Abstract
Background
Benign prostatic hyperplasia (BPH) affects 30% of men worldwide, folate is essential for life. However, few studies have investigated the relationship between folate levels and BPH. The present study aims to explore the relationship between red blood cell (RBC) folate, a better indicator of long-term folate intake, and BPH in United States (US) men.
Methods
We used statistics from four cycles of the “National Health and Nutrition Examination Survey” (NHANES2001-2008), RBC folate data come from laboratory data and BPH date come from questionnaire data. A multivariate conditional logistic regression model and subgroup analysis were using to assess the association between RBC folate and BPH.
Results
647 males from four survey cycles in the NHANES2001-2008, of which, 574 men (88.7%) had BPH. After adjusting for potential confounders, a considerable correlation was observed between RBC folate and BPH; With the first quintiles of RBC folate as the reference, multivariable-adjusted odds ratios (ORs) and confidence intervals (95% CIs) of the second, third, fourth, and the highest quintiles were 1.19 (0.58 ∼ 2.44), 1.39 (0.65 ∼ 2.97), 2.27 (0.96 ∼ 5.39), 2.26 (1.35 ∼ 3.76) and 5.37 (1.85 ∼ 15.59), respectively.
Conclusions
Individuals with high levels of RBC folate were associated with an increased risk of self-reported benign prostatic hyperplasia of US men.
1. Introduction
Benign prostatic hyperplasia (BPH) is a common phenomenon among older men and is frequently associated with bothersome lower urinary tract symptoms (LUTS) [Citation1,Citation2]. According to the Global Burden of Disease 2019, cases of BPH increased by 105.70% in 2019 compared with cases in 1990 [Citation3]. The disease prevalence increases with age. The histological prevalence of BPH at autopsy is 50–60% in men aged 60 years old and increases to 80–90% in those over 70 years old [Citation4]. Moreover, a worldwide survey showed that the quantified incidence of LUTS/BPH is substantial and is likely to increase with aging, thus rapidly increasing human burden for outweighing other urinary tract infections [Citation5]. BPH, similar to hypertension and diabetes, is common in middle-aged and elderly populations; however, it has received less attention, and the specific mechanisms underlying it remain unknown. The pathogenesis of the disease is thought to be influenced by androgens, estrogens, insulin, inflammation, proliferative reawakening, stem cells, and telomerase [Citation6,Citation7]. Nutritional factors [Citation8] may influence benign prostatic hyperplasia, and it is easy to regulate, such as micronutrients like zinc, vitamin D [Citation9] and β-carotenoids [Citation10]. Therefore, it is an important direction of BPH research to find relevant prevention and control methods and detection indicators.
Folate is a necessary water-soluble vitamin B for humans, they are essential for human health and development. It is involved in the methylation of biomolecules and has a protective effect against neural tube defects, ischemic events and cancer, therefore, many countries introduce food folic acid fortification [Citation11,Citation12]. Data from the post-fortification era provide strong evidence for a reduction in NTDS caused by folic acid fortification [Citation13,Citation14]. However, there is growing concern about the amount of synthetic folic acid in the human food supply, and some studies have found that excessive intake of folic acid can have adverse effects on some populations, especially the elderly [Citation15–17]. Basic research found that folic acid supplementation in large dose (2.0 mg/kg) of maternal mice significantly increased the proportion of prostatitis in offspring mice [Citation18]. Classic biomarker for assessing folate status include serum folate and red blood cell folate. Serum folate reflects the current folate content in the bloodstream, while red blood cell folate serves as a better indicator of long-term folate intake. Based on existing research, the relationship between folic acid and BPH has not been fully explored.
The National Health and Nutrition Examination Survey (NHANES) database, a major initiative of the National Center for Health Statistics (NCHS), collects data representing various aspects of the U.S. civilian population, including diet, health, and more, with a certain degree of universality. Therefore, we utilized the NHANES database from 2001 to 2008 to investigate the potential correlation between folic acid and BPH, aiming to contribute to the prevention and treatment of BPH.
2. Materials and methods
2.1. Data sources and study population
In this cross-sectional study, data from 4-year cycles of the NHANES 2001-2008 were used to assess the association between BPH and RBC folate. A total of 41658 individuals from the 4-year cycle participated in our study, and we established the following exclusion criteria: 1. Female; 2. Missing data on BPH and RBC folate; and 3. Missing data on age, race, or other covariates. Based on the exclusion criteria, 647 patients were ultimately included in the study.
The NHANES, conducted by the National Center for Health Statistics (NCHS) of the centers for disease control and prevention, collects data on the health and nutritional status of the non-institutionalized civilian population in the US. As a national cross-sectional survey, the NHANES includes a series of physical examinations, laboratory tests, and questionnaires to obtain information based on sampling probability. However, these data only represented the US population. All participants signed an informed consent form. The data used are available to the public on the NHANES website and the program was approved by the NCHS Research Ethics Review Board. This study follows STROBE reporting guidelines to enhance the reporting of observational epidemiological studies. We did not submit this work for human subject ethical review because it was a cross-sectional study based on non-clinical data.
2.2. BPH definition
If male participants answered the question “Are you ever been told you have an enlarged prostate?” And “Is enlargement hyperplasia of the prostate?” If they answered “yes” to both questions, they were diagnosed with an enlarged prostate. The following participants were excluded: men who did not know the answer, refused to answer the question, or had missing data [Citation6].
2.3. RBC folate definition
Whole blood and serum were treated and properly frozen (−20 °C) until transported to the National Centre for Environmental Health for analysis. For the analysis of RBC folate, the blood samples is first diluted. The sample is diluted 1:11 with a solution of 1 g/dL ascorbic acid in water and either incubated for 90 min prior to assay or frozen immediately for later assay. RBC folate was calculated from the whole blood folate concentration using microbiologic assay by adjusting for RBC volume and correcting for serum total folate concentration, which was calculated as the sum of individual folate forms [Citation19].
2.4. Potential confounders
Multiple potential covariates were assessed based on literature [Citation6,Citation20,Citation21] including age, marital status, race, educational level, supplement use, body mass index (BMI), waist circumference, C-reactive protein(CRP), total prostate specific antigen(PSA), smoking status, drinking behavior, and the presence of hypertension, diabetes, and activity status. Race was categorized as non-Hispanic white, non-Hispanic black, or Mexican American, or other race. Marital status was categorized as married, living with partners or living alone. Educational level was categorized as < 9 years, 9-12 years, or > 12 years. Dietary supplements were determined by asking about the supplements and medications received in the past month. BMI was derived from examination data and calculated based on height and weight. The determination of pre-existing conditions (hypertension and diabetes) was based on a question in the questionnaire that asked whether the physician had been informed of the condition in the past. Smoking and drinking status were determined based on the questions in the questionnaire “Smoke at least 100 cigarettes in a lifetime” and “Drink at least 12 drinks/year”. Activity status was determined based on the questions in the questionnaire “At least 10 min of heart-rate exercise a day”.
2.5. Statistical analysis
Categorical variables were represented as proportions (%), while continuous variables were described as mean (standard deviation) or median (interquartile interval). Unidirectional analyses of variance (normal distribution), Kruskal-Wallis tests (asymmetric distribution), and chi-square tests (categorical variables) were conducted to compare differences among the groups. Logistic regression models were used to determine the odds ratios (OR) and 95% confidence intervals (95% CIs) for the association between BPH and RBC folate. Model 1, which was our crude model, was adjusted for no variables; Model 2, Adjusted for age, race, education levels, family poverty levels, marital status. Model 3, Further adjusted dietary supplement, BMI, waist circumference, CRP, total prostate specific antigen based on modle2. Model 4, Adjusted for all Covariates, including age, race, education levels, family poverty levels, marital status, dietary supplement, BMI, waist circumference, CRP, total PSA, smoking status, drinking behavior, and the presence of hypertension, diabetes and activity status.
In addition, possible changes in the association between the consumption of RBC folate and BPH were examined, including the following variables: age (≤65 years vs. > 65 years), marital status (married or living with partners vs. living alone), educational level (≤12 years vs > 12 years) and family income (low vs. medium and high). Heterogeneity between subgroups was assessed using multivariate logistic regression, and interactions between subgroups and the consumption of erythrocyte folate were examined using likelihood ratio tests.
Since the sample size was determined solely based on the data presented, no statistical power estimates were considered a prior. Moreover, all analyses were performed using Free Statistics software, version 1.7. A descriptive study was conducted with all participants. A two-tailed test was used to determine a p-value of < 0.05 as significant.
3. Results
3.1. Study population
A total of 41658 participants completed the survey, of whom 21181 were female. Participants with missing data on BPH (n = 19264), participants with missing data on RBC folate (n = 284), and participants with other covariates (n = 282) were excluded. Ultimately, 647 participants from the NHANES were included in this cross-sectional study between 2001 and 2008. An extremely detailed inclusion and exclusion procedure is shown in .
Baseline characteristics
shows the distribution of the survey population characteristics (n = 647) of the whole sample. Of these 647 men, 574(88.7%) had BPH, and 73 (11.3%) did not have. The average age of the men included in this analysis was 68.1 ± 10.8 years. In this study, most of the population was non-Hispanic white. Moreover, participants with high RBC folate were older and more likely to had BPH and to be Non-Hispanic white, more than high school education level, of medium family monthly poverty level index, to have larger waist circumference, and to use dietary supplement.
Table 1. Baseline characteristics of the study participants.
3.3. Relationship between RBC folate and BPH
The multivariate linear regression results are presented in . These results indicated a significant relationship between RBC folate levels and BPH. Taking the first quintile of RBC folate as the reference category, the highest quintiles of RBC folate were associated with higher odds for BPH. Within the unadjusted model (Model 1), the OR for the highest quintile (OR = 5.37, 95% CI: 1.98,14.62) was significantly higher compared with that for the lowest quintiles,P for trend < 0.005. When we adjusted for some covariates, the correlation did not change, which includes age, race, education levels, family income, marital status, dietary Supplement, BMI(Body Mass Index), waist circumference, CRP(C-reactive protein),total prostate specific antigen, hypertension status, diabetes status, smoke status, drink status, activity status. With the first quintile category of RBC folate as the reference, the multivariable-adjusted ORs and 95% CIs of the, second, third, fourth, and the highest quintile categories were 1.19 (0.58 ∼ 2.44), 1.39 (0.65 ∼ 2.97), 2.27 (0.96 ∼ 5.39), and 5.37 (1.85 ∼ 15.59), respectively.
Table 2. Multivariable logistic regression to assess the association of red cell folate with benign prostatic hyperplasia.
3.4. Stratified analysis
In addition, in order to further analyze the relationship between high levels of erythrocyte folate and BPH, we divided Red RBC folate levels into <649(ng/mL) and ≥649(ng/mL) groups, and conducted stratified analysis according to age, BMI, marital status, education level and family poverty level. shows the forest map. Individuals who are >65 years of age, have a BMI <30, are married or living with a partner, have less than 12 years of education, and have a moderate to high household income have a higher risk of developing BPH. Subgroup analysis showed no significant interaction.
Figure 2. Subgroup regression to assess the relationship between Red RBC folate and benign prostatic hyperplasia.
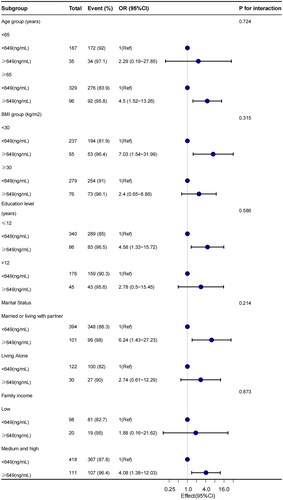
We adjusted for age, race, education levels, family poverty levels, marital status, dietary supplement, BMI(Body Mass Index), waist circumference, CRP(C-reactive protein), total prostate specific antigen, smoking status, drinking behavior, and the presence of hypertension, diabetes and activity status.
4. Discussion
This nationally representative cross-sectional study was conducted to examine whether RBC folate status is associated with BPH. The results showed a clear association between high RBC folate levels and BPH. We observed a significant dose-response trend, with OR increasing with increasing RBC folate dose compared to the trend in the lowest quintile. The highest quintile of RBC folate concentration increased the risk of BPH, after adjusting for all covariates, the results remained stable. In subgroup analysis, individuals aged >65 years, with BMI <30, married or cohabiting with a partner, with less than 12 years of education, and with moderately high family income have a higher risk of developing benign prostatic hyperplasia. No significant interaction was observed among these groups.
Folate is an essential microelement for life as it is involved in a range of processes including deoxyribonucleic acid methylation and mitochondrial translation. Moreover, Folic acid is a major aid in the one-carbon metabolic pathway and produces phospholipids, creatine, and epinephrine [Citation22]. It is absorbed from food and produced by microorganisms in the small intestine. Then, it is absorbed by the proximal duodenum and jejunum [Citation23]. After the transformation of enterocytes and hepatocytes, the bioactive folate THF and 5-methyl-THF play important roles in cellular monocarbonic acid metabolism. Folic acid is an essential cofactor for the synthesis of pyrimidine and purine, two compounds that are the basic building blocks of nucleotides that make up DNA and RNA. Folic acid is therefore essential for the synthesis, repair and replication of DNA. The pathogenesis of benign prostatic hyperplasia is closely related to the increase of cell proliferation and decrease of apoptosis as well as growth factors, and relevant studies have also shown that folic acid may have a complex relationship with these biological changes. Ren Huanhuan found that folate deficiency may hinder cell proliferation and induce apoptosis in L02 cells. Lv Fenghua discovered that the sole application of folate or combined with vitamin B12 can effectively elevate the levels of serum and vascular endothelial growth factor (VEGF) (p < 0.05), while also altering VEGF protein expression (p < 0.05).
Excessive folic acid intake is a problem. Enhanced folic acid treatment was introduced in the US in 1998 to prevent neurological malformations in people prone to neural tube defects [Citation24,Citation25]. The folic acid status is affected by many factors. However, the dietary folic acid intake alone cannot determine the risk population for folic acid deficiency [Citation26]. After entering the human body, dietary folic acid undergoes cellular transformation and metabolism, and every 10% increase in folic acid intake from natural foods increases the level of folic acid in the blood and RBC by 6%–7% [Citation27]. However, the program does not limit the amount and variety of fortified foods, and exceeding the tolerable upper limits of folic acid absorption and metabolism can have potentially adverse effects [Citation28], such as high folic acid consumption disrupting cholesterol homeostasis in the liver [Citation29], and folic acid supplementation and higher serum levels are associated with an increased risk of prostate cancer [Citation30].
A possible mechanism underlying the linear relationship between RBC folate and BPH is the difference in folate metabolism caused by aging. Study has shown that the folic acid content in the blood and RBCs increase when older American men use or do not use supplements containing folic acid, suggested that folate metabolism may be affected by aging [Citation31]. Hussain Mohamad Awwad [Citation32] reported that the possible mechanism of reduced plasma S-adenosylmethionine levels in older patients with advanced prostate cancer and younger patients with low-grade prostate cancer was the difference in folate metabolism. Folic acid metabolizing enzymes play a key role in folic acid metabolism, they affect the absorption, utilization and biological activity of folic acid. Study [Citation33] has shown that folic acid deficiency and excessive intake may interfere with cell replication and survival, and reduced enzyme efficiency may also interfere with nutrient metabolism and affect disease risk. Studies investigating the relationship between folic levels and BPH are limited. A case-control study from Italy have found that folic acid is not associated with the risk of benign prostatic hyperplasia [Citation34]. However, our study suggested that high levels of RBC folate may be a risk factor for BPH in the US.
In view of the linear positive correlation between high levels of erythrocyte folate and the risk of BPH in elderly men, elderly men in the United States should adjust their daily life by adjusting diet, supplemting folic acid, and regularly monitoring erythrocyte folic acid levels. First, eat a balanced diet, avoid excessive intake of folic acid rich foods, avoid excessive alcohol, because alcohol may interfere with folic acid absorption and metabolism; Avoid blind use of folic acid supplements, it is recommended taking them under the guidance of a doctor or dietitian to determine the appropriate dosage. At the same time, folic acid levels and folic acid metabolism enzymes should be monitored.
This study had several limitations. First, because of the design of the transverse trial, this analysis alone could not prove the cause of BPH. The definition of benign prostatic hyperplasia in this study was obtained through a questionnaire survey, which may have retrospective bias. Second, the change in red folate levels in patients with BPH remained unclear and required further investigation. Third, it was impossible to identify the interference factors that were not included in the trial. Despite the limitations of our study, it had some strengths. It explored the association of RBC folate, a better indicator of long-term folate intake,rather than dietary folate, with BPH.
5. Conclusions
In conclusion, our study showed a clear association of RBC folate levels with BPH. Interestingly, we observed a significant dose-response trend, in which the OR increased with increasing RBC folate concentrations, suggesting that high RBC folate may be a risk factor for BPH in US men. Although our results added to the limited evidence on this topic, further studies are needed, not only because of the limitations of our study, but also because of the potential for BPH status alteration by controlling high levels of RBC folate.
Ethical approval and patient consent statement
The “NHANES” campaign was approved by the Ethics Review Committee of the NCHS, and a personal informed consent was signed before participating in the study.
Authors’ contributions
YuanPeng Huang: drafted the work and revised it critically for important content; Tingting Chen: collected and analyzed the data, prepared figures and tables and Write the manuscript. All writers have read and approved the final manuscript.
Acknowledgments
We are grateful to Professor Liu Jie from the Renmin Hospital of Renmin University of China, who provided data support, research, and design consultation for this study and reviewed it.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
This provides a set of public data for this study. All data applied in this study can be searched on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm), and more detailed analysis data can be obtained by contacting the corresponding author.
Additional information
Funding
References
- Foo KT. What is a disease? What is the disease clinical benign prostatic hyperplasia (BPH)? World J Urol. 2019;37(7):1293–1296. doi: 10.1007/s00345-019-02691-0.
- Langan RC. Benign prostatic hyperplasia. Prim Care. 2019;46(2):223–232. doi: 10.1016/j.pop.2019.02.003.
- Zhu C, Wang D-Q, Zi H, et al. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil Med Res. 2021;8(1):64. doi: 10.1186/s40779-021-00359-8.
- Ng M, Baradhi KM. Benign Prostatic Hyperplasia. In StatPearls; StatPearls Publishing: treasure Island (FL), 2023.
- Launer BM, McVary KT, Ricke WA, et al. The rising worldwide impact of benign prostatic hyperplasia. BJU Int. 2021;127(6):722–728. doi: 10.1111/bju.15286.
- Yang L, Liu Z, Peng Z, et al. Exposure to di-2-ethylhexyl phthalate and benign prostatic hyperplasia, NHANES 2001-2008. Front Endocrinol. 2021;12:804457. doi: 10.3389/fendo.2021.804457.
- Devlin CM, Simms MS, Maitland NJ. Benign prostatichyperplasia - What do We know? BJU Int. 2021;127(4):389–399. doi: 10.1111/bju.15229.
- S, S, Ea P, I K, Wc, et al. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. A f Clin Nutr. 2002;75(4):689–697. doi: 10.1093/ajcn/75.4.689.
- Das K, Buchholz N. Benign prostate hyperplasia and nutrition. Clin Nutr ESPEN. 2019;33:5–11. doi: 10.1016/j.clnesp.2019.07.015.
- Zhou H, Xu M, Pan Y, et al. The association between several serum micronutrients and benign prostatic hyperplasia: results from NHANES 2003-2006. Prostate. 2024;84(2):212–220. doi: 10.1002/pros.24641.
- Shulpekova Y, Nechaev V, Kardasheva S, et al. The concept of folic acid in health and disease. Molecules. 2021;26(12):3731. doi: 10.3390/molecules26123731.
- Wald NJ, Hoffbrand AV. Mandatory UK folic acid fortification. Lancet. 2021;398(10315):1961–1962. doi: 10.1016/S0140-6736(21)02447-8.
- Crider KS, Qi YP, Yeung LF, et al. Folic acid and the prevention of birth defects: 30 years of opportunity and controversies. Annu Rev- Nutr. 2022;42(1):423–452. doi: 10.1146/annurev-nutr-043020-091647.
- Benavides-Lara A, Fernández-Sánchez O, Barboza-Argüello M, et al. Integrated surveillance strategy to support the prevention of neural tube defects through food fortification with folic acid: the experience of Costa Rica. Childs Nerv Syst. 2023;39(7):1743–1754. doi: 10.1007/s00381-023-05837-z.
- Field MS, Stover PJ. Safety of folic acid. Ann N Y Acad Sci. 2018;1414(1):59–71. doi: 10.1111/nyas.13499.
- Patel KR, Sobczyńska-Malefora A. The adverse effects of an excessive folic acid intake. Eur J Clin Nutr. 2017;71(2):159–163. doi: 10.1038/ejcn.2016.194.
- Selhub J, Rosenberg IH. Excessive folic acid intake and relation to adverse health outcome. Biochimie. 2016;126:71–78. doi: 10.1016/j.biochi.2016.04.010.
- Zhu J, Jia Y-L, Luo Y-W, et al. Effect of maternal folic acid supplementation on prostatitis risk in the rat offspring. Int Urol Nephrol. 2018;50(11):1963–1973. doi: 10.1007/s11255-018-1969-8.
- Zhou L, Wen X, Peng Y, et al. Red blood cell folate and severe abdominal aortic calcification: results from the NHANES 2013-2014. Nutr Metab Cardiovasc Dis. 2021;31(1):186–192. doi: 10.1016/j.numecd.2020.08.020.
- Inamura S, Ito H, Shinagawa T, et al. Serum C-reactive protein level is not associated with prostatic inflammation but with overactive detrusor in patients with benign prostatic hyperplasia. Neurourol Urodyn. 2019;38(6):1728–1736. doi: 10.1002/nau.24051.
- Wang Y-B, Yang L, Deng Y-Q, et al. Causal relationship between obesity, lifestyle factors and risk of benign prostatic hyperplasia: a univariable and multivariable mendelian randomization study. J Transl Med. 2022;20(1):495. doi: 10.1186/s12967-022-03722-y.
- Mentch SJ, Locasale JW. One-carbon metabolism and epigenetics: understanding the specificity. Ann N Y Acad Sci. 2016;1363(1):91–98. doi: 10.1111/nyas.12956.
- Malinowska AM, Schmidt M, Kok DE, et al. Ex vivo folate production by fecal bacteria does not predict human blood folate status: associations between dietary patterns, gut microbiota, and folate metabolism. Food Res Int. 2022;156:111290. doi: 10.1016/j.foodres.2022.111290.88
- Viswanathan M, Treiman KA, Kish-Doto J, et al. Folic acid supplementation for the prevention of neural tube defects: an updated evidence report and systematic review for the US preventive services task force. JAMA. 2017;317(2):190–203. doi: 10.1001/jama.2016.19193.
- Chen M-Y, Rose CE, Qi YP, et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr. 2019;109(5):1452–1461. doi: 10.1093/ajcn/nqz027.
- Dietary Intake of Folate and Assessment of the Folate Deficiency Prevalence in Slovenia Using Serum Biomarkers - PubMed. https://pubmed.ncbi.nlm.nih.gov/34836112/. (accessed 2023-07-20).
- Hiraoka M, Kagawa Y. Genetic polymorphisms and folate status. Congenit Anom. 2017;57(5):142–149. doi: 10.1111/cga.12232.
- Ledowsky C, Mahimbo A, Scarf V, et al. Women taking a folic acid supplement in countries with mandatory food fortification programs may be exceeding the upper tolerable limit of folic acid: a systematic review. Nutrients. 2022;14(13):2715. doi: 10.3390/nu14132715.
- Leclerc D, Jelinek J, Christensen KE, et al. High folic acid intake increases methylation-dependent expression of lsr and dysregulates hepatic cholesterol homeostasis. J Nutr Biochem. 2021;88:108554. doi: 10.1016/j.jnutbio.2020.108554.
- Pieroth R, Paver S, Day S, et al. Folate and its impact on cancer risk. Curr Nutr Rep. 2018;7(3):70–84. doi: 10.1007/s13668-018-0237-y.
- Rycyna KJ, Bacich DJ, O Keefe DS. Divergence between dietary folate intake and concentrations in the serum and red blood cells of aging males in the United States. Clin Nutr. 2016;35(4):928–934. doi: 10.1016/j.clnu.2015.07.002.
- Awwad HM, Ohlmann C-H, Stoeckle M, et al. Serum concentrations of folate vitamers in patients with a newly diagnosed prostate cancer or hyperplasia. Clin Biochem. 2018;56:41–46. doi: 10.1016/j.clinbiochem.2018.04.011.
- Marosi K, Agota A, Végh V, et al. [The role of homocysteine and methylenetetrahydrofolate reductase, methionine synthase, methionine synthase reductase polymorphisms in the development of cardiovascular diseases and hypertension]. Orv Hetil. 2012;153(12):445–453. doi: 10.1556/OH.2012.29326.
- Tavani A, Longoni E, Bosetti C, et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: a case-control study from Italy. Eur Urol. 2006;50(3):549–554. doi: 10.1016/j.eururo.2005.11.027.