Abstract
Introduction
Various approaches are required to prevent and treat heterogeneity-based prostate cancer. Here, we analyzed the anticancer effects of metformin, which has a good toxicity profile and is inexpensive.
Method
From January 2010 to December 2019, analysis was conducted retrospectively in a cohort from the National Health Insurance Service database. The wash-out period was set for cancer diagnosis in 2010 and 2011, and subjects (105,279) diagnosed with prostate cancer (ICD C61) from 2012 to 2014 were excluded The final subjects (105,216) were defined as the metformin administration group when they took metformin for 180 days or more from January 2012 to December 2019. The non-metformin group was defined as those who took less than 180 days from January 2012 to December 2019. The prevalence of prostate cancer according to metformin administration and the risk according to the cumulative duration of metformin were analyzed.
Results
A total of 105,216 people were included in this study, with 59,844 in the metformin group and 45,372 in the metformin non-administration group. When calculating HRs (Hazard Rate) according to the cumulative period of metformin administration, metformin administration period length was inversely associated with prostate cancer risk (Q2 HR = 0.791 95% CI: 0.773–0.81, Q3 HR = 0.634 95% CI: 0.62–0.649, Q4 HR = 0.571 95% CI: 0.558–0.585). HRs tended to decrease with the cumulative duration of metformin administration.
Conclusion
This study confirmed that prostate cancer risk decreased with increasing duration of metformin administration. Metformin should be considered as a new strategy in the treatment and prevention of prostate cancer characterized by heterogeneity.
Introduction
Prostate cancer (PCa) is a representative urological cancer, of which one-sixth of men are at risk [Citation1]. The incidence rate has increased significantly in Asian countries over the past few decades. Therefore, various approaches to preventing and treating PCa based on heterogeneity are needed.
Hyperinsulinemia associated with type 2 diabetes can play an important role in cancer and can be negatively related to cancer prognosis [Citation2,Citation3]. Increased insulin levels in obese men can exacerbate PCa [Citation3–5]. This is supported by laboratory evidence showing that hyperinsulinemia upregulates insulin receptors and increases tumor growth in PCa cells [Citation6]. However, diabetes is associated with a reduction in diagnosis of PCa, potentially mediated by low testosterone level [Citation7,Citation8].
Metformin, a non-guanid drug, is the most widely used oral hypoglycemic agent in type 2 diabetes, with a good toxicity profile and low cost. Metformin is currently approved by the U.S. Food and Drug Administration (FDA) as an exercise and dietary supplement to enhance blood sugar control in adults and children with type 2 diabetes [Citation9]. Its main mechanisms are to stimulate 50 AMP-activated protein kinase in the liver, inhibit gluconeogenesis, and reduce circulating insulin level [Citation10]. In addition to other anti-tumor properties, such as a decrease in the c-myc tumor gene, metformin can also reduce insulin-stimulated cancer growth [Citation11].
We hypothesized that metformin use would be associated with a reduced risk of PCa. Therefore, we conducted a large population-based study of metformin use and PCa. In addition, the effect of metformin on PCa prognosis was analyzed according to treatment duration to provide a basis for including metformin in new strategies for the treatment of PCa characterized by heterogeneity.
Materials and methods
Study cohort
This study was approved by the Institutional Review Board of Hanyang University Hospital in Seoul (HYUH2020-10-004). The National Health Insurance Service (NHIS) database includes only anonymized data, so participant consent was not required [Citation12]. The NHIS provides compulsory medical care to almost all Koreans and collects health data from nearly 50 million insured people, including hospitalization and outpatient visits, diagnosis, drug prescriptions, national health examination, and death data.
Classification of patient groups
This is a retrospective cohort study using the NHIS Database from January 2010 to December 2019. We set the washout period to exclude the canceller diagnosis with CCI for 2 years, from January 2010 to December 2011. Exclusion criteria included: (1) Those with a date of death before the diagnosis of cancer, (2) those younger than 20 years Subjects who met the inclusion criteria and who were newly diagnosed with prostate cancer (C61) between 2012 and 2014 were classified into a metformin-treated group and a group not treated with metformin. The metformin non-administration group was defined as subjects who had never been prescribed metformin during the study period (from January 2012 to December 2019) or those with a metformin usage period <180 days.
PCa was defined as a code C61 claim from 2012 to 2014 according to the International Classification of Diseases (ICD-10), the age group was classified using a 10-year interval starting from the age of 20, and income groups were classified into quartiles. Residential areas were divided into Seoul, other metropolitan cities (Busan, Daegu, Incheon, Gwangju, Daejeon, Ulsan, and Gyeonggi-do), and rural areas.
Statistics
Continuous variables were described as mean and standard deviation for normally distributed variables and as median (Q1, Q3) for non-normally distributed variables. Independent t-tests were used to compare normally distributed variables, and Wilcoxon rank-sum test was used to compare non-normally distributed variables. The chi-square and Fisher’s exact test were used to compare categorical variables. P values < 0.05 were considered statistically significant. Statistical analysis was performed using SAS v9.4 (SAS Institute, Cary, NC) and R software (version 4.0.4 or newer; R Project for Statistical Computing).
Results
During this study, a total of 105,279 patients were diagnosed with PCa, of whom 105,216 were included in this study after the exclusion criteria were applied (). The remaining patients were divided into the metformin group (n = 59,844) and the metformin non-administration group (n = 45,372). shows demographic characteristics including age, sex, income, residential area, comorbidities, and CCI(Charlson comorbidity index). The metformin non-administration group was older than the metformin group and had a higher income level, while the metformin group had higher CCI and comorbidity.
Figure 1. Flow chart of the study cohort.
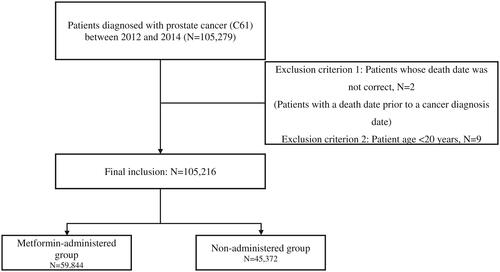
Table 1. Descriptive characteristics of the study population with PCa.
Survival rate was analyzed among the administration and non-administration groups by age, income, and residential area subgroups in stratified analysis (). When stratified by age, income, and residential area, the survival rate of the administration group was higher than that of the non-administration group.
Table 2. Overall survival rate.
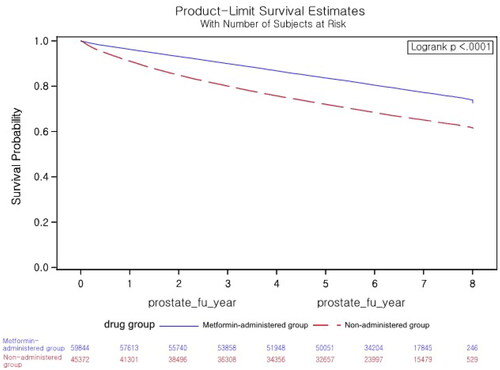
The survival curve during the follow-up period was estimated using the Kaplan-Meier method, and survival between groups was compared with the log-rank test. The survival rate varied significantly based on metformin use (p < 0.001) ().
Figure 2. Kaplan–Meier survival analysis of time to death.
Causes of death in the total population of patients with PCa were malignant neoplasm (14,632), PCa (5782), other (4316), and respiratory disease (2943) (). Causes of death in the metformin administration group were malignant neoplasm (5129), other (2272), cardiovascular disease (2016), respiratory disease (1570), endocrine disease (1075), and PCa (1039). Causes of death in the metformin non-administration group were malignant neoplasm (9503), prostate cancer (4743), others (2044), cardiovascular diseases (4678), and respiratory disease (1373). HRs for cancer-related death were lower in the metformin administration group than those for the metformin non-administration group [malignant neoplasm (HR = 0.381, 95% CI: 0.368–0.394), PCa (HR = 0.154, 95% CI: 0.144-0.165) bladder cancer (HR = 0.24), and kidney cancer (HR = 0.24, 95% CI: 0.188–0.308)].
Table 3. Hazard ratios for mortality in PCa patients.
The incidence of PCa is expressed as the number of events per 100,000 people (PY), as shown in . The incidence of urological cancer was 59,844 (56.88%) in the metformin non-administration group, and the incidence rate per 100,000 PY was 18300.33 in the metformin non-administration group and 16,981.7 in the metformin group. The metformin group had a higher risk of prostate cancerPCa than the metformin non-administration group (HR = 1.141, 95% CI: 1.127–1.156).
Table 4. Hazard ratios for prostate cancer incidence.
The longer the cumulative duration of administration of metformin, the lower the HR for the risk of prostate cancer (Q2 HR = 0.791 95% CI: 0.773–0.81, Q3 HR = 0.634 95% CI: 0.62–0.649, Q4 HR = 0.571 95% CI: 0.558–0.585). HR tended to decrease with the cumulative duration of metformin administration.
If the reference is group 1 of the non-treated group, it can be seen that HRs decrease according to the duration of treatment in comparison with the metformin-treated group quartile group (group 5: HR = 0.926 95% CI: 0.88–0.975, group 6: HR = 0.735 95% CI: 0.699–0.773, group 7: HR = 0.593 95% CI: 0.563–0.623, group 8: HR = 0.535 95% CI: 0.509–0.563) ().
Table 5. Hazard ratio for prostate cancer incidence by the group.
In relative 5-year survival rate, the 1-year, 3-year, 5-year, and 8-year survival rates of the metformin group were 99.7%, 94.7%, 88.2% and 78.5%, respectively, and those in the metformin non-administration group were 96.7%, 85.6%, 76.2%, and 65.4%, respectively ().
Table 6. Conditional relative 5-year survival.
Discussion
Using a large population-based longitudinal cohort study, we investigated prostate cancer risk according to the use and cumulative duration of metformin.
Studies on the link between metformin and PCa have shown that cancer rates have reduced diabetes and metformin’s risk of PCa by 44% [Citation13]. A Scottish study reported that diabetics taking metformin had a 23% lower overall risk of cancer compared to those not taking metformin [Citation14]. The study observed and reported risk reduction for the longest metformin use period.
In a large Finnish population-based registry study, a reduction in the risk of PCa was observed with the use of all diabetes drugs [Citation15]. The study showed that treatment of diabetes, rather than specific drugs, was inversely proportional to the risk of PCa. Other studies have reported differences between ethnic groups, including that African and European Americans showed a different association between PCa and diabetes [Citation16].
In the present study, the relative 5-year survival rate was higher in the metformin group than in the non-administration group. These findings are in agreement with the results of previous papers. This study presents the results of showing the anticancer effect of metformin on prostate cancer through the analysis of the incidence and survival rate of prostate cancer according to the cumulative dose of metformin.
The specific mechanism of action of metformin has not been identified, but it (1) inhibits cellular respiration in mitochondria, (2) activates AMPK, and (3) prevents increases in cAMP concentration induced by glucagon, consequently inhibiting protein kinase A activity and affecting normal gut bacteria [Citation17–19]. Metformin is involved in cellular apoptosis through the activation of AMPK and complete inhibition of phosphorylation, and it has been suggested that the apoptosis induced by energy stress due to AMPK is mediated by a combination of metformin and 2DG [Citation20]. p53 regulates the induction of apoptosis mediated by metformin/2DG, and the combination of metformin and 2DG strongly induces the expression of p53 [Citation20].
The drug biguanide was developed in Europe in the 1920s, but its interest has declined due to insulin and has returned to the spotlight. Metformin lowers blood sugar with few side effects and is inexpensive, making it the most cost-effective diabetes treatment currently available. For these reasons, it is widely used. Additional large-scale, big-data research is expected to provide a new treatment strategy for PCa based on heterogeneity.
The findings of this research indicated that the risk of PCa changes according to the cumulative quantity of metformin taken, with risk decreasing as the cumulative amount increases. The key result of this study is that the longer the cumulative period of metformin use is, the lower the risk of PCa, confirming a preventive effect of PCa through long-term cumulative use. The death rate from PCa was lower in the group taking metformin compared with the non-administration group. Such results may have been shown because PCa includes cancers with a relatively mild prognosis enough to be called turtle cancer, but significant differences in causes of death between the metformin and non-administration groups indicate that metformin has anticancer effects.
Metformin is not without disadvantages. Diarrhea, nausea, vomiting, drainage, and poor appetite are the most common side effects of metformin use. It has been reported that 41.7% of Koreans who took atrioventricular formulations of metformin before taking Western formulations experienced gastrointestinal side effects [Citation21]. However, these side effects are not serious, and the administration of metformin should be actively considered in applicable cases. In addition, in the case of the Western type, side effects are less likely. Since this study confirmed that the mortality rate from PCa decreases with metformin use, metformin treatment should be recommended regardless of stage or progression.
This study has several limitations. First, due to the characteristics of NHIS data, it was not possible to analyze the information on the test number, which is actual clinical information. No information was available on PSA level or Gleason score, both of which are important clinical indicators of PCa. Therefore, an analysis of biochemical recurrence could not be conducted. Second, the absence of clinical information prohibited the analysis of patient groups according to the stage. This is also linked to the limitations, and if there was an analysis according to the risk, it would be possible to know which patient group could be used more effectively. Despite these limitations, metformin was effective against the development of PCa as well as decreasing associated mortality. In addition, there were differences in effect depending on the cumulative amount of metformin taken. To overcome these limitations, additional large-scale studies using actual clinical data should be performed.
Conclusions
In a real-world setting, a large, population-based study confirmed that metformin use is associated with a lower risk of PCa and death from PCa. This decrease in risk corresponds to the cumulative dose of metformin. Here, we present the basis for further studies on metformin as a new strategy in the treatment and prevention of PCa characterized by heterogeneity.
Author contribution
JKJ designed the study; all authors participated in writing the manuscript.
Acknowledgements
The authors would like to thank HKS and YJK for providing excellent advice on this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ries L, Melbert D, Krapcho M, et al. 2008. SEER cancer statistics review, 1975–2005, based on November 2007 SEER data submission, posted to the SEER website. Available from: http://seer.cancer.gov/csr/1975_2005/.
- Hsing AW, Gao YT, Chua S, Jr, et al. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95(1):67–71.
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and Colon cancer: a review. Am J Clin Nutr. 2007;86(3):836S–842S.
- Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1977–1983.
- Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the shared equal access regional cancer hospital database study group. J Clin Oncol. 2004;22(3):446–453.
- Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99(23):1793–1800.
- Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47(6):1071–1078.
- Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow‐up study. Int J Cancer. 2009;124(6):1398–1403.
- U.S. Food and Drug Administration. Metformin Hydrochloride Tablets [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2016. [updated Apr 29, 2016; cited 2016 Mar 1]. Available from: www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf.
- Cusi K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes Rev. 1998;6(2):89–131.
- Akinyeke T, Matsumura S, Wang X, et al. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis. 2013;34(12):2823–2832.
- Lee J, Lee JS, Park S-H, et al. Cohort profile: the national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46(2):e15.
- Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–6752.
- Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305.
- Murtola TJ, Tammela TL, Lahtela J, et al. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol. 2008;168(8):925–931.
- Waters KM, Henderson BE, Stram DO, et al. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009;169(8):937–945.
- Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56(9):1898–1906.
- Burcelin R. The antidiabetic gutsy role of metformin uncovered? Gut. 2014;63(5):706–707.
- Madiraju AK, Erion DM, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–546.
- Ben Sahra I, Laurent K, Giuliano S, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70(6):2465–2475.
- Kim CH, Han KA, Oh HJ, et al. Safety, tolerability, and efficacy of metformin extended‐release oral antidiabetic therapy in patients with type 2 diabetes: an observational trial in Asia. J Diabetes. 2012;4(4):395–406.

