Abstract
Objective
This study aimed to summarize the current evidence regarding the feasibility of robot-assisted radical prostatectomy (RARP) in men aged over 75 years.
Method
A comprehensive search of four electronic databases (China National Knowledge Infrastructure, PubMed, Web of Science, and Cochrane Library) was performed to identify eligible comparative studies as of April 2022. Parameters, including perioperative results and oncological and functional outcomes, were evaluated.
Results
Seven articles with 7575 patients undergoing RARP were included in this study. Patients with prostate cancer were grouped by age ≥ 75 years versus < 75 years. Our results demonstrated that compared with the older group, the younger group had better potency (p < .00001). However, there were no significant differences in operation time (p = .29), estimated blood loss (p = .13), length of hospital stay (p = .48), complications (p = .22), continence (p = .21), positive surgical margin (p = .28), and biochemical recurrence (p = .74) between the groups.
Conclusion
Our study revealed that the perioperative, oncological, and functional outcomes in men aged over 75 years undergoing RARP were not significantly different from those of their younger counterparts. RARP is feasible in men aged over 75 years.
Introduction
According to previous epidemiological studies, prostate cancer (PCa) is the second most common tumor in men [Citation1]. Radical prostatectomy (RP) is the recommended definitive surgical treatment for organ-confined disease [Citation2]. Although open RP (ORP) and laparoscopic RP (LRP) are the main treatment modalities for PCa, with the update of prostatectomy technology and equipment, robot-assisted RP (RARP) has been widely adapted. Certainly, compared with LRP, robotic techniques offer several advantages, including three-dimensional visualization, more precise dissection, and improved dexterity [Citation3,Citation4]. Recently, numerous published studies have shown that RARP is feasible and safe for PCa treatment, and better preoperative, functional, and oncological outcomes have been observed in patients who received RARP compared with ORP and LRP [Citation5].
These surgical features suggest that RARP is suitable for elderly patients. However, to the best of our knowledge, the European Association of Urology and National Comprehensive Cancer Network guidelines set a life expectancy greater than 10 years as the standard for RP [Citation6,Citation7]. However, in terms of safety and worse biochemical recurrence (BCR) and metastatic progression-free survival rates associated with age, many doctors are reluctant to perform RARP in men aged over 75 years [Citation8,Citation9]. With the advent of better healthcare facilities, the number of people aged over 80 years will triple by 2050. While a greater proportion of men are older, the risk of PCa has increased [Citation10]. Except life expectancy, patients’ health status is also a factor affecting the decision-making of RP. For patients aged over 70 years, if the G8-tool score is > 14, RARP should be offered, similar with younger individuals [Citation11]. Therefore, we need to reevaluate patients who are suitable for radical surgery. Previous studies have proven the safety and excellent oncological and functional outcomes of RARP in this select group of elderly patients with PCa [Citation9,Citation12]. Although RARP logically suggests a potential advantage in the elderly, the feasibility of RARP in elderly patients remains controversial.
Therefore, this study aimed to use these data to evaluate the feasibility of RARP in patients aged over 75 years and to better guide clinical practice.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria were used in our study [Citation13].
Search strategy
To identify eligible studies, we systematically searched four databases (China National Knowledge Infrastructure, PubMed, Web of Science, and Cochrane Library) as of April 2022, with no language restrictions. The searching items were as follows: “robot,” “robotic,” “robot-assisted prostatectomy,” “older man,” “elderly,” “age 75 years and older,” and “prostate cancer.” Relevant references for the eligible studies were manually searched. The two reviewers reviewed the literature separately. Any discrepancies were resolved through discussion with a third reviewer.
Inclusion and exclusion criteria
Our included studies met the following criteria: (1) studies comprising patients with PCa requiring RARP; (2) randomized controlled trials (RCTs) and retrospective or prospective comparative studies between patients aged < 75 years and ≥ 75 years; and (3) studies assessing one or more of the following results: perioperative, functional, and oncological outcomes. The exclusion criteria were as follows: (1) studies on inaccurate age stratification; (2) comments, letters, case reports, reviewers, noncomparative studies, and unpublished articles; and (3) studies with unavailable data for analyses.
Data extraction
All relevant data were independently extracted from eligible studies by two reviewers. Standard tables were used to extract all collected interest outcomes for each included study: the first author’s name, country, study design, surgical procedure, patient age, sample size, follow-up time, body mass index (BMI), prostate-specific antigen (PSA) level, prostate size, risk classification, tumor stage, neurovascular bundle (NVB) preservation, operative time (OT), estimated blood loss (EBL), length of hospital stay (LOS), total complications, potency, positive surgical margin (PSM), BCR, catheterization time, and continence at 12 months. If available, we analyzed complications graded according to the Clavien–Dindo classification (CDC) system.
Study quality assessment
Based on preliminary search results, the Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of the included studies [Citation14]. In the current meta-analysis, a score of 7 was considered high-quality for non-RCTs.
The above steps were independently completed by two reviewers. After discussion, disagreements were resolved by a third author.
Statistical analyses
Review Manager version 5.4 (The Cochrane Collaboration, Oxford, UK) was used for statistical analyses. Odds ratios (ORs) and mean differences (MDs) or standardized MDs were calculated to evaluate dichotomous and continuous data, and 95% confidence intervals (CIs) and p-values were calculated. The Q and I2 tests were used to estimate the heterogeneity between studies. According to the test results of heterogeneity, I2 > 50% or p < .10 represented significant heterogeneity, and the random-effects model was used for pooled estimates. Otherwise, the fixed-effects models were used for analyses. When the data in the study were expressed as median (interquartile range), we calculated the mean ± standard deviation according to the method described by Hozo et al. [Citation15]. p < .05 was considered statistically significant.
Results
Description of studies
As shown in the PRISMA flowchart (), 146 records were retrieved from the four databases. Seven eligible studies with 7575 patients (1017 aged ≥75 years vs. 6558 aged < 75 years) were included in the meta-analysis according to preliminary screening and full-text review. The included articles were published between 2012 and 2022 and were conducted in three countries: Germany, India, and Japan. Six retrospective studies and one prospective study were included. The follow-up period for all studies was > 12 months. The basic characteristics of all the patients in each study are presented in .
Table 1. Characteristics and designs of the included studies.
Table 2. Basic characteristics of patients.
Quality assessment
The final study quality scores based on the NOS scoring rules are shown in .
Baseline parameters
In our meta-analysis, patients aged over 75 years had a higher PSA level (weighted mean difference [WMD], −5.73; 95% CI, −9.53 to −1.95; p = .003, Figure S1 B) and BMI (WMD, −0.79; 95% CI, −1.28 to −0.29; p = .002; Figure S1 A) than patients aged < 75 years. However, there were no significant differences in prostate size (p = .70; Figure S1 C), risk classification (low, intermediate, high) (p = .13, p = .34, and p = .49, respectively; Figure S2) , or NVB preservation (unilateral, bilateral) (p = .98 and p = .10, respectively; Figure S3) between the two groups (Supplementary Material).
Surgical outcomes
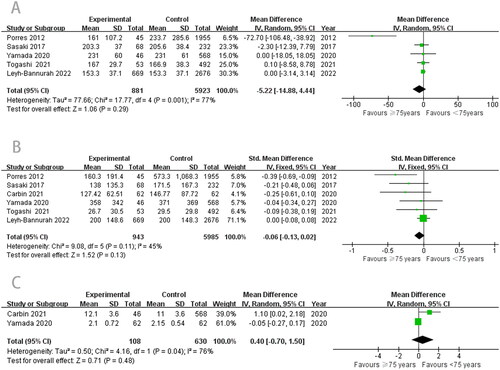
There was no statistical difference between the age < 75 years and ≥ 75 years groups (WMD, −5.22; 95% CI, −14.88 to −4.44; p = .29; ). Simultaneously, we evaluated the EBL and LOS of the two groups. The results also showed no statistical differences in EBL (WMD, −0.06; 95% CI, −0.13–0.02; p = .13; ) and LOS (WMD, 0.40; 95% CI, −0.70–1.50; p = .48; ) between the two groups.
Figure 2. Meta-analysis of perioperative parameters.
Fewer overall complications were found in the ≥ 75 years RARP group, but the difference did not reach statistical significance (OR, 1.15; 95% CI, 0.92–1.45; p = .22; ). Moreover, there were no differences between the groups in CDC grades I (OR, 0.98; 95% CI, 0.42–2.28; p = .96), II (OR, 1.28; 95% CI, 0.54–3.03; p = .57), and III (OR, 1.16; 95% CI, 0.39–3.42; p = .79) (). In addition, the length of catheterization between the groups was similar, with no significant statistical difference (WMD, −0.02; 95% CI, −0.07–0.04; p = .50; ).
Figure 3. Meta-analysis of perioperative parameters.
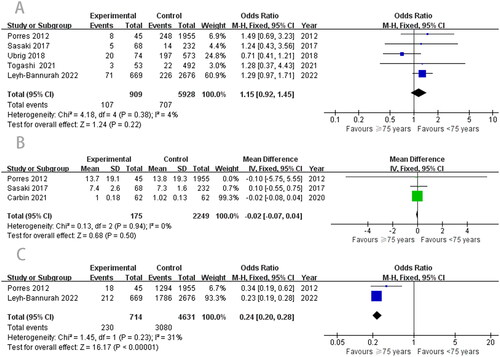
Table 3. Clavien–Dindo classification of complications in the studies.
Functional outcomes
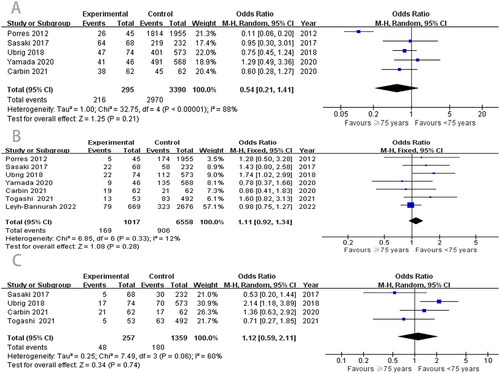
Among the five studies [Citation10,Citation16–19] on continence, there was high heterogeneity, and we used a random-effects model for the meta-analysis. The pooled results showed that there was no difference in postoperative continence (OR, 0.54; 95% CI, 0.21–1.41; p = .21; ) between the two groups. However, compared with the older group, the younger group had better potency (OR, 0.24; 95% CI, 0.20–0.28; p < .00001; ).
Oncological outcomes
PSM and BCR are important components of RARP. First, according to the meta-analysis results, the difference in PSM between the two groups was not statistically significant (OR, 1.11; 95% CI, 0.92–1.34; p = .28; ). Our data also showed that the ≥ 75 years group reported similar outcomes to the < 75 years group in BCR. No significant difference was observed between the two groups (OR, 1.12; 95% CI, 0.59–2.11; p = .74; ).
Quality of the study
According to the NOS, most of our included studies were considered of high quality, with only two studies having poor quality [Citation14].
Discussion
Although RP has been considered a standard and effective treatment option for clinically localized PCa in patients with a life expectancy of > 10 years, the survival benefit of elderly patients undergoing RP has been an area of concern for urologists, with elderly adults possibly being excluded from undergoing radical surgery [Citation20]. However, with the increase in elderly patients and widespread use of PSA tests, a growing number of elderly patients with PCa are being diagnosed, which also brings challenges to RP.
With the development of surgical technology and the da Vinci system approved by the Food and Drug Administration in 2018, RARP has been widely performed worldwide, and its safety and effectiveness have been confirmed in many studies [Citation4,Citation21]. A previous study reported that RP significantly improved life expectancy and quality-adjusted life years in elderly patients with few comorbidities [Citation22]. Simultaneously, many medical centers have identified that RARP may be a reasonable therapeutic option for patients older than 70 years and provide perioperative and functional outcomes comparable to those of younger patients [Citation20,Citation23]. In recent years, a number of studies have also evaluated the perioperative, functional, and oncological outcomes of RARP in elderly patients aged over 75 years [Citation8,Citation10,Citation16–19,Citation24]. However, whether RARP is feasible for the treatment of PCa in patients aged ≥ 75 years remains controversial.
Based on these background conditions, we performed the first meta-analysis to explore the feasibility of RARP in patients aged over 75 years. Based on strict screening criteria, seven articles with 7575 patients were included in our study. Our results showed that RARP had no significant effect on OT, EBL, total complications, PSM, BCR, catheterization time, and continence in older adult patients (≥ 75 years group) and younger patients (< 75 years group). We will perform further in-depth analyses of these study results.
The key factor for treatment selection in older adult patients is perioperative outcomes. First, regarding OT, our meta-analysis showed no significant difference between the two groups (p = .29), which is consistent with the results of several previously published studies. Sasaki et al. [Citation19] reported that RARP for older patients had similar mean OT to younger patients (203.3 vs. 205.6 min, p = .664). Yamada et al. [Citation8] confirmed this conclusion. A previous study confirmed that obesity increases the OT of RARP [Citation25]. Interestingly, we found that the BMI of older patients (aged ≥ 75 years) was higher than that of young patients (aged < 75 years) (p = .002), which did not prolong the surgery time. This may be related to the significant clinical experience of the operator. As the experience of the operator increases, the OT decreases accordingly [Citation4]. Second, our results showed no significant differences in EBL and LOS between the two cohorts. This finding is consistent with the results of the included studies.
Complications are also the focus of our surgeons, which is also a key factor affecting elderly patients with PCa who undergo RP. According to previous reports, our meta-analysis observed no difference in complications between the ≥ 75 years and < 75 years groups. Similar results were identified between the groups, regardless of the CDC grade. Fossati et al. [Citation26] performed a systematic review and revealed that pelvic lymph node dissection (PLND) in RP may be associated with more postoperative complications. However, all patients in our included studies underwent PLND, and most of the tumor stages were within stage T3, which may explain the similar overall complication rates between the two groups.
The duration of catheterization and continence after RP are closely related to the quality of life (QoL) of patients. The meta-analysis revealed no difference in the indwelling time and continence rate between the two groups after RARP. However, previous studies held different views. Carbin et al. [Citation10] found no difference in continence rates between the groups, but the time to continence (p = .02) was significantly shorter in younger men than in elderly men. In addition, Scosyrev et al. [Citation27] and Zorn et al. [Citation28] showed that older patients had worse continence rates than younger patients. The possible reasons are as follows: first, the definition of urinary incontinence varies from study to study [Citation10,Citation29]; second, continence may be related to preoperative lower urinary tract symptom burden measured by the International Prostate Symptom Score and QoL [Citation8]. Finally, underlying tumor characteristics, sample size, surgeon experience, and surgical approach and technique also influence continence [Citation29,Citation30].
In men, the most important aspect in the postoperative period is erectile function recovery. This study, with limited data, demonstrated that the erectile function recovery rates after RARP between elderly and younger patients were 32.2% and 66.5%, respectively, and there was a significant difference between the two groups. The natural incidence rate of erectile dysfunction is high in elderly patients. Leyh-Bannurah et al. [Citation8] also found that younger patients have a significantly better recovery of erectile function after RARP than older patients. Even if surgeons try their best to protect the nerves and vessels, the energy platform used during the operation may still cause nerve damage. Researchers have explained the reason for erectile dysfunction through a series of animal models and clinical experiments, and their studies have confirmed that increasing age will have a negative effect on peripheral nerve regeneration after injury, and that RARP will further lead to neurodegeneration [Citation31–33]. However, this is not a key factor affecting the performance of RARP in elderly patients.
Regarding oncological outcomes, we found that the older group had similar BCR and PSM rates to the younger group. A large high-volume center also reported that age did not significantly influence BCR and PSM [Citation8]. However, the outcomes have been controversial across studies. The largest study in Germany compared oncological outcomes after RP between patients aged < 75 and ≥ 75 years (13,732 vs. 265). The results demonstrated that older patients had significantly higher PSM rates than younger patients, and that older age was associated with a greater risk of BCR [Citation9]. Previous studies analyzed the clinicopathological results of 3350 elderly male patients with PCa and found that men aged ≥ 75 years had a higher risk of BCR (49.3 vs. 29.6% in younger men) [Citation34]. As far as we know, testosterone, as the principal circulating androgen in males, decreases with age [Citation35]. In the European Male Aging Study, it reported that the incidence of hypogonadism was 2.1% in the aged 40–79 years old men [Citation36]. Study showed that low serum testosterone levels (<300 ng/dL) were significantly associated with upgrading, upstaging, unfavorable disease and PSM [Citation37]. In addition, Ferro et al. [Citation38] suggested that low preoperative circulating testosterone level was significantly associated with high BCR rate after RP in patients with clinically localized PCa. However, Zorn et al. [Citation28] found that young men had more PSM than older men (p = .03), and they attributed this to smaller prostates and more nerve sparing in younger men than in elderly men. In our study, preoperative prostate volume and nerve sparing were not statistically significant, and this factor was avoided. In addition, PSM is highly correlated with the experience and technique of the surgeon and is recognized as a predictor of BCR. Since there was no difference in PSM after RARP in this study, this explains the similar BCR between the groups.
Although our meta-analysis provides meaningful guidance for clinical practice, it has some limitations. First, most of the included studies were retrospective and patients with carefully selected, which undoubtedly had a certain selection bias. However, it included a relatively large number of patients (7575), which represents our current evidence. Second, high heterogeneity was observed in some outcome parameters. Even if a random-effects model was used, these effects may still exist. Third, no detailed statistical analyses have been performed on the survival and prognosis of patients. Finally, some studies did not consider the comorbidity of patients, which may also affect the results after RARP.
Conclusions
In conclusion, our current evidence revealed that the perioperative, oncological, and functional outcomes in men aged over 75 years undergoing RARP were not significantly different from those of their younger counterparts. Therefore, we believe that it is feasible to perform RARP in men aged over 75 years. The inclusion level of the study limits the strength of our conclusions. Large-scale RCTs are required for further validation.
Author contributions
T. Tang and J. Wu are responsible for manuscript conceptualization. Z. Y. Xia and X. Q. Fu are responsible for manuscript writing and data analysis. J. Z. Li and X. Z. Yuan are responsible for data analysis. L. T. Tang is responsible for manuscript editing.
Supplemental Material
Download PDF (1 MB)Supplemental Material
Download MS Word (11.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gandaglia G, Leni R, Bray F, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4(6):877–892.
- Gurung PMS, Wang B, Hassig S, et al. Oncological and functional outcomes in patients over 70 years of age treated with robotic radical prostatectomy: a propensity-matched analysis. World J Urol. 2021;39(4):1131–1140.
- Garisto J, Bertolo R, Wilson CA, et al. The evolution and resurgence of perineal prostatectomy in the robotic surgical era. World J Urol. 2020;38(4):821–828.
- Du Y, Long Q, Guan B, et al. Robot-assisted radical prostatectomy is more beneficial for prostate cancer patients: a system review and meta-analysis. Med Sci Monit. 2018;24:272–287.
- Basiri A, de la Rosette JJ, Tabatabaei S, et al. Comparison of retropubic, laparoscopic and robotic radical prostatectomy: who is the winner? World J Urol. 2018;36(4):609–621.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262.
- Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134–143.
- Leyh-Bannurah SR, Wagner C, Schuette A, et al. Feasibility of robot-assisted radical prostatectomy in men at senior age ≥75 years: perioperative, functional, and oncological outcomes of a high-volume center. Aging Male. 2022;25(1):8–16.
- Mandel P, Kriegmair MC, Kamphake JK, et al. Tumor characteristics and oncologic outcome after radical prostatectomy in men 75 years old or older. J Urol. 2016;196(1):89–94.
- Carbin DD, Tamhankar AS, Ahluwalia P, et al. Robot-assisted radical prostatectomy in Indian men of age 75 years and above: a propensity score-matched analysis. J Robot Surg. 2022;16(4):799–806.
- Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172.
- Nakamura LY, Nunez RN, Andrews PE, et al. Older age does not impact perioperative complications after robot-assisted radical prostatectomy. J Robot Surg. 2011;5(3):201–208.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
- Porres D, Pfister D, Labanaris AP, et al. [Robot-assisted radical prostatectomy in elderly patients: surgical, oncological and functional outcomes]. Urologe A. 2012;51(10):1424–1431.
- Sasaki Y, Shiozaki K, Miyake T, et al. [ROBOT-assisted radical prostatectomy for men age 75 and older]. Nihon Hinyokika Gakkai Zasshi. 2017;108(1):12–16.
- Ubrig B, Boy A, Heiland M, et al. Outcome of robotic radical prostatectomy in men over 74. J Endourol. 2018;32(2):106–110.
- Yamada Y, Teshima T, Fujimura T, et al. Comparison of perioperative outcomes in elderly (age ≧ 75 years) vs. younger men undergoing robot-assisted radical prostatectomy. PLoS One. 2020;15(6):e0234113.
- Nishikawa M, Watanabe H, Kurahashi T. Safety and feasibility of robot-assisted radical prostatectomy for clinically localized prostate cancer in elderly Japanese patients. Prostate Int. 2017;5(1):13–16.
- Cornelius J, Mudlagk J, Afferi L, et al. Postoperative peripheral neuropathies associated with patient positioning during robot-assisted laparoscopic radical prostatectomy (RARP): a systematic review of the literature. Prostate. 2021;81(7):361–367.
- Alibhai SM, Naglie G, Nam R, et al. Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol. 2003;21(17):3318–3327.
- Greco KA, Meeks JJ, Wu S, et al. Robot-assisted radical prostatectomy in men aged > or =70 years. BJU Int. 2009;104(10):1492–1495.
- Togashi K, Hatakeyama S, Okamoto T, et al. Oncologic and patient-reported outcomes after robot-assisted radical prostatectomy in men aged ≥75 years. Urol Oncol. 2021;39(10):729.e17–729.e25.
- Gu X, Araki M, Wong C. Does elevated body mass index (BMI) affect the clinical outcomes of robot-assisted laparoscopic prostatectomy (RALP): a prospective cohort study. Int J Surg. 2014;12(10):1055–1060.
- Fossati N, Willemse PM, Van den Broeck T, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2017;72(1):84–109.
- Scosyrev E, Messing EM, Mohile S, et al. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118(12):3062–3070.
- Zorn KC, Mendiola FP, Rapp DE, et al. Age-stratified outcomes after robotic-assisted laparoscopic radical prostatectomy. J Robot Surg. 2007;1(2):125–132.
- Li J, Li Y, Cao D, et al. Outpatient versus inpatient robot-assisted radical prostatectomy: an evidence-based analysis of comparative outcomes. J Endourol. 2022;36(4):468–476.
- Singh R, Hicks JA. Robot-assisted radical prostatectomy in men aged > or =70 years (comment on Greco KA, Meeks JJ, Wu S, Nadler RB. BJU Int. 2009 Nov;104(10):1492–5.)
- Braun M, Wassmer G, Klotz T, et al. Epidemiology of erectile dysfunction: results of the 'cologne male survey. Int J Impot Res. 2000;12(6):305–311.
- Verdú E, Ceballos D, Vilches JJ, et al. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208.
- Seppan P, Muhammed I, Mohanraj KG, et al. Therapeutic potential of Mucuna pruriens (Linn.) on ageing induced damage in dorsal nerve of the penis and its implication on erectile function: an experimental study using albino rats. Aging Male. 2020;23(5):313–326.
- Brassell SA, Rice KR, Parker PM, et al. Prostate cancer in men 70 years old or older, indolent or aggressive: clinicopathological analysis and outcomes. J Urol. 2011;185(1):132–137.
- Yassin A, AlRumaihi K, Alzubaidi R, et al. Testosterone, testosterone therapy and prostate cancer. Aging Male. 2019;22(4):219–227.
- Efesoy O, Apa D, Tek M, et al. The effect of testosterone treatment on prostate histology and apoptosis in men with late-onset hypogonadism. Aging Male. 2016;19(2):79–84.
- Ferro M, Lucarelli G, Bruzzese D, et al. Low serum total testosterone level as a predictor of upstaging and upgrading in low-risk prostate cancer patients meeting the inclusion criteria for active surveillance. Oncotarget. 2017;8(11):18424–18434.
- Ferro M, Lucarelli G, de Cobelli O, et al. Circulating preoperative testosterone level predicts unfavourable disease at radical prostatectomy in men with international society of urological pathology grade group 1 prostate cancer diagnosed with systematic biopsies. World J Urol. 2021;39(6):1861–1867.