Abstract
Purpose
To evaluate the effect of thyroid function on male fertility, focusing on hypo- and hyperthyroidism.
Methods
A PubMed/MEDLINE, Web of Science, and Scopus research was performed. Original studies in English published online up to 31 May 2023 were selected and reviewed. The final reference list was defined based on the relevance of each paper to the scope of this review.
Results
The available data in animals (31 studies) and human (26 studies) showed conflicting results. However, thyroid dysfunction altered erection and ejaculation both in animal models than in men.
Conclusion
Both hypothyroidism and hyperthyroidism seem to cause ejaculation and erectile dysfunction. Hence, Guidelines recommend against the systematic screening for thyroid disorders in the men in sub-fertile couples, but only in men with ejaculation and erectile dysfunction and/or altered semen parameters.
Introduction
Infertility is defined as the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse, or due to an impairment of a person’s capacity to reproduce, either as an individual or with his/her partner [Citation1]. It is estimated to affect between 8% and 12% of reproductive-aged couples worldwide [Citation2]. Males are found to be solely responsible for 20–30% of infertility cases but contribute to 50% of cases overall [Citation3]. Multiple causes of male infertility have been identified; these include endocrine disorders (usually due to hypogonadism), sperm transportation disorders, primary testicular defects, and idiopathic causes [Citation4].
Thyroid impairments are quite common. Subclinical and overt hypo- and hyperthyroidism have a prevalence ranging, respectively, from 0.1% to 12.4% and from 0.2% to 10% in adults [Citation5–10]. Hashimoto’s thyroiditis and Graves’ disease are the two most frequent autoimmune thyroid diseases (AITD) inducing hypothyroidism and hyperthyroidism, respectively [Citation11,Citation12]. These conditions are often associated with other pathologies such as hematologic [Citation13–15], cardiovascular [Citation16,Citation17], gastrointestinal [Citation18,Citation19], metabolic [Citation20,Citation21], and, last but not least, reproductive [Citation22–64] abnormalities.
It is in fact well known that thyroid function has a close link and interplay with female fertility [Citation65]. In milder hypothyroidism, infertility usually does not occur, but there is an increased risk of complications during pregnancy; on the other hand, severe hypothyroidism has a direct inhibitory effect on ovulation, also reducing ovarian reserve, and affects the pituitary-testicular axis, leading to infertility [Citation66–68]. On the other hand, hyperthyroidism can also impair female fertility via multiple mechanisms [Citation69].
The impact of thyroid function on male fertility is still matter of discussion and not fully understood, in particular subclinical dysthyroidism. The aim of the present review is to evaluate the effect of thyroid function on male fertility, focusing on hypo- and hyperthyroidism.
Methods
A PubMed/MEDLINE, Web of Science, and Scopus research was performed, for free-text words and terms related to “fertility,” “infertility,” “male,” “hypothyroidism,” “hyperthyroidism,” “subclinical,” “sperm,” “gonadal function,” “thyroid,” “TSH.” Original studies in English published online up to 31 May 2023 were selected and reviewed. The final reference list was defined based on the relevance of each paper to the scope of this review.
Results
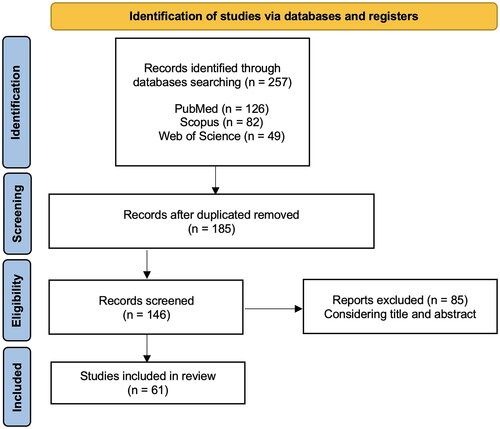
In the preliminary search, 257 studies were identified and 185 remained after removal of duplicates. A total of 146 articles were eligible for full-text screening and 61 full-text publications were included in the analysis. Specifically, 32 were performed in animal models; 29 were performed among human: 5 were case reports or case series, 2 were in vitro studies, and the remaining observational studies. The studies were published between 1962 and 2023. A flow diagram of the screening and selection process can be found in .
Animal studies
A total of 32 studies were found and summarized in .
Table 1. Summary of clinical studies and case reports about thyroid function and male fertility in animals in vivo.
Many studies evaluated the effects of thyroid hormone impairment on hypothalamic–pituitary–gonadal axis. Bruni et al. showed that hypothyroidism reduced luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release in rats with intact gonads (controls vs. hypothyroid: LH (ng/mL) 24 ± 6 vs. 8 ± 3, p < .005; FSH (ng/mL) 301 ± 51 vs. 203 ± 9, respectively, p < .005) and caused an abnormal increase in castrated rats (controls vs. hypothyroid: LH (ng/mL) 1163 ± 17 vs. 1683 ± 129, p < .001; FSH (ng/mL) 1090 ± 65 vs.1882 ± 48, respectively, p < .001). Moreover, replacement levothyroxine (LT4) therapy restored a “normal” LH and FSH in both groups [Citation22]. The same results were also reported by Valle et al. [Citation23], and reinforced by other authors who, in addition, provided evidence of a restoration of testosterone levels by introducing LT4 therapy [Citation24–26]. Moreover, Kala et al. showed a possible “double face” of hypothyroidism in terms of effect on the levels of testosterone in rats; persistent hypothyroidism diminished testosterone (4.2 ± 0.2 vs. 0.35 ± 0.02 ng/mL, p < .005), while transient hypothyroidism did not [Citation27]. Romano et al. tried to explain these mechanisms by showing that hypothyroidism alters post-transcriptional cascades in LH synthesis with a reduction in serum testosterone level, and probably with a direct effect on testicular function [Citation28]. El-Kashlan added that hormonal and testicular impaiment was also caused by testicular oxidative stress, DNA damage, and apoptotic activity [Citation29]. However, few authors showed normal serum levels of testosterone, LH and FSH in hypothyroid rats [Citation30,Citation73,Citation77].
In terms of gonads, studies have shown a possible reduction in the volume of testicles, seminal vesicles, and ventral prostate in hypothyroid rats [Citation23,Citation25,Citation28,Citation30,Citation75]. By contrast, larger seminal vesicles were observed by Bruni et al. [Citation22], whereas Umezu et al. described enlarged adult testes probably through the hypertrophy of Sertoli cells [Citation31]. On the other hand, some authors stated that hypothyroidism did not affect the weight of testicles, ventral prostate or seminal vesicles, or structure of seminiferous tubules [Citation26,Citation40,Citation71,Citation77]. Aruldhas et al. then reported volume normalization after LT4 replacement therapy [Citation25], although Choudhury et al. on the contrary, described a further decrease in volume of seminal vescicle after the administration of triiodiotironine to hypothyroid rats [Citation32]. Instead, Valle et al. reported no volume changes [Citation23].
The direct effect of hypothyroidism on gametogenesis, seminiferous tubules, and Leydig cells development has been reported [Citation33,Citation34]. These findings were confirmed by other authors showing the complete reversion of fertility after levothyroxine treatment [Citation29,Citation33,Citation35,Citation36]. In particular, Romano et al. and El-Kashlan et al. well described a significant decrease in total and daily sperm production, increased sperm transit time through the epididymis and altered semen characteristics compromising the fertilization process [Citation29,Citation36]. Korejo et al. added that concomitant diabetes exacerbated the issue [Citation34]. These data were confirmed by Chattopadhyay et al. adding that levothyroxine treatment is unable to restore normal fertility [daily testicular sperm production (No. ×106/g tissue): Euthyroid* 10.65 ± 0.87 vs. hypothyroid^ 3.99 ± 0.45 (p < 0.05)* vs. hypothyroid + T3 5.58 ± 0.61 (p < 0.05)*^; Epididymal sperm count (No.104/ml): Euthyroid* 1121.40 ± 137.86 vs. hypothyroid^ 553.80 ± 70.92 (p < 0.05)* vs. hypothyroid + T3 828.20 ± 56.73 (p < 0.05)*^] [Citation37]. On the contrary, a few authors saw no morphological changes in Leydig cells and reduction of fertility in hypothyroid mice [Citation40,Citation71,Citation73]. These disorders seem mediated by impaired antioxidant defense mechanisms [Citation29,Citation38], as suggested by Ibrahim et al. [Citation39].
Reduced libido in hypothyroid mice was first described by Karkun et al. [Citation40], whereas Jiang et al. showed its partial reversion after the intrdocution of LT4 therapy [Citation35]. These data were not supported by Chubb et al. [Citation76].
In the same way, hyperthyroidism was associated with the reduction of LH (−45% vs. euthyroidism, p < 0.001), FSH (−33.3%, p < 0.001), and serum testosterone levels (−34.3%, p < 0.001) in rats [Citation42,Citation74]. Schneider et al. hypothysized that it was due to the concomitant pituitary suppression and accelerated FSH metabolism [Citation43]. In addition, Asker et al. affirmed that thyrotoxicosis enhanced testicular oxidative stress (increase in malondialdehyde and nitric oxide concentrations by 19.0% and 44.4%, respectively, p < 0.01) causing testicular physiological impairment [Citation42]. Conversely, Jannini et al. showed a slight increase in FSH levels after inducing thyrotoxicosis by T3 therapy [Citation78], whereas Howland showed normal serum LH and FSH levels [Citation70,Citation72]. Only one study evaluated the effect of thyrotoxicosis on testicular volume, reporting a significant reduction in weight (−17%, p < 0.01) [Citation42]. Impaired sperm quality was reported by few authors (sperm count −40.4% and motility −37.7%, p < 0.01, by Asker et al.) [Citation36,Citation42]; low sperm count and motility could be due to the increase in testicular oxidative stress mediated by vimentin synthesis enhancement [Citation29,Citation44].
Human studies
A total of 29 studies were found and summarized in .
Table 2. Summary of clinical studies and case reports about thyroid function and male fertility in human.
Few data on the effects of thyroid hormone impairment on the hypothalamic–pituitary–gonadal axis are reported. In hypothyroid patients, Wortsman et al. reported both hypergonadotropic and hypogonadotropic hypogonadism [Citation45]. Serum FSH and LH levels were reported to rise after restoring euthyroidism by Jaya Kumar et al. in a small set of patients (LH (IU/l): hypothyroid 18.7 ± 7.3 vs. euthyroid 7.2 ± 2.0, p < 0.001; FSH (IU/l): hypothyroid 6.3 ± 2.0 vs. euthyroid 2.7 ± 0.9, p < 0.001) [Citation46]. By contrast, Ambigapathy et al. found that hypogonadotropic hypogonadism was more common than hypergonadotropic hypogonadism, with serum LH normalization upon restoring euthyroidism [Citation47]. In addition, in sub-fertile patients, Wortsman et al. reported that gonadal dysfunction preceded the development of hypothyroidism [Citation45].
With reference to the gonads, De La Balze et al. showed that prepuberal onset of hypothyroidism was associated with delayed testicular maturation and involution of adult characteristics (tubular content, tubular wall and intertubular connective tissue), mediated by gonadotropin secretion failure due to prolonged thyroid insufficiency [Citation49]. A positive correlation between fT3 level, seminal vesicle volume and inhomogeneous echotexture was reported by Lotti et al. [Citation48].
On the other hand, but physiologically in line with that reported above, Rehman et al. showed a correlation between altered semen parameters and subclinical hypothyroidism [Citation50]. In particular, the increase in thyroid stimulating hormone (TSH) levels was related to a significant reduction in normal morphology (% normal form: 5.79 ± 3.98 in euthyroid vs. 0.63 ± 0.92 in hypothyroid, p < 0.05), motility (% normal motility: 60.85 ± 21.00 in euthyroid vs. 20.18 ± 26.80 in hypothyroid, p < 0.05) and in sperm count (million sperm per mL: 88.85 ± 54.80 in euthyroid vs. 22.64 ± 31.39 in hypothyroid, p < 0.05) [Citation50]. The first datum was subsequently confirmed by Nikoobakht et al. Krassas et al. and Griboff et al. [Citation51,Citation53,Citation54], whereas impaired total and progressive sperm motility was confirmed by Ambigapathy et al. [Citation47]. With the exception of spermatozoa morphology, these data were previously reported in the 1990s by Corrales Hernandez et al. [Citation55]. Krassas et al. and Jaya Cumar et al. reported the normalization of morphology spermatozoa, improvement in sperm count and motility after LT4 therapy [Citation46,Citation53]. Mendeluk et al. conducted a semen analysis on spermatozoa incubated with LT4, finding a rapid and significant increase in the percentage of hyperactive sperm (8.93 × 106 ± 9.52 × 106 in control vs. 17.20 × 106 ± 21.16 × 106 after procedure, p < 0. 03) [Citation56]. Condorelli et al. showed that it was due to the beneficial effect of L-T4 on sperm mitochondrial function, oxidative stress, and DNA integrity [Citation57]. By contrast, Lotti et al. reported – in a cross-control study on 163 men in infertile couples – no association between hypothyroidism and semen parameters, as already reported by Trummer et al. [Citation48,Citation61].
In 5,401 infertile men, Zhao et al. found that serum TSH levels were positively associated with a DNA fragmentation index (DFI) over 25% [Citation58].
Among 2511 infertile couples, Rao et al. found that paternal subclinical hypothyroidism was associated with a poorer clinical outcome in in vitro fertilization (IVF) and intracytoplasmatic sperm injection (ICSI), for couples over 35 years old (total pregnancy rate 42% in euthyroid subjects vs. 32% in cases of hypothyroidism, p = 0.009) [Citation59].
Libido reduction and impaired erectile function was described in hypothyroid men [Citation46,Citation51]. In particular, Jaya Cumar et al. reported a prevalence of libido reduction in 37.5% of hypothyroid patients [Citation46]. Ambigapathy et al. confirmed sexual dysfunction by Androgen Deficiency in the Aging Male (ADAM) score in 80% of men and in 72.72% by Arizona Sexual Experience Scale (ASEX) score [Citation47]. Carani et al. showed hypoactive sexual desire, erectile dysfunction, and delayed ejaculation. Overall, ejaculation latency time declined significantly after L-T4 replacement therapy [Citation60].
Trummer et al. found a prevalence of thyroid antibodies in 7.5% of infertile men and added that elevated TPO-Ab were strongly correlated with pathozoospermia and asthenozoospermia [Citation61]. Instead, Poppe et al. and Binjandi et al. found no association between infertility and other thyroid function tests or TPOAb positivity [Citation66,Citation81,Citation85].
Examining hyperthyroidism, Hudson et al. reported greater levels of testosterone (hyperthyroid 67 ± 19 vs. euthyroid 23 ± 3.1 (nmol/L), p < 0.001), estradiol (hyperthyroid 237 ± 63 vs. euthyroid 99 ± 11 (pmol/L), p < 0.01), LH (hyperthyroid 24 ± 6 vs. euthyroid 8.8 ± 2.3 (IU/L), p < 0.001], and FSH [hyperthyroid 18 ± 6.1 vs. euthyroid 6.8 ± 2.4 (IU/L), p < 0.01] in patients affected by Graves’ disease [Citation62]. Clyde et al. explained that this was due to the increased binding capacity and affinity of testosterone-estradiol binding globulin in hyperthyroid status, and added that hormone levels return to normal values upon restoring euthyroidism [Citation63]. Röjdmark et al. found that pituitary gonadotrophs became “hypersensitive” to exogenous gonadotropin releasing hormone (GnRH) with LH and FSH response significantly higher in hyperthyroid patients because Leydig cells respond more powerfully to exogenous GnRH [Citation64].
In the study of gonads, only Krassas et al. reported a reduction in volume; in particular, 30% of hyperthyroid patients showed reduced size, which was restored after treatment [Citation52].
Clyde et al. and Krassas et al. reported oligospermia or borderline low count with decreased motility in thyrotoxicosis restored with medical therapy [Citation52,Citation63]. Similar results were found by Kidd et al. [Citation79].
Finally, Carani et al. reported a high prevalence of delayed ejaculation in hyperthyroid men, which reduced after thyroid hormone normalization from 50% to 15% [Citation60]. Krassas et al. reported sexual dysfunction associated with decreased libido in 56.5% of thyrotoxic patients, which improved after 6 months of treatment for hyperthyroidism [Citation52].
Discussion
The most recent Guidelines on Thyroid Disorders prior to and during Assisted Reproduction by the European Thyroid Association was published in 2021 [Citation86]. The present narrative review aims to apply the aforementioned Guidelines, adding the newest knowledge from 2021 to today.
It is well known that female fertility is impaired by thyroid disfunction, in particular by hypothyroidism; by contrast, we are still in the dark in terms of its effect on men, as the data available is less illuminating [Citation86]. A possible relationship has been reported between thyroid hormone impairment and male fertility, both in animal models and in humans; however, the precise effect on the pituitary-testicular axis, gonad structure, function, and sexual behavior are not completely clear. In fact, as Giano Bifronte revealed, some authors have reported that thyroid hormones are irrelevant to the male reproductive sphere [Citation23,Citation26,Citation30,Citation40,Citation48,Citation61,Citation70–73,Citation76,Citation77,Citation85], whereas other authoritative sources argue that they affect male fertility [Citation22–64]. This issue becomes more relevant for all sub-fertile couples, and particularly for those applying for Assisted Reproduction.
The available studies on animal models suggested an impaired “sexual” behavior from dysthyroidism. It has been widely demonstrated that hypothyroidism causes hypergonadotropic hypogonadism in rats, restored with the introduction of L-T4 therapy [Citation22–26]. The most convincing explanation suggests that hormonal and testicular impairment is due to testicular oxidative stress, DNA damage and apoptotic activity [Citation29]. In hypothyroid mice, reduced libido is observed along with a significant decrease of daily and total sperm production, as well as altered sperm characteristics compromising the fertilization process [Citation29,Citation35–40]. These data were recently confirmed by Panahandeh et al. showing that hypothyroidism directly impairs semen parameters, whereas maternal hypothyroidism has no significant effect on gonad function in offspring [Citation87]. Less evidence is available about the relationship between hyperthyroidism and fertility. In animal models, thyrotoxicosis seems associated with gonadotropin alterations and testicular oxidative stress, but the extent of its impact on fertility is yet to be demonstrated.
Referring to humans, the evidence on male subfertility and thyroid diseases is limited [Citation86]. Nevertheless, there is a known, close correlation between radioactive iodine treatment for thyroid malignancy and sperm quality and fertility. For this reason, the recent guidelines suggest that sperm banking should be offered in cases involving multiple doses of radioactive iodine [Citation86]. It is also conceivable that thyroid hormones may influence male fertility both prenatally and postnatally [Citation88,Citation89]. Indeed, it has been reported that hypothyroidism could lead to hypergonadotropic hypogonadism, which is in turn associated with testicular involution causing reduced sperm quality and alterations in normal morphology [Citation45–47,Citation50,Citation51,Citation53,Citation54]. Nevertheless, how and to what extent this may impair fertility is yet to be proven. Evidence of altered semen parameters correlated with hypothyroidism has been presented in terms of sperm count, motility, and morphology [Citation47,Citation50,Citation51,Citation53,Citation54,Citation61].
However, it is important to underline that hypothyroidism can impair pulsatile secretion of GnRH by prolactin increase, leading hypogonadotropic hypogonadism [Citation86,Citation90]. Although it is known that long-standing hypothyroidism can cause pituitary hyperplasia reversible by L-T4 treatment [Citation91], males with primary hypothyroidism seldom exhibit elevated serum prolactin concentrations [Citation90]. Anyway, prolactin levels should be always assessed in all hypothyroid patients to exclude a secondary hypogonadotropic hypogonadism [Citation86].
By referring to autoimmunity, few data are available. Trummer et al. reported that elevated thyroid peroxidase antibodies were significantly correlated with pathozoospermia and asthenozoospermia [Citation61]. Instead, Poppe et al. and Binjandi et al. found no association between infertility and other thyroid function tests or TPOAb positivity [Citation66,Citation81,Citation85]. Moreover, only Poppe et al. showed that the prevalence of thyroid autoimmunity is comparable between men with normal and abnormal semen characteristics [Citation81]. In light of this, Guidelines do not recommend screening for thyroid autoimmunity in men of subfertile couples [Citation86].
For these reasons, the Guidelines on Thyroid Disorders prior to and during Assisted Reproduction by the European Thyroid Association clearly suggests “screening for thyroid dysfunction (TSH) in men with ejaculation and erectile dysfunction and or altered semen parameters” [Citation86]. This suggestion is confirmed in the articles published after Guidelines by Zhao et al. and Rao et al. confirming higher DFI and poorer clinical outcome in assisted reproduction with an increase in TSH [Citation58,Citation59]. On the contrary, hyperthyroidism seems to be associated with higher levels of testosterone due to an increase in sex hormone binding globulin [Citation62,Citation63], but few data are reported on sperm impairment and no conclusions can be drawn.
In conclusion, hypothyroidism and hyperthyroidism could cause ejaculation and erectile dysfunction, but with little clinical impact. Guidelines recommend screening for thyroid disorders only in men with ejaculation and erectile dysfunction or with altered semen parameters, but not in the men in sub-fertile couples.
Author contributions
Valentina Anelli: Writing - Original draft preparation, Writing- Reviewing and Editing, Literature research, Data analysis. Elisa Gatta: Writing - Original draft preparation, Writing- Reviewing and Editing, Literature research, Data analysis. Ilenia Pirola: Supervision, Validation. Andrea Delbarba: Writing- Reviewing and Editing, Validation. Carlo Cappelli: Conceptualization, Project administration, Writing- Reviewing and Editing, Validation.
Disclosure statement
The authors have no competing interests to declare that are relevant to the content of this article.
All authors contributed to the article and approved the submitted version.
Additional information
Funding
References
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. doi:10.1016/j.fertnstert.2017.06.005.
- Ombelet W, Cooke I, Dyer S, et al. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14(6):605–621. doi:10.1093/humupd/dmn042.
- Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13(1):37. doi:10.1186/s12958-015-0032-1.
- Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am. 2014;41(1):195–204. doi:10.1016/j.ucl.2013.08.006.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. doi:10.1210/jcem.87.2.8182.
- Walsh JP, Bremner AP, Feddema P, et al. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab. 2010;95(3):1095–1104. doi:10.1210/jc.2009-1977.
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the whickham survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. doi:10.1111/j.1365-2265.1995.tb01894.x.
- Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. doi:10.1001/archinte.160.4.526.
- Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: national health and nutrition examination survey (NHANES 1999-2002). Thyroid. 2007;17(12):1211–1223. doi:10.1089/thy.2006.0235.
- Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the whickham survey. Clin Endocrinol (Oxf). 1977;7(6):481–493. doi:10.1111/j.1365-2265.1977.tb01340.x.
- Romagnani S. The Th1/Th2 paradigm and allergic disorders. Allergy. 1998;53(46):12–15. doi:10.1111/j.1398-9995.1998.tb04951.x.
- Orgiazzi J. Thyroid autoimmunity. Presse Med. 2012;41(12 p 2):e611-25–e625. doi:10.1016/j.lpm.2012.10.002.
- Squizzato A, Romualdi E, Büller HR, et al. Clinical review: thyroid dysfunction and effects on coagulation and fibrinolysis: a systematic review. J Clin Endocrinol Metab. 2007;92(7):2415–2420. doi:10.1210/jc.2007-0199.
- Green ST, Ng JP. Hypothyroidism and anaemia. Biomed Pharmacother. 1986;40(9):326–331.
- Scappaticcio L, Maiorino MI, Maio A, et al. Neutropenia in patients with hyperthyroidism: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2021;94(3):473–483. doi:10.1111/cen.14313.
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system: from theory to practice. J Clin Endocrinol Metab. 1994;78(5):1026–1027. doi:10.1210/jcem.78.5.8175954.
- Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249–1252. doi:10.1056/NEJM199411103311901.
- Shafer RB, Prentiss RA, Bond JH. Gastrointestinal transit in thyroid disease. Gastroenterology. 1984;86(5 Pt 1):852–855.
- Nordyke RA, Gilbert FI, Jr., Harada AS. Graves’ disease. Influence of age on clinical findings. Arch Intern Med. 1988;148(3):626–631. doi:10.1001/archinte.148.3.626.
- Diekman T, Lansberg PJ, Kastelein JJ, et al. Prevalence and correction of hypothyroidism in a large cohort of patients referred for dyslipidemia. Arch Intern Med. 1995;155(14):1490–1495. doi:10.1001/archinte.1995.00430140052004.
- Williams GR, Bassett JHD. Thyroid diseases and bone health. J Endocrinol Invest. 2018;41(1):99–109. doi:10.1007/s40618-017-0753-4.
- Bruni JF, Marshall S, Dibbet JA, et al. Effects of hyper- and hypothyroidism on serum LH and FSH levels in intact and gonadectomized male and female rats. Endocrinology. 1975;97(3):558–563. doi:10.1210/endo-97-3-558.
- Valle LB, Oliveira-Filho RM, Romaldini JH, et al. Pituitary-testicular axis abnormalities in immature male hypothyroid rats. J Steroid Biochem. 1985;23(3):253–257. doi:10.1016/0022-4731(85)90402-9.
- Wong CC, Döhler KD, von Zur Mühlen A. Effects of tri-iodothyronine, thyroxine and isopropyl-di-iodothyronine on thyroid-stimulating hormone in serum and pituitary gland and on pituitary concentrations of prolactin, growth hormone, luteinizing hormone and follicle-stimulating hormone in hypothyroid rats. J Endocrinol. 1980;87(2):255–263. doi:10.1677/joe.0.0870255.
- Aruldhas MM, Valivullah HM, Govindarajulu P. Specific effect of the thyroid on testicular enzymes involved in carbohydrate metabolism. I. Hypothyroidism. Int J Androl. 1982;5(2):196–204. doi:10.1111/j.1365-2605.1982.tb00248.x.
- Andó S, Panno ML, Beraldi E, et al. Influence of hypothyroidism on in-vitro testicular steroidogenesis in adult rats. Exp Clin Endocrinol. 1990;96(2):149–156. doi:10.1055/s-0029-1211004.
- Kala N, Ravisankar B, Govindarajulu P, et al. Impact of foetal-onset hypothyroidism on the epididymis of mature rats. Int J Androl. 2002;25(3):139–148. doi:10.1046/j.1365-2605.2002.00338.x.
- Romano RM, Bargi-Souza P, Brunetto EL, et al. Hypothyroidism in adult male rats alters posttranscriptional mechanisms of luteinizing hormone biosynthesis. Thyroid. 2013;23(4):497–505. doi:10.1089/thy.2011.0514.
- El-Kashlan AM, Nooh MM, Hassan WA, et al. Therapeutic potential of date palm pollen for testicular dysfunction induced by thyroid disorders in male rats. PLoS One. 2015;10(10):e0139493. doi:10.1371/journal.pone.0139493.
- Kalland GA, Vera A, Peterson M, et al. Reproductive hormonal axis of the male rat in experimental hypothyroidism. Endocrinology. 1978;102(2):476–484. doi:10.1210/endo-102-2-476.
- Umezu M, Kagabu S, Jiang JY, et al. Developmental hormonal profiles in rdw rats with congenital hypothyroidism accompanying increased testicular size and infertility in adulthood. J Reprod Dev. 2004;50(6):675–684. doi:10.1262/jrd.50.675.
- Choudhury S, Chainy GB, Mishro MM. Experimentally induced hypo- and hyper-thyroidism influence on the antioxidant defence system in adult rat testis. Andrologia. 2003;35(3):131–140. doi:10.1046/j.1439-0272.2003.00548.x.
- Chowdhury AR, Gautam AK, Chatterjee BB. Thyroid-testis interrelationship during the development and sexual maturity of the rat. Arch Androl. 1984;13(2–3):233–239. doi:10.3109/01485018408987522.
- Korejo NA, Wei Q, Zheng K, et al. Contemporaneous effects of diabetes mellitus and hypothyroidism on spermatogenesis and immunolocalization of claudin-11 inside the seminiferous tubules of mice. BMC Dev Biol. 2018;18(1):15. doi:10.1186/s12861-018-0174-4.
- Jiang JY, Umezu M, Sato E. Characteristics of infertility and the improvement of fertility by thyroxine treatment in adult male hypothyroid rdw rats. Biol Reprod. 2000;63(6):1637–1641. doi:10.1095/biolreprod63.6.1637.
- Romano RM, Gomes SN, Cardoso NC, et al. New insights for male infertility revealed by alterations in spermatic function and differential testicular expression of thyroid-related genes. Endocrine. 2017;55(2):607–617. doi:10.1007/s12020-016-0952-3.
- Chattopadhyay S, Choudhury S, Roy A, et al. T3 fails to restore mitochondrial thiol redox status altered by experimental hypothyroidism in rat testis. Gen Comp Endocrinol. 2010;169(1):39–47. doi:10.1016/j.ygcen.2010.07.014.
- Nascimento Gomes S, do Carmo Corrêa DE, de Oliveira IM, et al. Imbalanced testicular metabolism induced by thyroid disorders: new evidences from quantitative proteome. Endocrine. 2020;67(1):209–223. doi:10.1007/s12020-019-01989-8.
- Ibrahim W, Tousson E, Ali EM, et al. Folic acid alleviates oxidative stress and hyperhomocysteinemia involved in testicular dysfunction of hypothyroid rats. Gen Comp Endocrinol. 2011;174(2):143–149. doi:10.1016/j.ygcen.2011.08.012.
- Karkun JN, Das RP, Kar AB. Hypothyroidism and reproduction in male rats. Ann Biochem Exp Med. 1962;22:25–30.
- Aruldhas MM, Ramalingam N, Jaganathan A, et al. Gestational and neonatal-onset hypothyroidism alters androgen receptor status in rat prostate glands at adulthood. Prostate. 2010;70(7):689–700. doi:10.1002/pros.21101.
- Asker ME, Hassan WA, El-Kashlan AM. Experimentally induced hyperthyroidism influences oxidant and antioxidant status and impairs male gonadal functions in adult rats. Andrologia. 2015;47(6):644–654. doi:10.1111/and.12312.
- Schneider G, Kopach K, Ohanian H, et al. The hypothalamic-pituitary-gonadal axis during hyperthyroidism in the rat. Endocrinology. 1979;105(3):674–679. doi:10.1210/endo-105-3-674.
- Zamoner A, Barreto KP, Filho DW, et al. Hyperthyroidism in the developing rat testis is associated with oxidative stress and hyperphosphorylated vimentin accumulation. Mol Cell Endocrinol. 2007;267(1-2):116–126. doi:10.1016/j.mce.2007.01.005.
- Wortsman J, Rosner W, Dufau ML. Abnormal testicular function in men with primary hypothyroidism. Am J Med. 1987;82(2):207–212. doi:10.1016/0002-9343(87)90057-x.
- Jaya Kumar B, Khurana ML, Ammini AC, et al. Reproductive endocrine functions in men with primary hypothyroidism: effect of thyroxine replacement. Horm Res. 1990;34(5–6):215–218. doi:10.1159/000181828.
- Ambigapathy JS, Kamalanathan S, Sahoo J, et al. Effect of thyroxine replacement on Leydig cell and Sertoli cell function in men with hypothyroidism. Indian J Endocrinol Metab. 2020;24(3):265–269. doi:10.4103/ijem.IJEM_69_20.
- Lotti F, Maseroli E, Fralassi N, et al. Is thyroid hormones evaluation of clinical value in the work-up of males of infertile couples? Hum Reprod. 2016;31(3):518–529. doi:10.1093/humrep/dev338.
- De La Balze FA, Arrillaga F, Mancini RE, et al. Male hypogonadism in hypothyroidism: a study of six cases. J Clin Endocrinol Metab. 1962;22(2):212–222. doi:10.1210/jcem-22-2-212.
- Rehman R, Zafar A, Fatima SS, et al. Altered sperm parameters and subclinical hypothyroidism; a cross sectional study in Karachi, Pakistan. Int J Clin Pract. 2020;74(9):e13555. doi:10.1111/ijcp.13555.
- Nikoobakht MR, Aloosh M, Nikoobakht N, et al. The role of hypothyroidism in male infertility and erectile dysfunction. Urol J. 2012;9(1):405–409.
- Krassas GE, Pontikides N, Deligianni V, et al. A prospective controlled study of the impact of hyperthyroidism on reproductive function in males. J Clin Endocrinol Metab. 2002;87(8):3667–3671. doi:10.1210/jcem.87.8.8714.
- Krassas GE, Papadopoulou F, Tziomalos K, et al. Hypothyroidism has an adverse effect on human spermatogenesis: a prospective, controlled study. Thyroid. 2008;18(12):1255–1259. doi:10.1089/thy.2008.0257.
- Griboff SI. Semen analysis in myxedema. Fertil Steril. 1962;13(5):436–443. doi:10.1016/s0015-0282(16)34627-1.
- Corrales Hernández JJ, Miralles García JM, García Diez LC. Primary hypothyroidism and human spermatogenesis. Arch Androl. 1990;25(1):21–27. doi:10.3109/01485019008987590.
- Mendeluk GR, Rosales M. Thyroxin is useful to improve sperm motility. Int J Fertil Steril. 2016;10(2):208–214. doi:10.22074/ijfs.2016.4911.
- Condorelli RA, La Vignera S, Mongioì LM, et al. Thyroid hormones and spermatozoa: in VitroEffects on sperm mitochondria, viability and DNA integrity. J Clin Med. 2019;8(5):756. doi:10.3390/jcm8050756.
- Zhao S, Tang L, Fu J, et al. Subclinical hypothyroidism and sperm DNA fragmentation: a cross-sectional study of 5401 men seeking infertility care. J Clin Endocrinol Metab. 2022;107(10):e4027–e36. doi:10.1210/clinem/dgac458.
- Rao M, Yang Z, Su C, et al. Paternal subclinical hypothyroidism affects the clinical outcomes of in vitro fertilization/intracytoplasmic sperm injection. Thyroid. 2021;31(1):12–22. doi:10.1089/thy.2020.0154.
- Carani C, Isidori AM, Granata A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab. 2005;90(12):6472–6479. doi:10.1210/jc.2005-1135.
- Trummer H, Ramschak-Schwarzer S, Haas J, et al. Thyroid hormones and thyroid antibodies in infertile males. Fertil Steril. 2001;76(2):254–257. doi:10.1016/s0015-0282(01)01875-1.
- Hudson RW, Edwards AL. Testicular function in hyperthyroidism. J Androl. 1992;13(2):117–124. doi:10.1002/j.1939-4640.1992.tb01641.x.
- Clyde HR, Walsh PC, English RW. Elevated plasma testosterone and gonadotropin levels in infertile males with hyperthyroidism. Fertil Steril. 1976;27(6):662–666. doi:10.1016/S0015-0282(16)41896-0.
- Röjdmark S, Berg A, Kallner G. Hypothalamic-pituitary-testicular axis in patients with hyperthyroidism. Hormone Res Paediatr. 1988;29(5–6):185–190.
- Dosiou C. Thyroid and fertility: recent advances. Thyroid. 2020;30(4):479–486. doi:10.1089/thy.2019.0382.
- Poppe K, Glinoer D. Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum Reprod Update. 2003;9(2):149–161. doi:10.1093/humupd/dmg012.
- Vaquero E, Lazzarin N, De Carolis C, et al. Mild thyroid abnormalities and recurrent spontaneous abortion: diagnostic and therapeutical approach. Am J Reprod Immunol. 2000;43(4):204–208. doi:10.1111/j.8755-8920.2000.430404.x.
- Krassas GE. Thyroid disease and female reproduction. Fertil Steril. 2000;74(6):1063–1070. doi:10.1016/s0015-0282(00)01589-2.
- Mintziori G, Kita M, Duntas L, et al. Consequences of hyperthyroidism in male and female fertility: pathophysiology and current management. J Endocrinol Invest. 2016;39(8):849–853. doi:10.1007/s40618-016-0452-6.
- Howland BE, Ibrahim EA. Hyperthyroidism and gonadotropin secretion in male and female rats. Experientia. 1973;29(11):1398–1399. doi:10.1007/BF01922840.
- Vilchez-Martinez JA. Study of the pituitary-testicular axis in hypothyroid adult male rats. J Reprod Fertil. 1973;35(1):123–126. doi:10.1530/jrf.0.0350123.
- Howland BE, Ibrahim EA. Some effects of treatment with triiodothyronine(T3) on reproductive organ weights and gonadotropin levels in the serum and pituitary gland of the male rat. Horm Res. 1974;5(4):193–198. doi:10.1159/000178631.
- Chubb C, Nolan C. Animal models of male infertility: mice bearing single-gene mutations that induce infertility. Endocrinology. 1985;117(1):338–346. doi:10.1210/endo-117-1-338.
- Aruldhas MM, Valivullah HM, Govindarajulu P. Specific effect of thyroid hormone on testicular enzymes involved in carbohydrate metabolism. II. Hyperthyroidism. Biochim Biophys Acta. 1982;715(1):121–125. doi:10.1016/0304-4165(82)90057-5.
- Matsushima M, Kuroda K, Shirai M, et al. Spermatogenesis in snell dwarf, little and congenitally hypothyroid mice. Int J Androl. 1986;9(2):132–140. doi:10.1111/j.1365-2605.1986.tb00876.x.
- Chubb C, Henry L. The fertility of hypothyroid male mice. J Reprod Fertil. 1988;83(2):819–823. doi:10.1530/jrf.0.0830819.
- Weiss SR, Burns JM. The effect of acute treatment with two goitrogens on plasma thyroid hormones, testosterone and testicular morphology in adult male rats. Comp Biochem Physiol A Comp Physiol. 1988;90(3):449–452. doi:10.1016/0300-9629(88)90218-6.
- Jannini EA, Ulisse S, Piersanti D, et al. Early thyroid hormone treatment in rats increases testis size and germ cell number. Endocrinology. 1993;132(6):2726–2728. doi:10.1210/endo.132.6.8504773.
- Kidd GS, Glass AR, Vigersky RA. The hypothalamic-pituitary-testicular axis in thyrotoxicosis. J Clin Endocrinol Metab. 1979;48(5):798–802. doi:10.1210/jcem-48-5-798.
- Abalovich M, Levalle O, Hermes R, et al. Hypothalamic-pituitary-testicular axis and seminal parameters in hyperthyroid males. Thyroid. 1999;9(9):857–863. doi:10.1089/thy.1999.9.857.
- Poppe K, Glinoer D, Tournaye H, et al. Is systematic screening for thyroid disorders indicated in subfertile men? Eur J Endocrinol. 2006;154(3):363–366. doi:10.1530/eje.1.02098.
- Burns-Cox C. Sluggish sperms. Lancet. 2007;369(9570):1346. doi:10.1016/S0140-6736(07)60632-1.
- Hassani Y, Larroque B, Dos Santos S, et al. Fecundity in young adults treated early for congenital hypothyroidism is related to the initial severity of the disease: a longitudinal population-based cohort study. J Clin Endocrinol Metab. 2012;97(6):1897–1904. doi:10.1210/jc.2011-3286.
- Komiya A, Watanabe A, Kawauchi Y, et al. Severe oligozoospermia in a patient with myxedema coma. Reprod Med Biol. 2012;11(4):207–211. doi:10.1007/s12522-012-0129-6.
- Birjandi B, Ramezani Tehrani F, Amouzegar A, et al. The association between subclinical hypothyroidism and TPOAb positivity with infertility in a population-based study: Tehran thyroid study (TTS). BMC Endocr Disord. 2021;21(1):108. 10–1186. doi:10.1186/s12902-021-00773-y.
- Poppe K, Bisschop P, Fugazzola L, et al. 2021 European thyroid association guideline on thyroid disorders prior to and during assisted reproduction. Eur Thyroid J. 2021;9(6):281–295. doi:10.1159/000512790.
- Panahandeh F, Feizi F, Pourghasem M, et al. Hypothyroidism and fertility: an animal model follows up in the Second-Generation. Cell J. 2022;24(3):148–154. doi:10.22074/cellj.2022.8054.
- Holsberger DR, Cooke PS. Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res. 2005;322(1):133–140. doi:10.1007/s00441-005-1082-z.
- Mendis-Handagama SM, Ariyaratne HB. Effects of thyroid hormones on Leydig cells in the postnatal testis. Histol Histopathol. 2004;19(3):985–997. doi:10.14670/HH-19.985.
- Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702–755. doi:10.1210/er.2009-0041.
- Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273–288. doi:10.1210/jc.2010-1692.