolic syndrome. Atherosclerosis. 2014;233(1):272–277. atherosclerosis.2014.01.002
- Zhang M, Ni Z, Zhou W, et al. Undercarboxylated osteocalcin as a biomarker of subclinical atherosclerosis in non-dialysis patients with chronic kidney disease. J Biomed Sci. 2015;22:75.
- Tacey A, Smith C, Woessner MN, et al. Undercarboxylated osteocalcin is associated with vascular function in female older adults but does not influence vascular function in male rabbit carotid artery ex vivo. PLoS One. 2020;15(11):e0242774.
- Choi BH, Joo NS, Kim MJ, et al. Coronary artery calcification is associated with high serum concentration of undercarboxylated osteocalcin in asymptomatic Korean men. Clin Endocrinol. 2015;83(3):320–326.
- Smith C, Voisin S, Al Saedi A, et al. Osteocalcin and its forms across the lifespan in adult men. Bone. 2020;130:115085.
- Kanazawa I, Yamaguchi T, Sugimoto T. Relationship between bone biochemical markers versus glucose/lipid metabolism and atherosclerosis; a longitudinal study in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;92(3):393–399.
- Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21(12):1998–2003.
- Foresta C, De Toni L, Selice R, et al. Increased osteocalcin-positive endothelial progenitor cells in hypogonadal male patients. J Endocrinol Invest. 2010;33(7):439–442.

