Abstract
Background
The absence of even one parent has short- and long-term effects on the child’s current and future health. The purpose of the study was to determine whether there is a long-term relationship between the type of family in which men were raised and an individual’s adult social position, well-being in adulthood and their biological condition regardless of social status in adulthood.
Materials and methods
Data for 4528 males, aged 25–80 years, were selected from the archives of the Lower Silesian Medical Centre in Wrocław, Poland. A total of 329 men declared that they grew up in incomplete families. Height, weight, % fat, cardiovascular and respiratory systems, blood parameters, and health of men who grew up in complete or incomplete families were compared.
Results
Growing up in an incomplete family reduced chances for better education, decreased life satisfaction in adulthood, and negatively affected the final height. After taking into account the education achieved, the effect persisted only for diastolic blood pressure, creatinine, and serum phosphorus levels.
Conclusions
Growing up in an incomplete family has a significant impact on male’s socioeconomic position (SES), life satisfaction, and final height. A poorer quality of diet is proposed as an early life risk factor for adult health.
Introduction
Social variation in health status is a broad field of research. It has been found that people with lower socioeconomic status (SES) are characterized by subpar biological conditions, shorter life expectancy at birth, poorer overall health, and higher mortality rates (both all-cause and cardiovascular mortality) [Citation1]. This phenomenon is observed in adults as well as children, adolescents, and young adults in various countries regardless of the country’s overall SES [Citation2–5].
In recent years, studies have provided evidence indicating that one’s current health status is the outcome of the influence of factors that operate on people throughout their lives, from the fetal stages, onto childhood, adolescence, and finally adulthood. The formation of overall health as one ages is not linear, and the factors acting on health exert an influence of varying strengths, depending on the period and sensitivity of the body’s functions, especially in the so-called critical periods [Citation6–9]. Low social status in childhood, poor living conditions, and abnormal nutritional input are all factors that limit prenatal development and result in an increased risk of developing obesity, depression, cardiovascular disease, and diabetes in adulthood [Citation10–12].
Several models have been proposed to explain the influence of childhood SES on adult health [Citation13–16]. The three basic models are the timing model, the accumulation model, and the change model. The timing model, or latency model, focuses on the identification of sensitive periods when individuals are most vulnerable to exposure to psychosocial and physical or behavioral influences. This is based on the observation that the impact of exposure to certain risks or situations can be greater at a given period than during other periods [Citation17,Citation18], and that the sensitivity of the body and its systems change during development due to differing levels of maturity, plasticity, and adaptation of organisms, including humans. The accumulation model, as opposed to the timing model, suggests that not only certain sensitive periods of life have consequences for adult health, but deleterious effects accumulate throughout life. In addition, not only the duration of socioeconomic disadvantages but also their intensity is important [Citation19]. The change model assumes that the negative effects of low SES in childhood and adolescence are likely to be at least partially remediated if an upward shift in social position occurs [Citation20]. Consistently lowered social position during life can result in poorer overall health status in adulthood.
The growth and development of the child can be influenced by the structure of the family [Citation21], although the results of publications on different populations do not lead to clear conclusions [Citation17,Citation22–24]. Growing up in an incomplete family, regardless of the cause of the familial disruption: the death of one or both parents, divorce, separation, or birth by a single mother without a partner, can have a deteriorating effect on a child’s condition and health, and in extreme cases, can reduce the child’s chances of survival, especially at an earlier age [Citation25]. Of particular note are children in institutional care who, among other things, are diagnosed with growth abnormalities, resulting in lower final height compared to peers living in families with at least one present parent [Citation26]. Moreover, lower total brain volume in a dose-dependent way and alterations in adult brain structure in children with severe social deprivation in the first years of life are observed [Citation27]. American studies point to childhood social problems (broken families and conflicts) as the strongest factors that increase the risk of health disorders and mortality [Citation28]. On the other hand, the results of Finnish studies suggest that socioeconomic conditions in childhood are not the principal determinants of health during adulthood [Citation29]. In turn, research by Lipowicz et al. [Citation23] indicates the importance of subjective, self-perceived social status in childhood, relative to peers, rather than real social status, for health status in adulthood.
Single parenthood is currently a fairly common situation in Poland. Most often, it is a forced situation and not a parent’s choice (abandonment by the partner, divorce). Usually, a single child is raised by the mother, although legally, the same rights are given to the child’s father or legal guardian. The results of six national censuses, conducted in 1970, 1988, 1995, 2002, 2011, and 2021, show that the prevalence of single-parent families has gradually increased in recent decades. In 1970, the percentage of single parents with children was 12.7%. In 1988, single mothers made up 13.6% of all families, whereas single fathers made up 1.74% of all families. In 1995, single parents of both sexes already accounted for 16.8%, which includes 15% of single mothers and 1.8% of single fathers [Citation30]. In 2002, single mothers made up 17.2% of families, and single fathers made up 2.2% of families. In less than 10 years, by 2011, these frequencies had risen to 19.8% and 3%, respectively [Citation31]. The most recent data from 2021 showed that single parents accounted for 22.6%, of which 18.8% were single mothers, and 3.8% were single fathers [Citation32]. This means that at the beginning of the twenty-first century, the number of single parents reached 2.5 million in Poland [Citation30]. Poland is not the only country where an increase in the frequency of families with only one adult member has been observed in recent years. The highest proportions of single parents among households with children in the EU were found in Estonia, Denmark, Lithuania, and Latvia – all with more than 20%. Slovakia, Croatia, Greece, and Slovenia had the lowest proportions of single parents, all of which recorded percentages less than 5%. In the whole European Union, single parents accounted for 12.6% of households with children [Citation33].
In studies of the relationship between early life stages and health status in adulthood, a commonly used determinant of childhood status is parental education level [Citation11]. Although the impact of various other mechanisms related to school absenteeism, childhood adversity, and physical, emotional, and sexual abuse, and not necessarily linked to parental education level and income, have also been analyzed [Citation34,Citation35]. Growing up in an incomplete family is also often a marker of lower status, lower household income, and fewer educational opportunities [Citation36]. Therefore, the purpose of this study was to determine whether there was a long-term relationship between the type of family in which adult males were raised and their biological condition as determined by height, weight, and parameters of physiological systems, regardless of social status in adulthood. In addition, the association of growing up in incomplete families with social status, well-being, and biological condition of the individual in adulthood was estimated.
Materials and methods
The retrospective data analyzed were sourced from the paper archives of the Lower Silesian Medical Centre (DOLMED) in Wrocław, Poland. The database collected is designed as a non-interventional study (survey study). In DOLMED, routine periodic examinations of employees of various local institutions of the state, municipal, and private sectors, requested and funded by employers, as part of mandatory regular examinations were performed in the 1980–90s. All participants agreed to the survey and were familiar with the measurement procedures used. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The data are fully anonymized and do not include medical diagnosis, description of medical procedures undertaken, or other sensitive information about individual participants.
For this study, data for 4528 males aged 25–80 years with complete sets of information were selected. All subjects showed no signs of overt disease. Men up to 60 years of age were considered occupationally active, and men over 60 years old were considered retired. The subjects were divided into four groups based on education: tertiary (16 years of schooling), secondary (12 years of schooling), basic vocational (10–11 years of schooling), and primary (7–8 years of schooling). During this time in Poland, basic vocational education persisted for two or three years beyond primary school and instructed in practical trades such as mechanic, electrician, or railway man. Two groups pertaining to marital status were created: currently married and unmarried, the latter of which includes single, separated, divorced, and widowed. The categories for the individuals’ place of residence were cities (with a population of more than 100,000 inhabitants), towns (no more than 100,000 inhabitants), and villages (settlements placed in rural areas without city rights). Lifestyle markers included information on cigarette smoking (never/quit/smoke), alcohol drinking (never/occasionally, 1–3 times a month/often, 1–3 times per week), and physical activity levels (passive/non-regular, 1–3 h per week/regular, ≥4 h per week). Men rated their satisfaction with their lives as satisfied or unsatisfied. The self-assessment of health had four categories (good/good, although not recently/chronic disease/bad).
In the questionnaires, individuals gave information about their childhood family life. Subjects answered questions concerning both parents: Is your father/mother: (a) alive, and he/she is healthy; (b) alive, and he/she is ill; (c) he/she is dead; (d) I do not know anything about my father/mother. When the individual knew the condition of both parents (healthy, ill, dead) was classified as “raised in a complete family” (n = 4199). Choosing the option: “I don’t know anything about my father/mother” was interpreted that the respondent was unaware whether his father/mother is alive or dead. Participants with these responses were classified as “growing up in a single-parent family,” when he did not know anything only about one parent – fatherless (n = 215) or motherless (n = 25), or classified as “parentless,” when he did not know anything about either parent and was most likely brought up in an orphanage (n = 89). Next, these two groups were combined into one, designated as having “grown up in incomplete families” (n = 329).
To represent a wide range of physiological functions, 21 biological and physiological parameters were selected:
Body build: height (cm), body mass index (BMI; kg/m2), and % body fat [Citation37];
Respiratory characteristics: vital capacity (VC) and forced expiratory volume in one second (FEV1), measured using a Collins apparatus;
Cardiovascular characteristics: heart rate, systolic and diastolic blood pressures measured twice in the sitting position, on the left arm at heart level, after at least a 5-min rest period. The two readings were averaged;
Sensory characteristics: visual acuity was evaluated for both eyes without glasses using the Snellen optotype reading chart;
Hematologic characteristics: red blood cell (RBC) count, white blood cell (WBC) count in 1 mm3, haemoglobin (HB) g/dl, and erythrocyte sedimentation rate (ESR) mm/h;
Biochemical characteristics: glucose (mg/dl), total cholesterol (mg/dl), calcium (mg/dl), phosphorus (mg/dl), total protein (g/dl), bilirubin (g/dl), creatinine (mg/dl), and alkaline phosphatase (ALPase; mg/dl).
Blood was obtained in the morning following laboratory procedures. Participants were requested to be fasting.
All biological variables were standardized for age by applying a simple linear regression and presented in standard deviation units. Then, the absolute values of differences in biological characteristics between the studied groups of men were calculated, and results were presented graphically as profiles. If mean values for men raised in single-parent families were lower, a minus sign was inserted. If mean values were higher for men from complete families, a plus sign was added. Next, the obtained differences were presented in figures according to ascending values. The significance of differences by type of distribution was assessed using a Mann–Whitney U test or Fisher test. To determine whether there was a statistically significant difference between the expected and the observed frequencies a chi-square test was employed. Finally, to estimate the independent effect of childhood family life and current SES, for variables showing significant variation by family type, 2-way analyses of variance were performed, where the independent variables were family type and current educational level of men. A p value <0.05 was considered statistically significant.
Results
contains the distribution of socioeconomic characteristics, lifestyle behaviors, and self-assessment of health status and life for all men in two separate groups distinguished based on their family type while growing up. The men raised in incomplete families were significantly less educated as compared with men from complete families; more than 40% of men without at least one parent during childhood finished primary school, and about 8% achieved a university education, whereas those values were observed to be 23.6% and 14.6%, respectively for men raised with both parents. As opposed to education, adult men from both types of families did not differ significantly in marital status or place of residence. Concerning lifestyle elements, respondents from the two types of family environments differed only in their drinking habits; adult men raised in incomplete families were significantly more likely not to drink alcohol than adult men raised in complete families. Additionally, adult men raised in incomplete families were more likely, although not statistically significant, to rate their lives as unsatisfactory.
Table 1. The distribution of baseline characteristics, lifestyle behaviors, and health and life self-assessment for men aged 25–80 years, growing up in two types of families.
presents raw data of biological and physiological variables for the two groups of men. Men raised in incomplete families were significantly older and shorter than men from complete families (171.2 cm v. 172.0 cm), had significantly lower lung capacity as expressed by VC and FEV1, lower RBC count, higher ESR, significantly higher serum phosphorus, lower total protein, lower bilirubin, and higher ALPase levels (). Since most of the biological and physiological variables show significant correlations with age, further analyses were performed after standardizing the values of the variables to the age of the individuals.
Table 2. Raw data of biological and physiological variables for men raised in complete and incomplete families.
present the differences in biological characteristics between the studied groups of men. When men from complete and incomplete families were compared, out of 21 variables, the differences were significant in only four cases. Men from incomplete families (single-parent and parentless families) were significantly leaner and had lower levels of bilirubin, higher levels of ALPase, and significantly higher levels of phosphorus (). When men raised in complete families were compared with men from single-parent families, the latter were significantly leaner and had significantly higher levels of ALPase and phosphorus in the blood (). Men raised without both parents were significantly shorter and possessed lower blood creatinine levels, as compared with men from complete families (). Finally, a comparison of two groups of incomplete families was carried out ().
Figure 1. The differences between the standardized mean values of the analyzed health parameters of men raised in complete and incomplete families.
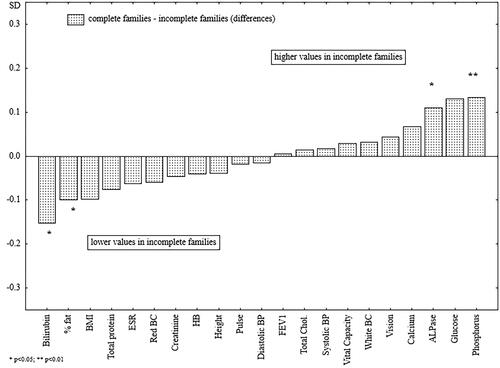
Figure 2. The differences between the standardized mean values of the analyzed health parameters of men raised in complete and single-parent families.
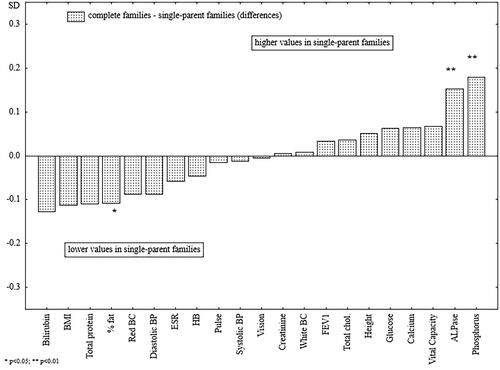
Figure 3. The differences between the standardized mean values of the analyzed health parameters of men raised in complete families and parentless men.
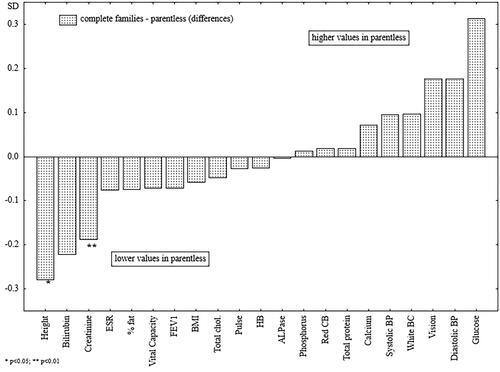
Figure 4. The differences between the standardized mean values of the analyzed health parameters of men raised in single-parent families and parentless men.
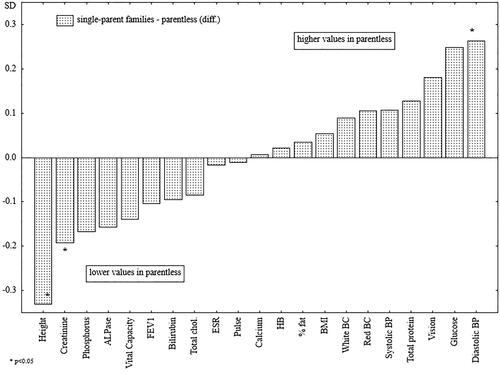
The men raised without both parents were significantly shorter (), had lower creatinine levels, and had significantly higher diastolic blood pressure as compared with the men raised in single-parent families. In the case of diastolic blood pressure, creatinine levels, and serum phosphorus, family type appeared to be a factor acting independently of the men’s educational levels at the time of the study ().
Figure 5. Standardized values of height of men raised in different types of families.
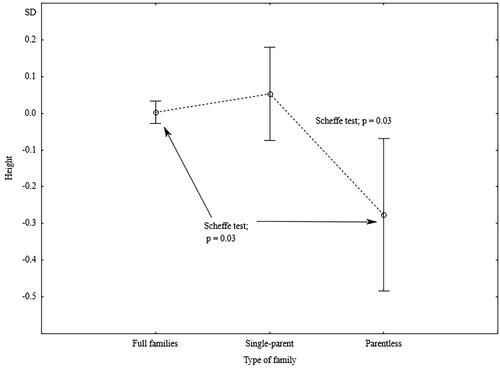
Table 3. The net effects of family type as a determinant of childhood SES status and current SES of adult men – results of two-way analyses of variance.
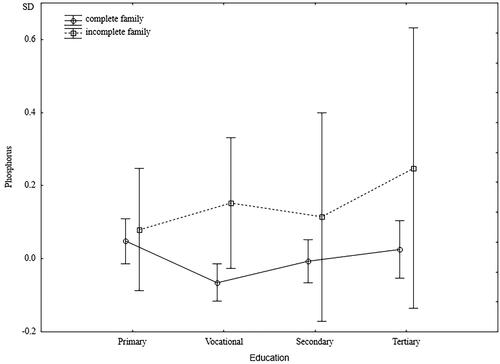
Regardless of their education level, men from incomplete families had significantly lower diastolic blood pressure. However, a significant interaction indicated that while the lowest diastolic blood pressure was characterized by men with higher education and raised in incomplete families, men from incomplete families and with low levels of education had the highest diastolic blood pressure (). The independent effect of family type on creatinine levels was close to borderline significance (p = 0.06), and a significant interaction indicated higher creatinine levels in better-educated men from incomplete families and lower creatinine levels in men with lower education who were raised in incomplete families (). Serum phosphorus levels did not depend on the men’s education levels. In each group’s education level, men raised in incomplete families had significantly higher phosphorus levels ().
Figure 6. Standardized values of diastolic blood pressure of men raised in different types of families by current level of education.
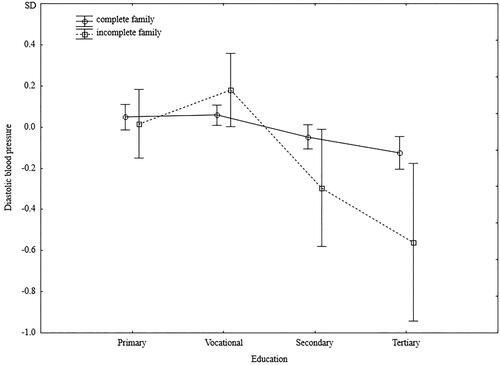
Figure 7. Standardized values of creatinine of men raised in different types of families by current level of education.
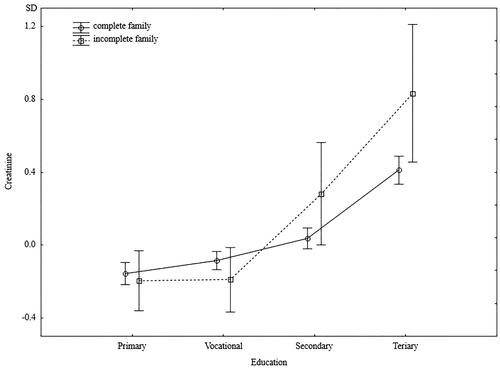
Figure 8. Standardized values of phosphorus of men raised in different types of families by current level of education.
Discussion
This study on the impact of family type on an individual’s education level, well-being, and biological condition of adult males in Poland demonstrates that growing up in an incomplete family reduced chances for better education, made upward social mobility more difficult, decreased life satisfaction in adulthood, and negatively affected final height. In turn, generally, the study does not confirm a clear, significant effect on the function of most physiological systems, except for body fat percentage, diastolic blood pressure, as well as levels of bilirubin, ALPase, creatinine, and phosphorus. In sum, the influence of family of origin on biological condition in adulthood, after taking into account the level of education achieved, lost significance. The importance of the lack of at least one parent persisted only for diastolic blood pressure, creatinine, and serum phosphorus levels.
A review of the literature indicated that there is stronger evidence of a causal effect of the absence of a father on educational attainment, particularly for high school graduation [Citation38]. A family disruption, for example, families with an absent father had mixed effects with test scores, including tests of verbal, math, and general ability. In addition, the magnitude of the relationships analyzed depended on the type of analysis performed and the data design. Most research on the importance of childhood conditions for later years is focused on early life stages (childhood, adolescence, and early adulthood [Citation39]) and since changes in social position can also occur in later years, it would be important to assess the relationship with SES in later adulthood as well. The studied adult Poles, born between 1904 and 1968, who grew up in incomplete families were significantly more likely to have completed their education at the elementary or vocational school level. Similarly, Finnish children born out of wedlock between 1933 and 1943, when being born in marriage was a norm, attained a lower SES in adulthood [Citation36]. During the years when the absence of one or both parents was stigmatized, the functioning of children from single-parent families in an classroom environment might have led to the presence of increased stress levels, the risk of school bullying and violence, more frequent school absenteeism [Citation40], and the decision to leave education and enter the workforce sooner [Citation35]. Thus, the quality of education and school environment can be, in addition to poor material resources, mediating elements in the mechanisms that explain the link between childhood and adulthood SES, and, consequently, with adult health [Citation36].
Numerous studies by psychologists, pedagogists, and epidemiologists present the link between a parent’s absence from a child’s or adolescent’s life (for various reasons) and youths’ emotional and mental health [Citation38,Citation41,Citation42]. However, they emphasize that such an effect also depends on the quality of the family’s relationships, and point out that the absence of toxic relationships is sometimes better for a child’s mental and emotional health than the presence of a disruptive parent in the family [Citation39,Citation41]. This study showed that the effect of a single-parent family was long-lasting and reduced life satisfaction in adult men. The disappearance or absence of a parent is a significant stressor for a child and can cause anxiety symptoms later on in adulthood [Citation43], depression [Citation44], and various mental health disorders [Citation45]. Underlying the relationship between exposure to stress early in childhood and adult health condition lies in the dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis’ function [Citation46,Citation47]. In addition, a significant indirect pathway, through which greater adversity during childhood was linked to a flatter cortisol slope via self-esteem, was observed [Citation48]. It is exactly the self-esteem that has attributed measurable influence on the activity of the HPA axis across an individual’s life span [Citation48], and the emergence of stress-related disorders. The observed relationship may be partly explained by poorer material status in adulthood and reduced educational attainment of children from single-parent families [Citation49,Citation50], which simultaneously determines the level of psychological distress and may be a consequence of living under higher stress in childhood [Citation50].
Adult men who were raised without both parents, significantly, were the shortest, while men raised with one parent did not differ significantly in height from men raised in complete families. Height is a well-established determinant of nutritional status and living conditions during childhood and is also a measure of social differences during growth [Citation51–53], as such, the lower final height of men raised without both parents confirms their inadequate nutritional status, possible poorer health, and deprived socioeconomic circumstances during childhood. Life history theory links low adult male body height to early childhood familial disruption. Sheppard et al. [Citation54] analyzing U.S. data, showed that this relationship is partially mediated by later puberty of boys. Perceived psychological stress raises level of cortisol and inhibits testosterone production, delaying pubertal onset [Citation55] and childhood disruption may constrain the male’s ability to catch up in height [Citation54].
Examples of extreme social deprivation resulting in low body height (psychosocial dwarfism) are presented in the paediatric endocrinology literature [Citation26], where developmental and medical problems documented in institutionalized children are described [Citation56,Citation57]. Among these children, growth, and development may be limited despite sufficient nutritional intake, and inhibitory factors are indicated by endocrine disorders (e.g. impairment of the growth hormone insulin-like growth factor axis or increased levels of stress hormones [Citation26]).
Among the 21 parameters used to assess the health and function of various physiological systems of adult men, the men’s family of origin had a significant independent effect in only three cases: diastolic blood pressure, creatinine level, and blood phosphorus. Cardiovascular function expressed by diastolic blood pressure was the worst in men in whom the effect of family of origin and one’s own achieved education was superimposed: the co-occurrence of growing up in a single-parent family and the maximum education level being at most vocational. Thus, it can be assumed that these men had an increased risk of developing cardiovascular disease and premature death [Citation58,Citation59]. Men raised in incomplete families, regardless of current SES, also had significantly lower creatinine levels. Lower creatinine levels can be linked to malnutrition [Citation60], meatless diets, low dietary protein intake [Citation61], and low muscle mass [Citation62]. Also, an association between decreased creatinine clearance (eGFR) and increasing lean mass index was described in men and women [Citation63]. Variation in creatinine level by education level was also shown in a study in rural Malawi, where those without any formal education had the lowest creatinine levels compared to those with primary and secondary schooling, which could be linked to poverty and presumably lower meat consumption [Citation64]. A less healthy diet and poorer quality of food consumed by men raised in single-parent families may also be indicated by an elevated serum phosphorus level. Elevated phosphorus levels may be a consequence of the consumption of inexpensive, highly processed foods. What leads to high phosphorous levels is the excessive intake of highly absorbable forms of dietary phosphorus, used, among other things, as preservatives. High amounts of preservatives and additive phosphate are included in soft drinks, frozen meals, cereals, snack bars, processed cheeses, and refrigerated bakery products, among others [Citation65–67]. Gutiérrez et al. [Citation68] analyzed the association of SES with serum phosphate concentrations among NHANES III participants and showed that increasing poverty was independently linked with increased serum phosphate and a higher likelihood of hyperphosphatemia. Additionally, participants from less affluent strata more frequently consumed meat products (particularly highly processed items such as hot dogs, bacon, and sausage), eggs, cereals, beans/peanuts, and non-diet colas, and less frequently consumed dairy products, seafood, fruits, and vegetables [Citation68]. Of particular interest as a source of toxic phosphorus, is the consumption of soft drinks or jams with food-grade phosphoric acid (additive E338) [Citation67], which is used to acidify beverages and foods and is also a significant risk factor for the development of hypocalcaemia [Citation69,Citation70].
One of the mechanisms that may be involved in the mediation of the relationship between living conditions in single-parent families and nutrition (beginning in the prenatal period of the child of a single mother) and mental and physical health may be the change in the activity of genes responsible for the development of diseases (expression or suppression) underlying epigenetics and nutrigenomics [Citation71]. For example, inadequate nutritional intake of a pregnant single mother can reflect on the fetus leading to an increased risk of cardiovascular and metabolic diseases as well as mental disorders in adulthood [Citation72]. Also, changes in the epigenome in the postnatal period can affect the tempo of aging processes and disease progression [Citation73]. According to principles of epigenetics, living in a single-parent family, probably full of prolonged stressful situations, through epigenetic changes can lead to dysregulation of the HPA axis, which can manifest as hyperreactivity to stress and increased susceptibility to affective disorders, changes in circadian rhythms, neurotransmitter imbalances in the brain and impaired immune function [Citation74].
Potential limitations of this study should be noted. The first of which concerns the criterion for determining the type of family of origin. It was assumed that the lack of any knowledge of the biological parent indicates that the man grew up in an incomplete family. Unfortunately, there was no information on whether, in the absence of a biological parent, a step-parent was involved in the upbringing, and whether the family was truly incomplete. Second, there was lack of information on the quality of family relationships, neither in childhood (family of origin) nor current family. The quality of relationships in the family is a very important element affecting a child’s growth and development. Toxic relationships might touch a portion of the men surveyed who were raised in full families, which could also have influenced their health. It is possible that if the quality of family relationships were taken into account, the observed differences could be even greater. Next, the level of education, the parental income of the individuals during childhood, or even the number of siblings of the individual, which could have a greater impact on biological condition than family type, were unknown. Also, one of the limitations is the lack of information on fat distribution. Increased visceral tissue is, among other things, a consequence of elevated levels of stress hormones, as well as a modifier of biological health parameters [Citation75]. However, the analyzed measures of total body fatness (BMI, %fat) did not show statistically significant variation, confirming that they are not good measures of visceral fatness. Finally, the study included men considered to be healthy, with no signs of overt disease. Therefore, the presented ranges of physiological parameters do not refer to diagnosed diseases or pathological conditions. Rather, it is possible to speak of health trends and an increased risk of health disorders.
In sum, growing up in an incomplete family reduces chances for better education, makes upward social mobility more difficult, decreases life satisfaction in adulthood, and negatively affects final height. However, no clear significant independent effects of incomplete family of origin during childhood were observed on the function of most physiological systems in adulthood, except those connected with poorer nutritional status (lower height and body fat percentage) and a poorer quality of diet. The consumption of an excess of inexpensive, highly processed foods, meatless diets, low dietary protein intake, and low consumption of dairy products, fruits, and vegetables can be indicated as an early life risk factor for health in adulthood, in addition to other recognized risk factors, such as the probably more severe working conditions of less-educated men from single-parent families, a possibly lower health risk factor knowledge, or the long-term effect of experienced childhood stress as a result of a dysregulated HPA axis.
The problem presented is not losing its relevance, even though it concerns men surveyed many years ago. The number of one-headed families is rising from decade to decade. So although acceptance of the phenomenon is increasing, the impact on health, through stress and frequent economic constraints, will continue.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Willcox S, Sweeny K. Chronic diseases in Australia: blueprint for preventive action the influence of childhood circumstances on adult health. Commissioned report No. 01/2014. Australian Health Policy Collaboration; 2015. Available from: https://www.vu.edu.au/sites/default/files/AHPC/pdfs/Influence-of-childhood-circumstances-on-adult-health.pdf.
- Krzyzanowska M, Boryslawki K. Number of siblings and children of short and long living individuals. Int J Anthropol. 2002;17(3–4):173–180. doi: 10.1007/BF02446213.
- Dow WH, Rehkopf DH. Socioeconomic gradients in health in international and historical context. Ann NY Acad Sci. 2010;1186:24–36. doi: 10.1111/j.1749-6632.2009.05384.x.
- Łopuszańska-Dawid M, Szklarska A, Kołodziej H, et al. The relationship between: occupational status, biological condition and androgen hormone level among Polish adult men: the Wroclaw Male Study. Aging Male. 2016;19(4):231–238. doi: 10.1080/13685538.2016.1220519.
- Suder A. Body fatness and its social and lifestyle determinants in young working males from Cracow, Poland. J Biosoc Sci. 2009;41(1):139–154. doi: 10.1017/S0021932008002873.
- Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955.
- Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002;35:159–184. doi: 10.1002/ajpa.10183.
- Kim P, Evans GW, Chen E, et al. How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan. In: Halfon N, Forrest CB, Lerner RM, editors. Handbook of life course health development. Cham, Switzerland: Springer International Publishing; 2017, p. 463–497. doi: 10.1007/978-3-319-47143-3_19.
- Colombo J, Gustafson KM, Carlson SE. Critical and sensitive periods in development and nutrition. Ann Nutr Metab. 2019;75(1):34–42. doi: 10.1159/000508053.
- Barker DJP, Osmond C, Kajantie E, et al. Growth and chronic disease: findings in the Helsinki birth cohort. Ann Hum Biol. 2009;36(5):445–458. doi: 10.1080/03014460902980295.
- Suglia SF, Saelee R, Guzmán IA, et al. Child socioeconomic status, childhood adversity and adult socioeconomic status in a nationally representative sample of young adults. SSM Popul Health. 2022;18:101094. doi: 10.1016/j.ssmph.2022.101094.
- Pollitt RA, Kaufman JS, Rose KM, et al. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health. 2008;62(6):484–491. doi: 10.1136/jech.2006.054106.
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7.
- Montez JK, Hayward MD. Cumulative childhood adversity, educational attainment, and active life expectancy among US adults. Demography. 2014;51(2):413–435. doi: 10.1007/s13524-013-0261-x.
- Suder A. Socioeconomic and lifestyle determinants of body fat distribution in young working males from Cracow, Poland. Am J Hum Biol. 2008;20(1):100–109. doi: 10.1002/ajhb.20687.
- Pavalko EK, Caputo J. Social inequality and health across the life course. Am Behav Sci. 2013;57(8):1040–1056. doi: 10.1177/0002764213487344.
- Hulanicka B, Lipowicz A, Kozieł S, et al. Relationship between early puberty and the risk of hypertension/overweight at age 50: evidence for a modified barker hypothesis among polish youth. Econ Hum Biol. 2007;5(1):48–60. doi: 10.1016/j.ehb.2006.12.001.
- Halfon N, Larson K, Lu M, et al. Lifecourse health development: past, present and future. Matern Child Health J. 2014;18(2):344–365. doi: 10.1007/s10995-013-1346-2.
- Cohen S, Janicki-Deverts D, Chen E, et al. Childhood socioeconomic status and adult health. Ann NY Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x.
- Jankowska EA, Szklarska A, Lipowicz A, et al. Inter-generation social mobility modifies framingham risk score in polish middle-aged men, but not in women. J Biosoc Sci. 2008;40(3):401–412. doi: 10.1017/S0021932007002635.
- Hannan C, Halpin B, Coleman C. Growing up in a one-parent family : the influence of family structure on child outcomes. A report for the family support agency. Department of Sociology, University of Limerick; 2014. Available from: https://core.ac.uk/download/pdf/59352478.pdf.
- Poulton R, Caspi A, Milne BJ, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3.
- Lipowicz A, Kozieł S, Hulanicka B, et al. Socioeconomic status during childhood and health status in adulthood: the Wrocław growth study. J Biosoc Sci. 2007;39(4):481–491. doi: 10.1017/S0021932006001799.
- Karlamangla AS, Singer BH, Williams DR, et al. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: the CARDIA study (USA). Soc Sci Med. 2005;60(5):999–1015. doi: 10.1016/j.socscimed.2004.06.056.
- Li J, Vestergaard M, Cnattingius S, et al. Mortality after parental death in childhood: a nationwide cohort study from three Nordic countries. PLOS Med. 2014;11(7):e1001679. doi: 10.1371/journal.pmed.1001679.
- Johnson DE, Gunnar MR. Children without permanent parents: research, practice, and policy: IV. Growth failure in institutionalized children. Monogr Soc Res Child Dev. 2011;76(4):92–126. doi: 10.1111/j.1540-5834.2011.00629.x.
- Mackes NK, Golm D, Sarkar S, et al. Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc Natl Acad Sci USA. 2020;117(1):641–649. doi: 10.1073/pnas.1911264116.
- Bryan RH. Getting to why: adverse childhood experiences’ impact on adult health. J Nurse Pract. 2019;15(2):153–157.e1. doi: 10.1016/j.nurpra.2018.09.012.
- Lynch JW, Kaplan GA, Cohen RD, et al. Childhood and adult socioeconomic status as predictors of mortality in Finland. Lancet. 1994;343(8896):524–527. doi: 10.1016/s0140-6736(94)91468-0.
- Statistical Yearbook of the Republic of Poland. 2001 [cited 2023 Mar 20]. Available from: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-the-republic-of-poland-2001,2,13.html?contrast=default.
- Statistical Yearbook of the Republic of Poland. 2020 [cited 2023 Mar 20]. Available from: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-the-republic-of-poland-2020,2,22.html.
- Statistics Poland. Families – preliminary results of the 2021 census; 2023 [cited 2023 Mar 20]. Available from: https://stat.gov.pl/spisy-powszechne/nsp-2021/nsp-2021-wyniki-wstepne/rodziny-wyniki-wstepne-nsp-2021,9,1.html.
- Eurostat. Household composition statistics. Eurostat, Stat Explain; 2021, p. 1–9 [cited 2023 Mar 20]. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Household_composition_statistics#Increasing_number_of_households_consisting_of_adults_living_alone%0Ahttp://ec.europa.eu/eurostat/statistics-explained/index.php/Household_composition_stati.
- Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411.
- Kearney CA. School absenteeism and school refusal behavior in youth: a contemporary review. Clin Psychol Rev. 2008;28(3):451–471. doi: 10.1016/j.cpr.2007.07.012.
- Mikkonen HM, Salonen MK, Häkkinen A, et al. The lifelong socioeconomic disadvantage of single-mother background – The Helsinki Birth Cohort Study 1934–1944. BMC Public Health. 2016;16(1):817. doi: 10.1186/s12889-016-3485-z.
- Crook GH, Bennett CA, Norwood WD, et al. Evaluation of skin-fold measurements and weight chart to measure body fat. J Am Med Assoc. 1966;198(1):157–162. doi: 10.1001/jama.198.1.157.
- McLanahan S, Tach L, Schneider D. The causal effects of father absence. Annu Rev Sociol. 2013;39:399–427. doi: 10.1146/annurev-soc-071312-145704.
- Leturcq M, Panico L. The long-term effects of parental separation on childhood multidimensional deprivation: a lifecourse approach. Soc Indic Res. 2019;144(2):921–954. doi: 10.1007/s11205-018-02060-1.
- Dronkers J, Veerman GJM, Pong SL. Mechanisms behind the negative influence of single parenthood on school performance: lower teaching and learning conditions? J Divorce Remarriage. 2017;58(7):471–486. doi: 10.1080/10502556.2017.1343558.
- East L, Jackson D, O’Brien L. Father absence and adolescent development: a review of the literature. J Child Health Care. 2006;10(4):283–295. doi: 10.1177/1367493506067869.
- Rosenberg J, Wilcox WB. The importance of fathers in the healthy development of children. Child abuse and neglect user manual series. US Department of Health and Human Services; 2006. Available from: https://www.childwelfare.gov/pubpdfs/fatherhood.pdf.
- Lähdepuro A, Savolainen K, Lahti-Pulkkinen M, et al. The impact of early life stress on anxiety symptoms in late adulthood. Sci Rep. 2019;9(1):4395. doi: 10.1038/s41598-019-40698-0.
- Pesonen AK, Räikkönen K, Heinonen K, et al. Depressive symptoms in adults separated from their parents as children: a natural experiment during World War II. Am J Epidemiol. 2007;166(10):1126–1133. doi: 10.1093/aje/kwm254.
- Räikkönen K, Lahti M, Heinonen K, et al. Risk of severe mental disorders in adults separated temporarily from their parents in childhood: the Helsinki Birth Cohort Study. J Psychiatr Res. 2011;45(3):332–338. doi: 10.1016/j.jpsychires.2010.07.003.
- Adam EK, Quinn ME, Tavernier R, et al. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018.
- Young ES, Farrell AK, Carlson EA, et al. The dual impact of early and concurrent life stress on adults’ diurnal cortisol patterns: a prospective study. Psychol Sci. 2019;30(5):739–747. doi: 10.1177/0956797619833664.
- Zilioli S, Slatcher RB, Chi P, et al. Corrigendum to: childhood adversity, self-esteem, and diurnal cortisol profiles across the life span. Psychol Sci. 2018;29:161–165.
- Ely M, Richards M, Wadsworth M, et al. Secular changes in the association of parental divorce and children’s educational attainment – evidence from three British birth cohorts. J Soc Pol. 1999;28(3):437–455. doi: 10.1017/S0047279499005693.
- Lacey RE, Bartley M, Pikhart H, et al. Parental separation and adult psychological distress: material and relational pathways. BMC Public Health. 2014;14(1):272. doi: 10.1186/1471-2458-14-272.
- Wronka I, Pawlinska-Chmara R. Childhood environment and adult height among Polish university students. Coll Antropol. 2009;33:1039–1045.
- Krzyzanowska M, Umławska W. The relationship of Polish students’ height, weight and BMI with some socioeconomic variables. J Biosoc Sci. 2010;42(5):643–652. doi: 10.1017/S0021932010000180.
- Lopuszanska-Dawid M, Kołodziej H, Lipowicz A, et al. Social class-specific secular trends in height among 19-year old Polish men: 6th national surveys from 1965 till 2010. Econ Hum Biol. 2020;37:100832. doi: 10.1016/j.ehb.2019.100832.
- Sheppard P, Garcia JR, Sear R. Childhood family disruption and adult height: is there a mediating role of puberty? Evol Med Public Health. 2015;2015(1):332–342. doi: 10.1093/emph/eov028.
- Bribiescas RG. Reproductive ecology and life history of the human male. Am J Phys Anthropol. 2001;33:148–176. doi: 10.1002/ajpa.10025.abs.
- Routray S, Kumar Meher B, Tripathy R, et al. Growth and development among children living in orphanages of Odisha, an Eastern Indian state. IOSR J Dent Med Sci Ver I. 2015;14:2279–2861.
- Valge M, Meitern R, Hõrak P. Anthropometrics of Estonian children in relation to family disruption. Evol Med Public Health. 2021;9(1):276–286. doi: 10.1093/emph/eoab022.
- Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch Intern Med. 1993;153(5):598–615. doi: 10.1001/archinte.153.5.598.
- Boutitie F, Gueyffier F, Pocock S, et al. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med. 2002;136(6):438–448. doi: 10.7326/0003-4819-136-6-200203190-00007.
- Hari P, Bagga A, Mahajan P, et al. Effect of malnutrition on serum creatinine and cystatin C levels. Pediatr Nephrol. 2007;22(10):1757–1761. doi: 10.1007/s00467-007-0535-x.
- Bartholomae E, Knurick J, Johnston CS. Serum creatinine as an indicator of lean body mass in vegetarians and omnivores. Front Nutr. 2022;9:996541. doi: 10.3389/fnut.2022.996541.
- Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016;8(5):E305–E311. doi: 10.21037/jtd.2016.03.62.
- Al-Qaoud TM, Nitsch D, Wells J, et al. Socioeconomic status and reduced kidney function in the Whitehall II study: role of obesity and metabolic syndrome. Am J Kidney Dis. 2011;58(3):389–397. doi: 10.1053/j.ajkd.2011.04.017.
- Kohler IV, Soldo BJ, Anglewicz P, et al. Association of blood lipids, creatinine, albumin, and CRP with socioeconomic status in Malawi. Popul Health Metr. 2013;11(1):4. doi: 10.1186/1478-7954-11-4.
- Calvo MS. Dietary considerations to prevent loss of bone and renal function. Nutrition. 2000;16(7–8):564–566. doi: 10.1016/s0899-9007(00)00313-0.
- Bell RR, Draper HH, Tzeng DYM, et al. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107(1):42–50. doi: 10.1093/jn/107.1.42.
- Erem S, Razzaque MS. Dietary phosphate toxicity: an emerging global health concern. Histochem Cell Biol. 2018;150(6):711–719. doi: 10.1007/s00418-018-1711-8.
- Gutiérrez OM, Isakova T, Enfield G, et al. Impact of poverty on serum phosphate concentrations in the third national health and nutrition examination survey. J Ren Nutr. 2011;21(2):140–148. doi: 10.1053/j.jrn.2010.03.001.
- Mazariegos-Ramos E, Guerrero-Romero F, Rodríguez-Morán M, et al. Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in children: a case-control study. J Pediatr. 1995;126(6):940–942. doi: 10.1016/s0022-3476(95)70215-6.
- Chen L, Liu R, Zhao Y, et al. High consumption of soft drinks is associated with an increased risk of fracture: a 7-year follow-up study. Nutritients. 2020;12(2):530. doi: 10.3390/nu12020530.
- Taylor-Baer , Herman D. From epidemiology to epigenetics: evidence for the importance of nutrition to optimal health development across the life course. In: Halfon N, Forrest CB, Lerner RM, editors. Handbook of life course health development. Cham, Switzerland: Springer; 2018.
- Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473.
- Latham KE, Sapienza C, Engel N. The epigenetic lorax: gene-environment interactions in human health. Epigenomics. 2012;4(4):383–402. doi: 10.2217/epi.12.31.
- Silberman DM, Acosta GB, Zorrilla Zubilete MA. Long-term effects of early life stress exposure: role of epigenetic mechanisms. Pharmacol Res. 2016;109:64–73. doi: 10.1016/j.phrs.2015.12.033.
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415.

