Abstract
Purpose
To describe the effects of consistent levels of testosterone in a pellet form and it’s potential to reverse osteoporosis.
Methods
This is a descriptive case report of a 54 year male with a spontaneous fracture and osteoporosis in the presence of what many consider a normal male testosterone level.
Results
After discovering and documenting osteoporosis by DXA scan, the patient was shown to reverse the diagnosis of osteoporosis in a year on pelleted testosterone therapy. Consistent levels of 943 ng/dL were achieved; the patient also experienced improvements in quality of life and sleep apnea.
Conclusion
Testosterone deficiency (TD) is a clinical syndrome and osteoporosis can be found in levels above standard “criteria” of 300. This patient did not realize a benefit on injections both physical and clinically and both improved on pelleted testosterone. This should be further studied and considered for TD in men.
Introduction
Each year, more than 8 million men are diagnosed with osteoporosis or osteopenia [Citation1]. Men with osteoporosis are treated with lifestyle modifications, drug therapy, and hormonal therapy if they have been diagnosed with testosterone deficiency (TD). However, normal testosterone ranges remain a somewhat controversial area. Integrity of our skeletal system is maintained by a remodeling process, which is regulated by three bone cell types: bone-forming osteoblasts, bone-resorbing osteoclasts, and mechanically sensitive osteocytes. These are under regulation by many processes, which involve testosterone and androgen receptors as well as the effect of estrogen receptors that are known to be influenced by testosterone levels. Osteoporosis risk factors are best documented in women due to the acute estrogen loss at menopause, but they also consistently lose testosterone similar to men. Fracture risk is higher in women, but men suffering from fractures carry a greater mortality risk over women [Citation2,Citation3]. Administrative therapy also plays a key role. Problems in both obtaining and maintaining effective testosterone levels to achieve the desired therapeutic effect are critical in the process and clinical outcome [Citation4].
We describe a case of an osteoporotic patient with what many clinicians would consider a normal testosterone level who was successfully treated with subcutaneous testosterone—(pellet) achieving higher sustained and consistent levels, resulting in almost complete recovery of osteoporosis after 1 year of treatment.
Case
A 54-year-old male triathlete had a non-fall-related tibial plateau fracture on 1 December 2018 while stepping out of his ski boot, after a normal day of snow skiing.
Except for a back injury in a motor vehicle accident, his medical history was non-contributory. Surgical history included two previous wrist fractures sustained while mountain biking and a left meniscus arthroscopy to repair a meniscal tear. He had received intramuscular testosterone supplementation in his early 40’s to improve his overall performance. He was on this for a year and then stopped after consulting with a physician who expressed concern about his elevated blood count.
Following his tibial fracture, the patient saw an orthopedic surgeon and had casting and bracing for his left tibia for 3 months. He then underwent DEXA scan on 3 January 2019 with his results showing osteoporosis in the spine and femoral neck ().
Spine T −2.6, Z-2.1 osteoporosis
Total hip −1.8 Z-1.4 osteopenia
Fem neck T −2.7, Z-1.8 osteoporosis.
Figure 1. (a) Hip MRI report with patient demographics. (b) Radiography of right femoral neck and plotted t score. (c) Date of study and report. (d) T and Z score improved from −1.4 to 1.3. (e) The 6.8% improvement in 1 year data. (f) FRAX risk not calculated because of normal findings.
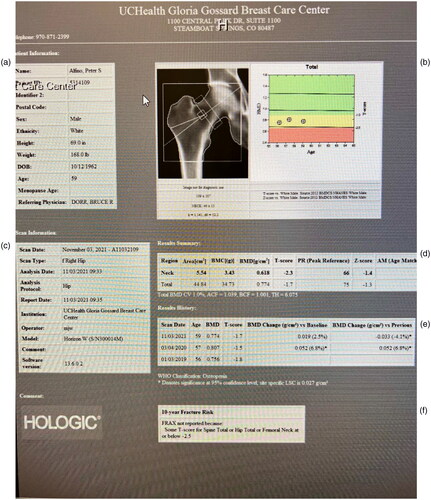
The patient was offered traditional etidronate treatment by his primary care physician, but he declined treatment. Patient sought counseling on 19 March 2019 due to his interest in addressing his osteoporosis with hormonal treatment. His initial Testosterone (T) was 473 ng/dL, vitamin D was 39 ng/mL, Prostate-Specific Antigen; 4 ng/mL, estradiol 14 ng/dL, Hga1c 4.9%, and his BMI was 26.3.
After counseling the patient about the pros and cons of testosterone therapy and possible side effects, we initiated testosterone pellet therapy. He was also started on 10,000 IU/day of a vitamin d3k2 nutraceutical formulation and DIM (diindoyl methane) 300 mg—which is also nutraceutical grade formula known to prevent aromatization. After 3 months, his testosterone level was up to 943 ng/dL, free testosterone 116 ng/dL, Hematocrit (Hct) 51%, and estradiol 15 ng/dL. The patient was checked regularly every 3–4 months by physical exam and lab workup. After 6 months, his repeat vitamin D level was 52 ng/mL and his estradiol was 29 ng/dL.
He continued testosterone therapy, and at the year interval, rather than the standard 2 year interval at the recommendation of his endocrinologist, a repeated DEXA scan was obtained on 3 April 2020 with results of:
Spine T -2.2/-1.6 6% increase—osteopenia
Total hip T-1.5/.8 6.8% increase—normal
Fem neck T-2.4/-1.5 7.2% increase—osteopenia
Figure 2. (a) DXA report and images of LS Spine. (b) Graph of t scores and normalization of parameters. (c) Demographic data of study. (d) Z and t scores of L1-4 and average value of −1.3. (e) 6% BMD improvement in one year.
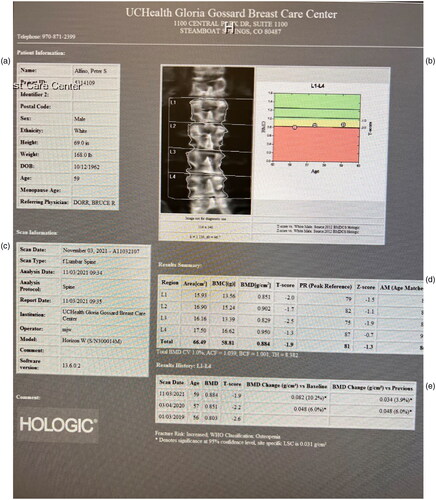
The patient, at 1 year of hormone therapy, improved his composite bone density substantially normalizing his total hip bone density and improving his quality of life and returned to performing triathlons in 1 year. He felt better in many ways and found it easier to maintain his therapy with the pellet administration. Normal assessment in our clinic would have even a 2 year interval for the DXA scan.
Discussion
There is a lack of standardization of TD diagnosis [Citation5]. Generally, TD is diagnosed when patients exhibit symptoms of TD followed by lab confirmation. The Joint international society guidelines propose an upper limit of normal as 350 ng dL, above which treatment is usually not beneficial [Citation6,Citation7]. The 2006 U.S. Endocrine Society guidelines recommended a total serum testosterone level of 300 ng/dL as the diagnostic threshold for treatment [Citation8]. However, there is no clear T threshold that predicts patients who will and not respond to treatment [Citation9]. Rigid interpretation of T ranges should not dictate clinical decision making. This is further noted by the International Society for the Study of the Aging Male that testosterone replacement therapy (TRT) may be reasonably offered to symptomatic patients above 350 ng/d [Citation10]. In an ideal setting, TRT should be based first on symptoms and secondarily on lab values. In our case, his fracture may have been prevented with more appropriate therapy and counseling. In addition, it would have maintained his libido, mood, and lean body mass—all of which were issues prior to his fracture.
Several systematic reviews showed conflicting data regarding the effect of T on bone mineral density (BMD). The Endocrine Society guideline published in 2010 stated that although testosterone had no effect on vertebral, hip, and femoral BMD, it was associated with an increase in lumbar BMD [Citation11]. A recent systematic review showed that the effect of testosterone supplementation on BMD and the risk of falls or fracture remain inconclusive [Citation12]. The authors acknowledged that their systematic review included a very small number of patients with osteoporosis. In contrast, there are many previous studies suggesting the beneficial effect of TRT on osteoporosis/osteopenia, A meta-analysis of 1083 patients from 29 randomized controlled studies showed that TRT could improve BMD at the lumbar spine by +3.7% compared with placebo [Citation13]. A recent narrative review revealed 13 studies showing the beneficial effect of TRT on osteoporosis/osteopenia [Citation14]. In our case, we successfully report the use of what some would consider supra-physiologic levels of T by pelleted bio-identical testosterone under FDA oversight from a 503b certified compounding pharmacy to treat osteoporosis. Review of the literature did not show any published cases looking at higher levels of T in enhancing bone strength except in rats [Citation15].
Adverse effects of elevated testosterone remain controversial. Recent systematic review by Zhang did not show an increase in cardiovascular events, all-cause mortality, or prostatic events in patients with TD treated with T [Citation12]. However, levels of synthetic T may induce vascular dysfunction, which may contribute to cardiac events [Citation16]. Previous trials showed including elevated hematocrit, leg edema, and increased incidence of prostatic events [Citation17]. In our case, the patient did not have any adverse events and a recently published 9-year study demonstrated extremely low rates of complications from subcutaneous testosterone administration TRT [Citation18]. Short term administration of testosterone and compliance can be a limiting factor in achieving desired results [Citation2].
A recognized complication of TRT can be aromatization leading to abnormal elevation of estradiol. His estradiol did not appreciably change (possibly due to the administration of DIM, a natural aromatase inhibitor) hinting that testosterone was the effective agent.
Given the incidence of pre diabetes and type 2 diabetes in our society, this can also negatively impact BMD [Citation4]. Diabetes can lead to osteopathy and decreased blood flow to the bone. In a recent study, even a diagnosis of prediabetes (Hga1c of 5.7–6.4) conferred an increased risk of osteopenia or osteoporosis [Citation19]. We did not observe this effect in our study as his level was normal.
Although this patient achieved both effective and therapeutic total and free testosterone levels, it is essential to achieve free hormone levels to activate receptor driven cellular processes. So, it is imperative to be sure that effective free testosterone levels are maintained.
Also, the administration of vitamin D is a confounding variable. Vitamin D plays a known beneficial role in bone formation and remodeling. It is essential in absorbing calcium form the gut and provides optimal circumstances for bone mineralization. This created a better environment for the hormonal effect of testosterone [Citation20].
Lastly, BMI can play a role in osteoporosis, A subset of the EARTH trial (Effects of androgen replacement therapy on hypogonadal men) showed that men given testosterone enanthate injections had significant increase in BMD [Citation21]. It was the first study to demonstrate a beneficial effect of TRT on BMD in osteoporotic Japanese men. They also showed that low BMI—probably due to the lower mechanical loading, was an independent risk factor for osteoporosis. Our patient had a BMI of 26.2, which, if anything, should have been a deterrent in the development of osteoporosis.
Conclusion
This case report noted pelleted bioidentical testosterone replacement using an FDA regulated 503b compounded pharmacy resulted in significant improvement of bone density. This affect was related to the significant elevation of what many would consider a normal testosterone level. This patient was able to strengthen bone, remove supportive fracture hardware in one year and had increased energy, recovery, and benefits to his sleep apnea without the significant side effects of standard etidronate therapy.
This case report raises two important questions. Is it safe and more efficacious to administer higher than normal testosterone doses in men suffering from issues related to loss of bone density and is the best mode of delivery in such situations as subcutaneous pellet versus transdermal or intramuscular administration? There is a tremendous need for more data that could address these questions, most notably a prospective randomized trial.
Disclosure statement
Dr. Bruce Dorr is an educational consultant for BioTE. Dr. Ahmed Abel Aziz has no disclosures. Dr. Mickey Karram is a research consultant for BIoTE.
Additional information
Funding
References
- Nb W, Ra A, Jp B, et al. Osteoporosis in men: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802–1822.
- de Paiva Gonçalves V, Cabrera-Ortega AA, Carvalho JS, et al. Physiologic testosterone replacement on male aged rats with orchiectomy induced osteoporosis in advanced stage: a tomographic and biomechanics pilot study. Aging Male. 2021;24(1):139–147.
- Mendoza FA, Le Roux M, Ahmed I. Primary osteoporosis in men: an unmet medical need. Fertil Steril. 2019;112(5):791–798.
- Groti Antonič K. Impact of testosterone therapy on bone turnover markers in obese males with type 2 diabetes and functional hypogonadism. Aging Male. 2022;25(1):269–277.
- Morgentaler A, Khera M, Maggi M, et al. Commentary: who is a candidate for testosterone therapy? A synthesis of international expert opinions. J Sex Med. 2014;11:1636–1645.
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009;55:121–130.
- Buvat J, Maggi M, Gooren L, et al. Endocrine aspects of male sexual dysfunction. J Sex Med. 2010;7:1627–1656.
- Paduch DA, Brannigan RE, Fuchs EF, et al. The laboratory diagnosis of testosterone deficiency. Urology 2014;83:980–988.
- Morgentaler A, Zitzmann M, Traish AM, et al. Fundamental concepts regarding testosterone deficiency and treatment: International expert consensus resolutions. Mayo Clin Proc. 2016;91(7):881–896.
- Park H, Ahn S, Moon D, et al. Evolution of guidelines for testosterone replacement therapy. JCM. 2019;8(3):410.
- Seftel AD, Kathrins M, Niederberger C. Critical update of the 2010 endocrine society clinical practice guidelines for male hypogonadism: a systematic analysis. Mayo Clin Proc. 2015;90:1104–1115.
- Zhang Z, Kang D, Li H. The effects of testosterone on bone health in males with testosterone deficiency: a systematic review and meta-analysis. BMC Endocr Disord. 2020;20(1):12.
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism, and serum lip profile in male-age men: meta analysis. Clin Endocrinol (Oxf). 2005;63(3):280–293.
- Shigehara K, Izumi K, Kadono Y, et al. Testoerone and bone health for me: a narrative review. JCM. 2021;10(3):530.
- Yarrow JF, Conover CF, Purandare AV, et al. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab. 2008;295(5):E1213–E1222.
- Alves JV, Costa R D, Pereira CA, et al. Supraphysiological levels of testosterone induce vascular dysfunction via activation of the NLRP3 inflammasome. Front Immunol. 2020;11:1647.
- Rabijewski M, Papierska L, Piątkiewicz P. An association between bone mineral density anabolic hormone in middle age and elderly men with pre diabetes. Aging Male. 2017;20(3):205–213.
- Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688.
- Lips P, van Schoor NM. The effect of vitamin d on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25(4):585–591.
- Shigehara K, Konaka H, Koh E, et al. Effects of testosterone replacement therapy on hypogonadal men with osteopenia or osteoporosis: a subanalysis of a prospective randomized controlled study in Japan (EARTH study). Aging Male. 2017;20(3):139–145.
- Donovitz GS. Low complication rates of testosterone and estradiol implants for androgen and estrogen replacement therapy in over 1 million procedures. Ther Adv Endocrinol. 2021;12:204201882110152.

