Abstract
Objective
As people get older, the innate and acquired immunity of the elderly are affected, resulting in immunosenescence. Prealbumin (PAB), transferrin (TRF), and albumin (ALB) are commonly used markers to monitor protein energy malnutrition (PEM). However, their relationship with the immune system has not been fully explored.
Methods
In our study, a total of 93 subjects (≥65 years) were recruited from Tongji Hospital between January 2015 and February 2017. According to the serum levels of these proteins (PAB, TRF, and ALB), we divided the patients into the high serum protein group and the low serum protein group. Then, we compared the percent expression of lymphocyte subsets between two groups.
Results
All the low serum protein groups (PAB, TRF, and ALB) had significant decreases in the percentage of CD4+ cells, CD3+CD28+ cells, CD4+CD28+ cells and significant increases in the percentage of CD8+ cells, CD8+CD28− cells. PAB, TRF, and ALB levels revealed positive correlations with CD4/CD8 ratio, proportions of CD4+ cells, CD3+CD28+ cells, CD4+CD28+ cells, and negative correlation with proportions of CD8+ cells, CD8+CD28− cells.
Conclusions
This study suggested PAB, TRF, and ALB could be used as immunosenescence indicators. PEM might accelerate the process of immunosenescence in elderly males.
1. Introduction
The average human lifespan is steadily rising around the world, leading to an increase in the elderly population [Citation1,Citation2]. It is predicted that by 2050, the number of people over 65 will reach about 1.6 billion, accounting for almost 16.6% of the world’s population [Citation3]. Affected by the aging process, the immune system undergoes various alterations, resulting in innate and adaptive immunity dysfunction, which is termed immunosenescence [Citation4].
Immunosenescence results in substantial changes in almost all lymphocyte subsets within the immune system, especially T lymphocytes, which represent the cellular immune population [Citation5]. Previous studies have indicated that aging brings an inversion of the CD4/CD8 ratio and loss of CD28 expression [Citation6,Citation7]. In addition, immunosenescence makes older people more prone to infections, cancers, neurodegenerative, and autoimmune diseases [Citation8,Citation9], leading to a high mortality risk and has caused extensive concern.
Functional immune response is associated with lipid and glucose metabolism, protein homeostasis, and inflammation activity [Citation10,Citation11]. High glucose can induce enhanced concentrations of reactive oxygen species (ROS) in human senescent CD8+ T cells via enhancing capacity to use glycolysis [Citation12]. Lipid metabolism could change inflammatory gene expression and H2O2 production of different immune cells and affect the immune response of the body [Citation13]. Patients with hypoproteinemia had significantly lower numbers and functions of CD4+, CD8+ T cells, and NK cells than healthy controls [Citation14]. Up to now, many studies have shown that protein energy malnutrition (PEM) is associated with immunosenescence [Citation15,Citation16].
PEM is common in older adults because of multiple acute/chronic illnesses, disabilities, physical, and environmental changes [Citation17–19]. As reported, PEM patients are more likely to have atrophy of the thymus and lymphatic tissue, leading to increased immature CD2+CD3− and decreased mature CD3+ T lymphocytes [Citation20]. In addition, PEM leads to a relatively insufficient concentration of glutamine in the body, which can affect lymphocyte RNA and protein synthesis through concanavalin A [Citation21]. Many studies have also shown that PEM leads to decreased CD4 subsets and preserved CD8 subsets [Citation22,Citation23].
Serum prealbumin (PAB), transferrin (TRF), and albumin (ALB) can partially reflect PEM and host immune status [Citation24–27]. However, their potential relationship with immunosenescence has not been fully explored. In this study, we comprehensively evaluated the changes of lymphocyte subsets at different serum protein (PAB, TRF, and ALB) levels in elderly males. It is hoped that this study will offer a better understanding of the relationship between these proteins and immunosenescence, and facilitate the early evaluation of immunosenescence.
2. Materials and methods
2.1. Study subjects
Our subjects were recruited from the Department of Geriatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. This retrospective study made use of the data from January 2015 to February 2017. We enrolled patients over the age of 65 who were admitted to our department for different reasons. The exclusion criteria were as follows: (1) age <65 years, (2) females, (3) HIV infection, (4) severe hepatic or renal dysfunction, (5) immunosuppressive drug therapy, (6) chronic hematological diseases, (7) autoimmune or rheumatic disease, and (8) known or suspected malignancy. Initially, a total of 221 males were selected for further qualification. After applying our exclusion criteria, 128 males were excluded and 93 remained. The study protocol was approved by the ethical committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. All patients gave written informed consent.
Sarcopenia was defined as the presence of low skeletal muscle index (SMI) <8.87 kg/m2 and grip strength <27 kg in men [Citation28]. Sarcopenic obesity was defined if sarcopenia was accompanied by >25% of total body fat for men [Citation29]. Frailty status was defined on the basis of at least 3 of the following factors: slowness, exhaustion, myopenia, weakness, and low energy expenditure [Citation30].
2.2. Laboratory tests
Heparin anti-coagulating peripheral blood samples of patients were collected by vacuum blood collection tubes. According to the manufacturer’s instructions, PAB (normal range, 200–400 mg/L), TRF (normal range, 2.00–3.60 g/L), and ALB (normal range, 35–52 g/L) were tested using ROCHE COBAS 8000 (Mannheim, Germany). Serum PAB >200 mg/L, serum TRF >2.00 g/L, and serum ALB >35.0 g/L were defined as high level and all others were defined as low level.
2.3. Flow cytometric analysis
The main characteristics of lymphocytes were determined by flow cytometer. FACSCanto flow cytometer (BD Biosciences, San Jose, CA) was used to acquire data and Diva software (BD Biosciences) to calculate percentages as well as absolute numbers of lymphocyte subsets. Of 50 uL of whole blood was stained with 5 μL of the following antibodies: CD3 APC-H7-A, CD4 Pacific Blue-A, CD8 APC-A, CD25 PE-Cy7-A, CD28 FITC-A, CD45 Pacific Orange-A, CD56 ECD-A, and CD127 PE-A (BD Biosciences). It was then incubated in the dark at room temperature for 15 min and added 450 μL of BD FACSTM lysing solution. Finally, BD TrueCount™ tubes were used to accurate lymphocyte numbers.
2.4. Statistical analysis
All statistical analyses were conducted by using SPSS version 24.0 (Chicago, IL). Student’s t-test was used for comparisons between two independent groups. Results were expressed as the mean ± standard deviation (SD) and frequency (percentage). Correlation analysis was performed using the Pearson correlation test. The level of statistically significant was set at p < 0.05 (two-sided).
3. Results
3.1. Percent expression of lymphocyte subsets for individuals with CD4/CD8 ratio less than or greater than 1
At baseline, a total of 93 elderly males (≥65 years) were included in this study. The age ranged from 65 to 99 years with the mean (81.28 ± 8.65) years. The mean age of the individuals with CD4/CD8 ratio greater than 1 was younger than individuals with CD4/CD8 ratio less than 1 (p = 0.004). BMI, CMV infection, sarcopenia, sarcopenic obesity, waist circumference, and frailty status did not differ between the two groups. Subjects with CD4/CD8 ratio less than 1 showed a significant decrease in serum PAB levels (p = 0.034). Subjects with CD4/CD8 ratio less than 1 also showed significant decreases in the percentage of CD4+ cells (p < 0.001), CD3+CD28+ cells (p < 0.001), CD4+CD28+ cells (p < 0.001), and significant increases in CD8+ cells (p < 0.001), CD8+CD28− cells (p < 0.001) ().
Table 1. Differences between CD4/CD8 > 1 group and CD4/CD8 < 1 group.
3.2. Percent expression of lymphocyte subsets categorized by serum PAB, TRF, and ALB levels
The mean age of the subjects with high serum PAB levels was younger than subjects with low serum PAB levels (p = 0.004). Sarcopenia (p = 0.001), sarcopenic obesity (p = 0.026), and frailty status (p < 0.001) occurred significantly less frequently in the high PAB group. The low PAB group showed significant decreases in CD4/CD8 ratio (p = 0.019), CD4+ cells (p = 0.014), CD3+CD28+ cells (p = 0.004), CD4+CD28+ cells (p = 0.010) and significant increases in CD8+ cells (p = 0.002), CD8+CD28- cells (p = 0.002) ().
Table 2. Percent expression of lymphocyte subsets in elderly males categorized by their serum prealbumin levels.
Compared to the low-level TRF group, the high-level TRF group was younger (p < 0.001) and had fewer sarcopenia (p = 0.006), sarcopenic obesity (p = 0.017), and frailty status (p = 0.006). Subjects with low serum TRF showed significantly lower CD4/CD8 ratio (p = 0.006) and proportions of CD4+ cells (p = 0.001), CD3+CD28+ cells (p = 0.001), CD4+CD28+ cells (p = 0.001) and significantly higher proportions of CD8+ cells (p = 0.002), CD8+CD28− cells (p = 0.002) ().
Table 3. Percent expression of lymphocyte subsets in elderly males categorized by their serum transferrin levels.
Participants with high serum ALB levels were younger (p = 0.002) and had fewer sarcopenia (p < 0.001), sarcopenic obesity (p = 0.001) and frailty status (p = 0.024) than those with low serum ALB levels. The low serum ALB group showed significantly lower proportions of CD4+ cells (p = 0.032), CD3+CD28+ cells (p = 0.008), CD4+CD28+ cells (p = 0.013) and higher proportions of CD8+ cells (p = 0.017), CD8+CD28− cells (p = 0.012) ().
Table 4. Percent expression of lymphocyte subsets in elderly males categorized by their serum albumin levels.
3.3. Correlations between PEM parameters, age, and T-cell subsets
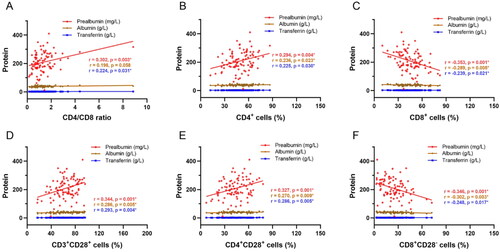
The effect of PEM on the different cell populations is shown in . PAB levels were positively correlated with CD4/CD8 ratio (p = 0.003), proportions of CD4+ cells (p = 0.004), CD3+CD28+ cells (p = 0.001), CD4+CD28+ cells (p = 0.001) and negatively correlated with proportions of CD8+ cells (p = 0.004), CD8+CD28− cells (p = 0.001). Likewise, TRF, ALB levels revealed positive correlations with CD4+ cells (p = 0.030, p = 0.023), CD3+CD28+ cells (p = 0.004, p = 0.005), CD4+CD28+ cells (p = 0.005, p = 0.009) and negative correlation with CD8+ cells (p = 0.021, p = 0.005), CD8+CD28− cells (p = 0.017, p = 0.003).
Figure 1. Correlation analysis between PEM and T-cell subsets. A–F) Correlation between PEM parameters (prealbumin, transferrin, and albumin) and CD4/CD8 ratio, proportions of CD4+ cells, CD8+ cells, CD3+CD28+ cells, CD4+CD28+ cells, and CD8+CD28− cells. Red indicates prealbumin, blue indicates transferrin, and brown indicates albumin. PEM: protein energy malnutrition.
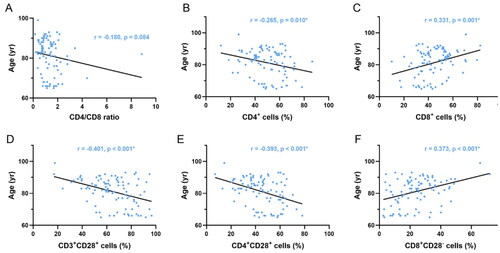
Dynamic changes in PEM parameters with age are shown in . Participants demonstrated significant decreases in the serum PAB (p = 0.007), TRF (p < 0.001), and ALB (p < 0.001) levels with increasing age. Correlation analysis between lymphocyte subsets and age is shown in . A decreasing trend in the proportions of CD4+ cells (p = 0.010), CD3+CD28+ cells (p < 0.001), and CD4+CD28+ cells (p < 0.001) was found to be associated with aging in subjects. In contrast, proportions of CD8+ cells (p = 0.001) and CD8+CD28− cells (p < 0.001) were all positively correlated with aging.
Figure 2. Correlation analysis between PEM and age. A–C) Correlation between PEM parameters (prealbumin, transferrin, and albumin) and age. Red indicates prealbumin, blue indicates transferrin, and brown indicates albumin. PEM: protein energy malnutrition.
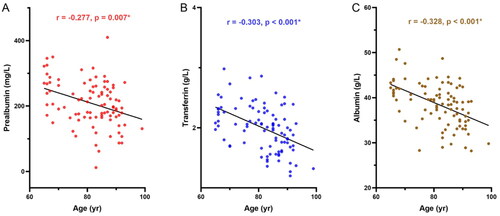
Figure 3. Correlation analysis between age and proportions of T-cell subsets. A–F) Correlation between age and CD4/CD8 ratio, proportions of CD4+ cells, CD8+ cells, CD3+CD28+ cells, CD4+CD28+ cells, and CD8+CD28− cells.
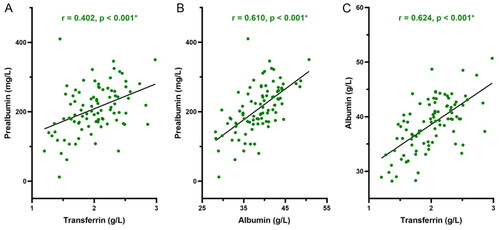
A correlation between different PEM parameters with one another is shown in . Serum PAB was significantly positively correlated with TRF (p < 0.001) and ALB (p < 0.001). Similarly, strong positive correlations were also detected between serum TRF and ALB (p < 0.001).
4. Discussion
More and more people are becoming recognized that the immune system declines with age, which is known as immunosenescence [Citation4]. The geriatric population with PEM contributes to the increased risk of immune aging [Citation15,Citation16]. This study investigated association of PEM parameters (PAB, TRF, and ALB) with immunosenescence among elderly males. Our results showed that PAB, TRF, and ALB are clinical indicators for evaluating immunosenescence.
Inversion of the CD4/CD8 ratio is a frequent finding in older age, considered as an important indicator of immunosenescence. Our results showed that the inverted CD4/CD8 ratio was associated with advanced age and loss of CD28 expression in T cells. In addition, the accumulation of CD28− T cells in the CD8 subsets is one of the most prominent changes in aging humans and represents impaired T-cell immunity [Citation9,Citation31]. CD28 is a B7 ligand and a co-stimulatory receptor expressed on the T-cell surface, it is vital for the second signal generated from CD28-B7 ligand interactions to activate and sustain the activity of T cells [Citation32,Citation33]. Repeated antigen stimulation could lead to loss of CD28 expression in T cells with age [Citation34].
PEM is a worldwide health problem and is frequently observed among adults aged over 65 years [Citation17,Citation35]. PEM is associated with reduced immune responses and can drastically impair T-cell proliferation and cytokine production. PAB, TRF, and ALB are commonly used in clinic to evaluate protein nutritional status and monitor therapeutic effects. This study showed that low serum PAB and TRF groups had a significant decrease in CD4/CD8 ratio and all the low serum protein groups (PAB, TRF, and ALB) had a CD28 loss in T cells. This finding is consistent with the lymphocyte phenotype of immunosenescence, indicating that PEM might participate in immunosenescence [Citation16,Citation22,Citation36]. Furthermore, immunosenescence markers, such as the proportions of CD8+CD28− cells, CD4 cells, and the CD4/CD8 ratio were also associated with cytomegalovirus (CMV) infection [Citation37]. Similar to that reported elsewhere, our subjects appear to constitute a higher risk of CMV infection and sero-prevalence rates approach 100% in old age [Citation38]. Hence, we are not able to evaluate the impact of secondary/tertiary CMV infection on serum protein levels (PAB, TRF, and ALB) in this study.
The prevalence of PEM has previously been reported associated with age, sarcopenia, sarcopenic obesity, and frailty status. In line with the above studies, our results revealed all the low serum protein groups (PAB, TRF, and ALB) were older and more likely to have sarcopenia, sarcopenic obesity, and frailty status. The underlying mechanism of sarcopenia, sarcopenic obesity, and frailty is superimposed pathologically to some extent, including gut dysbiosis, endotoxemia, chronic inflammation, and endocrine disturbance [Citation39]. Immune aging accompanied by changes in metabolism functions of immune cells is responsible for the environment of the skeletal muscle, eventually causing fibrosis, atrophy, and other consequences of muscle [Citation40]. Our findings highlight a fact that sarcopenia, sarcopenic obesity and frailty status may be involved in the reduction of PAB, TRF, and ALB levels favoring the immunosenescence. However, how these different complications alter the immunonutritional status require further investigation.
There are a few limitations in this article. First, this is a single-center study with a small sample size. Further studies including females and larger populations are needed to demonstrate the validity of our results. Second, serum PAB, TRF, and ALB levels are not sufficient to indicate malnutrition. Future research should also apply nutrition scales to assess nutritional status, such as the Subjective Global Assessment, Mini-Nutritional Assessment, and Detailed Nutritional Assessment [Citation41–43]. Third, due to the retrospective design, cytokines and sex hormones cannot be complemented for detection and need further exploration. In addition to these limitations, we believe that our study is valuable in evaluating accelerated immunosenescence. In clinical practice, the monitoring of PAB, TRF, and ALB is cheaper and more convenient compared with flow cytometric analysis, which provides the possibility for future individualized and large-scale clinical applications.
5. Conclusion
This study indicated that low serum PAB, TRF, and ALB levels were associated with T-cell immunosenescence phenotypes, such as decreased CD4/CD8 ratio and loss of CD28 expression in T cells. PAB, TRF, and ALB could be used as markers for evaluating accelerated immunosenescence in elderly males.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethical committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (protocol code TJ-C20141112 and 27 November 2014). All participants provided written informed consent. No experimental interventions were performed.
Author contributions
Conceptualization, XQQ, RDZ, and CTZ; methodology, SQJ, LR, FJY, and RDZ; formal analysis, SQJ and RDZ; data curation, SQJ, LR, FJY, and RDZ; writing-original draft preparation, RDZ, FJY, and SQJ; writing-review and editing, XQQ and CTZ; supervision, XQQ and CTZ; funding acquisition, XQQ and CTZ.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sander M, Oxlund B, Jespersen A, et al. The challenges of human population ageing. Age Ageing. 2015;44(2):185–187. doi: 10.1093/ageing/afu189.
- Xiong Y, Zhang Y, Zhang F, et al. Prevalence and associated factors of metabolic syndrome in Chinese Middle-aged and elderly population: a national cross-sectional study. Aging Male. 2021;24(1):148–159. doi: 10.1080/13685538.2021.1998432.
- Crichton M, Craven D, Mackay H, et al. A systematic review, meta-analysis and meta-regression of the prevalence of protein-energy malnutrition: associations with geographical region and sex. Age Ageing. 2019;48(1):38–48.
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–446. doi: 10.1111/j.1365-2567.2007.02555.x.
- Lioulios G, Fylaktou A, Papagianni A, et al. T cell markers recount the course of immunosenescence in healthy individuals and chronic kidney disease. Clin Immunol. 2021;225:108685. doi: 10.1016/j.clim.2021.108685.
- Weinberger B, Lazuardi L, Weiskirchner I, et al. Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Hum Immunol. 2007;68(2):86–90. doi: 10.1016/j.humimm.2006.10.019.
- Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013.
- Batatinha HAP, Diniz TA, de Souza Teixeira AA, et al. Regulation of autophagy as a therapy for immunosenescence-driven cancer and neurodegenerative diseases: the role of exercise. J Cell Physiol. 2019;234(9):14883–14895. doi: 10.1002/jcp.28318.
- Prcina M, Novak M, Cigankova V, et al. Immunosenescence - the role in the immunotherapy of older population. Bratisl Lek Listy. 2018;119(4):217–220. doi: 10.4149/BLL_2018_040.
- Gleeson LE, Sheedy FJ. Metabolic reprogramming & inflammation: fuelling the host response to pathogens. Semin Immunol. 2016;28(5):450–468. doi: 10.1016/j.smim.2016.10.007.
- Bertoni G, Minuti A, Trevisi E. Immune system, inflammation and nutrition in dairy cattle. Anim Prod Sci. 2015;55(7):943–948. doi: 10.1071/AN14863.
- Yi H-S, Kim SY, Kim JT, et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019;10(3):249. doi: 10.1038/s41419-019-1494-4.
- Mabrouk N, Lecoeur B, Bettaieb A, et al. Impact of lipid metabolism on antitumor immune response. Cancers (Basel). 2022;14(7):1850. doi: 10.3390/cancers14071850.
- Luo Y, Xie Y, Zhang W, et al. Combination of lymphocyte number and function in evaluating host immunity. Aging (Albany NY). 2019;11(24):12685–12707. doi: 10.18632/aging.102595.
- Liao X, Bian H, Zheng X, et al. Association of the inflammatory potential of diet and lower urinary tract symptoms among men in the United States. Aging Male. 2021;24(1):72–79. doi: 10.1080/13685538.2021.1920911.
- Tannou T, Koeberle S, Manckoundia P, et al. Multifactorial immunodeficiency in frail elderly patients: contributing factors and management. Med Mal Infect. 2019;49(3):167–172. doi: 10.1016/j.medmal.2019.01.012.
- Agarwal E, Marshall S, Miller M, et al. Optimising nutrition in residential aged care: a narrative review. Maturitas. 2016;92:70–78. doi: 10.1016/j.maturitas.2016.06.013.
- Dwyer JT, Gahche JJ, Weiler M, et al. Screening community-living older adults for protein energy malnutrition and frailty: update and next steps. J Community Health. 2020;45(3):640–660. doi: 10.1007/s10900-019-00739-1.
- Fang Y, Liu MJ, Zhang WW, et al. Nutrition support practices of hematopoietic stem cell transplantation centers in mainland China. Curr Med Sci. 2020;40(4):691–698. doi: 10.1007/s11596-020-2231-z.
- Lesourd BM, Laisney C, Salvatore R, et al. Decreased maturation of T-cell populations in the healthy elderly: influence of nutritional factors on the appearance of double negative CD4-, CD8-, CD2+ cells. Arch Gerontol Geriatr. 1994;19(1):139–154. doi: 10.1016/s0167-4943(05)80059-7.
- Calder PC, Yaqoob P. Glutamine and the immune system. Amino Acids. 1999;17(3):227–241. doi: 10.1007/BF01366922.
- Lesourd BM, Mazari L. Immune responses during recovery from protein-energy malnutrition. Clin Nutr. 1997;16(1):37–46. doi: 10.1016/s0261-5614(97)80047-7.
- Fulop T, Pawelec G, Castle S, et al. Immunosenescence and vaccination in nursing home residents. Clin Infect Dis. 2009;48(4):443–448. doi: 10.1086/596475.
- Hassanein el SA, Assem HM, Rezk MM, et al. Study of plasma albumin, transferrin, and fibronectin in children with mild to moderate protein-energy malnutrition. J Trop Pediatr. 1998;44(6):362–365. doi: 10.1093/tropej/44.6.362.
- Constans T, Bacq Y, Bréchot JF, et al. Protein-energy malnutrition in elderly medical patients. J Am Geriatr Soc. 1992;40(3):263–268. doi: 10.1111/j.1532-5415.1992.tb02080.x.
- Singh DKA, Manaf ZA, Yusoff NAM, et al. Correlation between nutritional status and comprehensive physical performance measures among older adults with undernourishment in residential institutions. Clin Interv Aging. 2014;9:1415–1423. doi: 10.2147/CIA.S64997.
- Devoto G, Gallo F, Marchello C, et al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem. 2006;52(12):2281–2285. doi: 10.1373/clinchem.2006.080366.
- Dodds RM, Granic A, Davies K, et al. Prevalence and incidence of sarcopenia in the very old: findings from the Newcastle 85+ study. J Cachexia Sarcopenia Muscle. 2017;8(2):229–237. doi: 10.1002/jcsm.12157.
- Moroni A, Perna S, Azzolino D, et al. Discovering the individualized factors associated with sarcopenia and sarcopenic obesity phenotypes—a machine learning approach. Nutrients. 2023;15(21):4536. doi: 10.3390/nu15214536.
- Gehle SC, Kleissler D, Heiling H, et al. Accelerated epigenetic aging and myopenia in young adult cancer survivors. Cancer Med. 2023;12(11):12149–12160. doi: 10.1002/cam4.5908.
- Fukushima Y, Minato N, Hattori M. The impact of senescence-associated T cells on immunosenescence and age-related disorders. Inflamm Regen. 2018;38(1):24. doi: 10.1186/s41232-018-0082-9.
- Ganesan A, Moon TC, Barakat KH. Revealing the atomistic details behind the binding of B7-1 to CD28 and CTLA-4: a comprehensive protein-protein modelling study. Biochim Biophys Acta Gen Subj. 2018;1862(12):2764–2778. doi: 10.1016/j.bbagen.2018.08.010.
- Levy R, Rotfogel Z, Hillman D, et al. Superantigens hyperinduce inflammatory cytokines by enhancing the B7-2/CD28 costimulatory receptor interaction. Proc Natl Acad Sci U S A. 2016;113(42):E6437–E6446.
- Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205(1):158–169. doi: 10.1111/j.0105-2896.2005.00256.x.
- Leij-Halfwerk S, Verwijs MH, van Houdt S, et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis. Maturitas. 2019;126:80–89. doi: 10.1016/j.maturitas.2019.05.006.
- Ozkan H, Olgun N, Saşmaz E, et al. Nutrition, immunity and infections: t lymphocyte subpopulations in protein–energy malnutrition. J Trop Pediatr. 1993;39(4):257–260. doi: 10.1093/tropej/39.4.257.
- Strindhall J, Skog M, Ernerudh J, et al. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (Dordr). 2013;35(3):985–991. doi: 10.1007/s11357-012-9400-3.
- Yi F, Zhao J, Luckheeram RV, et al. The prevalence and risk factors of cytomegalovirus infection in inflammatory bowel disease in Wuhan, Central China. Virol J. 2013;10(1):43. doi: 10.1186/1743-422X-10-43.
- Buch A, Carmeli E, Boker LK, et al. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—an overview. Exp Gerontol. 2016;76:25–32. doi: 10.1016/j.exger.2016.01.008.
- Zhang X, Li H, He M, et al. Immune system and sarcopenia: presented relationship and future perspective. Exp Gerontol. 2022;164:111823. doi: 10.1016/j.exger.2022.111823.
- Baker JP, Detsky AS, Wesson DE, et al. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med. 1982;306(16):969–972. doi: 10.1056/NEJM198204223061606.
- Persson MD, Brismar KE, Katzarski KS, et al. Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc. 2002;50(12):1996–2002. doi: 10.1046/j.1532-5415.2002.50611.x.
- Azad N, Murphy J, Amos SS, et al. Nutrition survey in an elderly population following admission to a tertiary care hospital. CMAJ. 1999;161(5):511–515.