Abstract
Objectives
To evaluate the efficacy of a novel approach to achieve the optimal penile erection during the penile doppler ultrasound (PDU) examination, which was oral sildenafil combined alprostadil injection.
Materials and Methods
A total of 60 ED patients were enrolled in our prospective study, and they were randomly assigned to two group with different PDU order. The approaches assisted the PDU included two models, mode A meaning injection of 15 μg alprostadil and model B meaning oral sildenafil 100 mg plus injection of 15 μg alprostadil. The PDU parameters were measured continuously before induced erection, and 5, 10, 15, 20, 25 min.
Results
Each group included 30 ED patients with similar clinical characteristics. After pooling the results together, the PSV, EDV, and RI were all improved significantly, when adding the oral sildenafil administration to assist PDU. Also, the clinical response of oral sildenafil administration plus alprostadil injection was better than that in alprostadil injection alone (p = 0.016). The arterial ED were decreased from 31.67% to 15.00% with the P value 0.031, and the mixed ED was also decreased statistically (23.33% vs 8.33%, p = 0.024).
Conclusion
Oral sildenafil administration plus alprostadil injection could improve the diagnostic accuracy of PDU.
Introduction
Erectile Dysfunction (ED), characterized by the repeated or continuous inability to achieve and/or maintain a penile erection adequate for satisfactory sexual intercourse [Citation1], represents a global issue affecting male sexual health. It has been reported that the prevalence of ED was approximately 150 million in 1995, with projections indicating an increase to 322 million by the year 2035 [Citation2]. Initially, scholars believed that Erectile Dysfunction (ED) solely impacted patients’ self-esteem and the relationship between couples. Over time, an increasing number of studies have linked ED with future cardiovascular diseases (CVD) [Citation3], viewing it as a sentinel symptom of subclinical coronary artery disease (CAD) and a risk factor for CVD [Citation4]. Among all causes of ED, ED of vascular origin, known as vasculogenic ED, is the most significant, accounting for 60%-80% of all cases of ED [Citation5]. The Penile Doppler Ultrasound (PDU) is regarded as the “gold standard” for assessing penile hemodynamics and categorizing subtypes of vasculogenic Erectile Dysfunction (ED) [Citation6], such as arterial ED (AED), venogenic ED (VED), and mixed-vasculogenic ED [Citation7]. Crucially, several studies have reported a strong association between PDU hemodynamic parameters, including Peak Systolic Velocity (PSV), End Diastolic Velocity (EDV), and Resistive Index (RI), and the risk of future cardiovascular diseases (CVD), with PSV being particularly emphasized [Citation8]. Therefore, conducting precise PDU examinations is crucial for ED patients when a potential vasculogenic cause of ED is suspected (e.g. diabetes mellitus, renal transplantation, multiple concomitant CV risk factors and/or overt peripheral vascular disease, and poor responders to oral therapy) [Citation9].
The PDU examination requires patients to achieve sufficient penile erection. Optimal erectile hardness is primarily attained through the injection of vasoactive agents into the penis. Unfortunately, anxiety, fear, and other psychological factors often hinder patients from achieving optimal erectile hardness during PDU examinations [Citation10]. To overcome this, several methods have been employed to assist in achieving the best quality erection, which include switching from injection of vasoactive agents to oral administration of phosphodiesterase type 5 inhibitors (PDE5i), increasing the dosage of vasoactive agents, administering additional doses of the agents, and providing audio-visual sexual stimulation (AVSS) [Citation11–13]. Multiple studies have confirmed the effectiveness of AVSS in assisting PDU examinations [Citation6,Citation11]. However, findings regarding the use of vasoactive agents and PDE5i have been contradictory. On one hand, larger dosages of vasoactive agents may lead to serious side effects such as prolonged erection and ecchymosis. On the other hand, the sole use of PDE5i might not effectively induce an erection [Citation12]. Therefore, there is an urgent need to identify a more effective assistant method for PDU examinations to enhance diagnostic accuracy for vasculogenic ED, beyond the use of AVSS.
In 2010, Yang et al. introduced a novel approach for inducing penile erection during PDU examinations, involving the oral administration of tadalafil 20 mg combined with a low dose of the vasodilator papaverine [Citation14]. Compared to tadalafil, sildenafil offers a quicker onset of action when taken orally on an empty stomach and maintains its effect for approximately 4–6 h [Citation15]. For intra-cavernous injection (ICI), alprostadil (also known as PGE1) demonstrates similar efficacy in inducing penile erection as papaverine, a nonspecific PDE inhibitor [Citation16]. However, alprostadil is associated with fewer required doses and lesser side effects than papaverine, while achieving comparable efficacy [Citation12,Citation17]. Considering these factors, our plan is to use oral sildenafil combined with alprostadil injection as a method to induce penile erection during PDU examinations, aiming to enhance the diagnostic accuracy for vasculogenic ED.
In this prospective study, we aim to evaluate the effectiveness of combining oral sildenafil with alprostadil injection as an adjunct to PDU examinations, specifically to see if this combination can alter PDU hemodynamic parameters and affect final diagnoses. Our hypothesis is that the combination of oral sildenafil and alprostadil injection exhibits superiority in facilitating PDU examinations, thereby altering hemodynamic parameters and final diagnoses. We conducted the present study in accordance with the STARD reporting checklist.
Materials and methods
Study population
The present prospective study was approved by our hospital’s ethical committee (Quick-PJ2020-07-15), and study implementation strictly followed the approved protocol. All enrolled patients have signed the informed consent for the study in advance. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). A total of 60 men complaining of ED were randomly selected from our andrology and urology outpatient department from September 2019 to November 2021. All patients should be 18 years old or elder, had a fixed heterosexual partner with a regular intercourse (at least once a week), and had a history of ED for at least 6 months. The potentially eligible patients would be excluded from the study if they met anyone of the following exclusion criteria: (1) they had advanced age (≥80 y); (2) the 5-question short version of the International Index of Erectile Function (IIEF-5) score was greater than 21; (3) they had any contraindications of sildenafil or alprostadil. All enrolled patients went medical and sexual history taking, physical examination, and blood sampling (checking fasting glucose, serum cholesterol, triglycerides, and total testosterone). Of course, the IIEF-5 was also completed by all patients in an interview-based fashion.
Sample size calculation
The required sample size of the present diagnostic study [Citation18] was calculated based on previous studies of PDU diagnostic accuracy, and an effect size (Cohen’s d) of 0.5 or more with 80% power (two-sided significance level of 5%) [Citation11]. Eventually, a minimum of 68 PDU examinations were needed to achieve the adequate sensitivity. We enrolled 60 patients performing 120 PDU examinations, enough to verify our hypothesis, and also considered the possibility of failure to complete our 2 times PDU tests.
Study protocol
All patients went two sessions of PDU with an interval of at least a week, and the penile erection was induced by two different modes. For model A, each patient was injected with 15 μg of alprostadil before PDU. For model B, each patient would receive 100 mg Sildenafil one hour before PDU, and would be injected with 15 μg of alprostadil when conducting PDU. All the patients should be fasting before taking sildenafil citrate. During both models, the audio-video sexual stimulation would be supplied to all patient by a glasses-type video monitor with earphones, allowing patients choosing the types of pornographic films casually. During PDU tests, the penile vascular parameters would be measured and recorded continuously before alprostadil administration and 5, 10, 15, 20, and 25 min afterwards until achieving the maximum erection. Meanwhile, we used the erection hardness score (EHS) to measure the penile rigidity. Actually, the EHS was based on the Schramek grading system (1990) [Citation19]. The original rigidity was classified from grade V (complete erection) to Grade I (no response). The EHS was classified from grade I to grade IV. After adjustment with the EHS, Patients were considered as Schramek Grade V when an Erection Hardness Score (EHS) was Grade IV lasted for more than 10 min, and Grades IV, III, and II when EHS Grades III, II and I, respectively, lasted for more than 10 min [Citation16]. After the PDU examinations, all patients were stayed for 4 h to observe the emergence of this adverse events. To minimizing the sequential bias of different modes, patients were randomly divided into 2 groups with different examination sequences, and each group had 30 patients as follows: group 1, model A to model B and group 2, model B to model A. The randomization allocation was achieved by using sealed opaque envelop method containing the assigned groups. When comparing the PDU parameters and diagnostic accuracy, all data from the two group would be pooled together. It means that the difference between model A and model B would be calculated rather than group 1 and group 2, ultimately.
Penile doppler ultrasound protocol
The PDU was conducted by a senior radiologist using an Aixplorer™ Ultrasound System (Supersonic Imagine S.A., Aix-en-Provence, France) with a SuperLinear™SL15- 4 probe (Frequency: 4–15 MHz). To avoid the subjective influences on the results, the radiologist was blinded to the patients’ groups and induction models. All examinations were performed in a quiet, isolated, and warm room with gentle lights. Before examinations, the patient could preview the device supplying the pornographic films and choose his favorite videos. Then the patients lied in a supine position, with the penis stretched to the abdomen sightly. The ultrasound probe was placed at the penoscrotal junction ventrally, and the cavernous arterial blood flow was measured along the longitudinal plane. First, the penis was screened carefully to exclude corporeal fibrosis or Peyronie’s plaques. Then, the PSV, EDV and RI of bilateral cavernous artery were measured before penile erection. After penile erection induced by different models, these parameters were measured and recorded at times of 5-min intervals. For each measurement, a mean value of the PDU parameters of left and right cavernous artery would be calculated and recorded. For each patient, the highest PSV and corresponding EDV during PDU test were noted as diagnostic basis. For PSV, it was the highest spot along the length region of the spectrum, and the EDV is the lowest spot of the spectrum. The RI was calculated as PSV-EDV/PSV, representing the arterial vascular resistance and compliance. The cavernous arterial inflow was considered normal if the highest PSV of cavernous arteries was greater than 35 cm/s. The cavernous veno-occlusive function was considered normal if the PSV was greater than 35 cm/s and the lowest EDV was less than 5 cm/s and/or the RI greater than 0.85. The AED was diagnosed by PDU when the highest PSV was less than 35 cm/s and the lowest EDV was less than 5 cm/s. The VED was diagnosed by PDU when the highest PSV was greater than 35 cm/s and the lowest EDV was greater than 5 cm/s. The mixed-vasculogenic ED was diagnosed by PDU when the highest PSV was less than 35 cm/s and the lowest EDV was greater than 5 cm/s.
Statistical analysis
The quantitative variables are expressed as Mean ± SD when normally distributed, and as the median (quartiles) when non-normal distributed. The Kolmogorov–Smirnov test was used to evaluate variable distributions. For comparison between two groups, the independent sample Student’s t-test was used for parametric variants and the Mann-Whitney test was used for non-parametric variants. For comparison between two models, the paired t-test was used. The ED subtypes diagnosed by PDU with different models were compared by chi-square test. All the tests were considered statistically significant when the P value was less than 0.05. The statistical analyses were performed by the SPSS software (SPSS; v. 25; IBM, Chicago, USA).
Results
Population characteristics
Finally, a total of 60 ED patients completed the PDU studies with a mean age of 40.77 ± 6.73 years, equally divided into the two groups. The mean IIEF-5 score in study population was 13.42 ± 3.53. The baseline characteristics between the two groups were similar with no obviously statistical differences. The results were shown in .
Table 1. Participant characteristics (N = 60).
Penile doppler ultrasound parameters
There were significant increases of PDU parameters between model A and model B. The highest PSV under model A was 37.87 ± 13.37 cm/s. While under model B, it increased to 45.56 ± 15.79 cm/s with the P value <0.001. For EDV and RI, the independent effect of model B still presented (EDV was 3.31 ± 4.79 cm/s in model A and 1.52 ± 5.46 cm/s in model B [p < 0.001]; RI was 0.89 ± 0.13 in model A and 0.95 ± 0.11 in model B [p < 0.001]). The detailed penile hemodynamic parameters were showed in . The clinical responses assessed by the EHS were also recorded during PDU. The EHS showed 16 (26.7%) patients achieved a full erection (Grade IV) in model A, less significantly than 32 (51.7%) patients in model B. For grade III and II, the number of patients in model A was 23 (38.3%) and 21 (35.0%) respectively, while the number of patients in model B was 13 (21.7%) and 15 (26.7%). In order to exclude the sessions order on the PDU parameters, we compared the PDU parameters between two groups with different model order simultaneously. The session orders did not have independent effect on PDU parameters, and the results were showed in . Firstly, the panel A, panel B, and panel C showed the maximum PSV, minimum EDV, and maximum RI values between subgroup 1 and subgroup 2. No difference was found among these PDU parameters both in subgroup 1 and in subgroup 2. And then, the panel A compared the maximum PSV values between model A and model B both in subgroup 1 and subgroup 2. The comparisons were also conducted in minimum EDV and maximum RI as showed in panel B and panel C. Contrary to the former, statistical difference was found among these PDU parameters both in model A and in model B.
Figure 1. PDU parameters according group and model. (Panel A: maximum PSV; Panel B: minimum EDV; Panel C: RI).
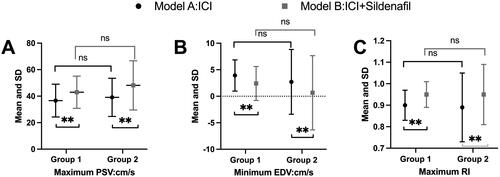
Table 2. PDU parameters between 2 models.
ED subtype diagnoses
The final diagnosis of ED subtype under different models were demonstrated in . The proportion of patients with different PDU diagnoses was changed significantly after model B intervention. For model A, the number of patients that were diagnosed as AED, VED, Mix-vasculogenic ED, and non-vasculogenic ED was 19(31.67%), 14(23.33%), 14(23.33%), and 13(21.67%) respectively. While under model B, the number was changed to 9(15.00%), 23(38.33%), 5(8.33%), and 23(38.33%). In details, the distribution of AED between two models was statistically different with the P value as 0.031. Similarly, the distribution of Mix-vasculogenic ED also showed significant difference with the P values as 0.024. Apart from the two subtypes, the distribution of VED and non-vascular ED did not show obvious differences, and the P value was 0.075 and 0.066 respectively.
Table 3. Diagnosis classification between 2 models.
Complications due to different assistant models
We also recorded the complications due to different assistant models during PDU. The unavoidable side effect of PDU test was prolonged erection. Only four patients in model A and five patients in model B experienced it but de-tumesce after 2 h observation without pharmacological treatments. For other side effects of sildenafil including dizziness and flushing, four patients complained these side effects during model B tests. The adverse effects secondary to the different models were showed in .
Table 4. Complications due to different models.
Discussion
The PDU was first introduced as a diagnostic tool for vascular ED by Lue et al. in 1985, utilizing the injection of papaverine to induce penile erection [Citation20]. The PDU provides dynamic evaluation of penile vessels beyond the routine anatomical assessment achievable by other imaging modalities such as CT or MRI [Citation21]. Over time, this technology has become the “gold standard” for measuring penile hemodynamic parameters to evaluate penile arterial function and veno-occlusive function [Citation6]. This is in contrast to selective internal pudendal arteriography and dynamic infusion cavernosometry and cavernosography (DICC), which, despite their effectiveness, are characterized by significant invasiveness and complexity [Citation12]. However, achieving optimal erection with the injection of vasoactive agents can be challenging for patients, resulting in a high rate of false positive results, estimated at 47% [Citation22].
Building upon existing strategies for inducing sufficient penile erection [Citation14,Citation23,Citation24], we have introduced a novel approach in our study to enhance penile erection adequacy and improve diagnostic accuracy. This novel approach involves the combination of oral sildenafil administration with alprostadil injection. Our study demonstrated that compared to the standard method of inducing erection with alprostadil injection alone, oral sildenafil administration combined with alprostadil injection significantly improved all penile hemodynamic parameters measured during PDU examinations in ED patients. Moreover, the diagnostic classification of vascular ED was statistically altered, with a decrease in AED from 31.67% to 15.00% and in Mixed-ED from 23.33% to 8.33%. Additionally, the clinical response, as assessed by the EHS, was statistically superior with oral sildenafil administration plus alprostadil injection compared to alprostadil injection alone (p = 0.016). Furthermore, our study implemented appropriate AVSS assistance during PDU examinations, creating an examination environment that closely resembles the patient’s home scenario, thereby alleviating anxiety [Citation25]. Notably, AVSS is a crucial factor for the effectiveness of sildenafil citrate in inducing penile erection in ED patients, as it inhibits sympathetic tone and increases parasympathetic influence [Citation26,Citation27].
Sequential bias refers to a situation where the results of a second PDU examination with ICI appear better than those of the first examination with the same ICI in the same patients. In our study, to mitigate sequential bias, all patients were randomly divided into two subgroups with different orders of PDU tests. Our findings indicated that the results of PDU examination were consistent when using the same assistance mode. Similar observations were reported in studies by Pescatori et al. (2000) [Citation28] and Carneiro et al. (2020) [Citation11]. These results underscore the notion that repeated PDU examinations may not lead to statistically significant changes in PDU results. The minimal alterations observed in PDU parameters could be attributed to patients’ adaptation to ICI over time.
The guidelines in urology and andrology do not universally recommend PDU for all ED patients due to the effectiveness of oral PDE5i such as sildenafil and tadalafil [Citation9,Citation29]. However, PDU can provide valuable insights for aging patients to further investigate related CVD risks [Citation30]. The dynamic penile PSV value, whether in a flaccid state or an erectile state, can accurately identify associated vascular diseases with an accuracy of approximately 80% [Citation31,Citation32]. Some scholars advocate for the use of the RigiScan test, intended to differentiate between psychogenic and organic ED [Citation33], to diagnose and classify vascular ED and its subtypes [Citation34]. However, there is a lack of reliable evidence connecting RigiScan test parameters with future CVD risks, diminishing the prognostic value of PDU for CVD, despite the RigiScan test being more reliable and less invasive [Citation35]. In a recent article, Morgado et al. raised questions about the utility of PDU compared to ICI tests [Citation36]. They compared the prognostic value of ICI tests and PDU for treatment efficacy and patient satisfaction with sildenafil administration. The study found that the prognostic value of PDU did not exceed that of ICI tests. However, ICI tests are still less likely to demonstrate the patient’s vascular status and its connection with future CVD.
Sildenafil, a type of PDE5i, is considered the first-line therapy for ED due to its effectiveness, ranging from 60% to 80% [Citation37]. Penile erection is stimulated by NO-cGMP, where increased cGMP levels lead to intracellular calcium reduction, resulting in penile smooth muscle relaxation [Citation38]. Sildenafil enhances the relaxant effect of cGMP by inhibiting its degradation in corporeal smooth muscle cells [Citation39]. Alprostadil, also known as prostaglandin E1 (PGE1), is the first and only drug approved for ED treatment via ICI, with a success rate of approximately 85% [Citation9]. Alprostadil’s pharmacology involves binding to the PGE1 receptor in corporeal smooth muscle cells, activating adenylate cyclase enzymes, and increasing ATP conversion to cAMP, leading to intracellular calcium reduction and subsequent smooth muscle relaxation in the corpus cavernosum [Citation16]. AVSS induces penile erection mainly through neurological mechanisms, increasing parasympathetic tone to release NO, which in turn relaxes smooth muscle cells via the NO-cGMP pathway [Citation27]. AVSS closely mimics the physiological process of erection during sexual intercourse with a partner. However, achieving optimal erection during PDU solely with AVSS assistance is challenging, potentially compromising the reliability of PDU results. Therefore, various combinations of the aforementioned approaches have been explored in ED patients to improve diagnostic accuracy. In our study, we combined these approaches to develop a novel method for inducing erection. Our findings demonstrate a statistically significant improvement in the diagnostic accuracy of PDU, supported by scientific evidence.
Prior to our study, Yang et al. proposed a method combining tadalafil 20 mg and papaverine 15 mg to augment Penile Doppler Ultrasound (PDU) examinations [Citation14]. They found that the PSV were improved a lot comparing to the tadalafil and ICI of papaverine (39.36 ± 11.27 cm/s [tadalafil plus papaverine], 37.17 ± 17.14 cm/s [papaverine], 28.91 ± 17.04 cm/s [tadalafil]). However, they did not report changes in the classification of vasculogenic ED. Additionally, they only measured PDU parameters for 15 min, which may not have been the optimal time for patients to achieve the best erection [Citation24]. In our study, we opted to use sildenafil instead of tadalafil, as studies have shown that sildenafil induces effective erections sooner than tadalafil [Citation13]. Similarly, we replaced papaverine with alprostadil due to its higher response rate compared to papaverine [Citation40] (alprostadil 67.2% vs. papaverine 32.8%).
The improved accuracy of the mixed model can be attributed to elevated levels of cAMP and cGMP, the second messengers responsible for inducing relaxation of smooth muscle cells in the corpus cavernosum. Park et al. conducted a study measuring cAMP and cGMP levels in cavernous and peripheral venous blood, finding that the mixed model generated higher levels of cAMP and cGMP compared to single models alone. This resulted in a more effective erection induction with the mixed model [Citation41].
We bolstered our conclusions with several strengths. Firstly, session orders were randomized to minimize sequential bias, aligning with previous related studies. Secondly, we expanded the sample size beyond that of related studies. Thirdly, all PDU examinations were conducted by the same senior radiologist, assisted by the same urologist, with the radiologist blinded to patient characteristics. These measures were taken to enhance the authenticity of results, mitigating the influence of subjective factors and reducing variability among observers. However, our study also had limitations. Firstly, sildenafil citrate placebo was not utilized in Group A to exclude placebo effects, a primary objective of our study. Secondly, we did not compare examination results between ICI of alprostadil and oral sildenafil administration due to concerns about the lower efficacy of oral sildenafil and the increased examination frequency for ED patients. Thirdly, vasoactive agents were not re-administered for PDU examinations. Additionally, the lack of significant findings in the diagnostic accuracy of PDU, particularly regarding “non-vascular ED,” may be attributed to the inadequate statistical power resulting from the sample size. Moving forward, further large-scale studies are needed to validate our conclusions and extend this novel assistant approach to the standardized operation process of PDU
Conclusion
In conclusion, the combination of oral sildenafil administration and alprostadil injection has shown promise in enhancing the diagnostic accuracy of Penile Doppler Ultrasound (PDU). This approach not only increases hemodynamic parameters but also alters the final subtype diagnoses of vasculogenic Erectile Dysfunction (ED). The novel method presents a potential alternative for assisting PDU examinations, offering improved erection quality akin to that experienced during intercourse.
Ethics approval and consent to participate
All procedures used in the present study were approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Quick-PJ2020-07-15).
Author contributions
YY Zhang, H J, XL Feng, and XS Zhang designed the study. YY Zhang, H J, XL Feng, GD Liu, and XW collected, analyzed and interpreted the clinical data. YY Zhang, H J, XL Feng, X W, and GD Liu wrote and revised the manuscript. YY Zhang, X W, and GD Liu conducted PDU tests. All authors confirmed the integrity of the prospective data and analysis. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to everyone participating in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data used for the present study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- NIH consensus conference. Impotence. NIH consensus development panel on impotence. Jama. 1993;270(1):83–90.
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences [research support, U S gov’t, P H S]. BJU Int. 1999;84(1):50–56. doi:10.1046/j.1464-410x.1999.00142.x.
- Zhao B, Hong Z, Wei Y, et al. Erectile dysfunction predicts cardiovascular events as an independent risk factor: a systematic review and meta-analysis [meta-analysis research support, non-U S gov’t systematic review]. J Sex Med. 2019;16(7):1005–1017. doi:10.1016/j.jsxm.2019.04.004.
- Montorsi P, Ravagnani PM, Galli S, et al. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease [review]. Am J Cardiol. 2005;96(12B):19M–23M. doi:10.1016/j.amjcard.2005.07.006.
- Lue TF, Mueller SC, Jow YR, et al. Functional evaluation of penile arteries with duplex ultrasound in vasodilator-induced erection [research support, non-U S gov’t]. Urol Clin North Am. 1989;16(4):799–807. doi:10.1016/S0094-0143(21)01813-9.
- Kuo YC, Liu SP, Chen JH, et al. Feasability of a novel audio-video sexual stimulation system: an adjunct to the use of penile duplex doppler ultrasonography for the investigation of erectile dysfunction. J Sex Med. 2010;7(12):3979–3983. doi:10.1111/j.1743-6109.2009.01583.x.
- Li M, Ma Z, Zhang XL, et al. Significance of blood lipid parameters as effective markers for arteriogenic erectile dysfunction. Andrology. 2020;8(5):1086–1094. doi:10.1111/andr.12776.
- Mulhall J, Teloken P, Barnas J. Vasculogenic erectile dysfunction is a predictor of abnormal stress echocardiography. J Sex Med. 2009;6(3):820–825. doi:10.1111/j.1743-6109.2008.01087.x.
- Salonia A, Bettocchi C, Boeri L, et al. European association of urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80(3):333–357. doi:10.1016/j.eururo.2021.06.007.
- Aversa A, Sarteschi LM. The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. J Sex Med. 2007;4(5):1437–1447. doi:10.1111/j.1743-6109.2007.00546.x.
- Carneiro F, Nascimento B, Miranda EP, et al. Audiovisual sexual stimulation improves diagnostic accuracy of penile doppler ultrasound in patients with erectile dysfunction. J Sex Med. 2020;17(2):249–256. doi:10.1016/j.jsxm.2019.11.263.
- Nascimento B, Miranda EP, Terrier JE, et al. A critical analysis of methodology pitfalls in duplex doppler ultrasound in the evaluation of patients with erectile dysfunction: technical and interpretation deficiencies. J Sex Med. 2020;17(8):1416–1422. doi:10.1016/j.jsxm.2020.05.023.
- Yang Y, Hu JL, Ma Y, et al. Pharmaco-induced erections for penile color-duplex ultrasound: oral PDE5 inhibitors or intracavernosal injection? [comparative study randomized controlled trial research support, non-U S gov’t]. Int J Impot Res. 2012;24(5):191–195. doi:10.1038/ijir.2012.15.
- Yang Y, Hu JL, Ma Y, et al. Oral tadalafil administration plus low dose vasodilator injection: a novel approach to erection induction for penile color duplex ultrasound [comparative study randomized controlled trial research support, non-U S gov’t]. J Urol. 2011;186(1):228–232. doi:10.1016/j.juro.2011.02.2691.
- Gong B, Ma M, Xie W, et al. Direct comparison of tadalafil with sildenafil for the treatment of erectile dysfunction: a systematic review and meta-analysis. Int Urol Nephrol. 2017;49(10):1731–1740. doi:10.1007/s11255-017-1644-5.
- Xuan XJ, Bai G, Zhang CX, et al. The application of color doppler flow imaging in the diagnosis and therapeutic effect evaluation of erectile dysfunction. Asian J Androl. 2016;18(1):118–122. doi:10.4103/1008-682X.155533.
- Chochina L, Naudet F, Chéhensse C, et al. Intracavernous injections in spinal cord injured men with erectile dysfunction, a systematic review and Meta-Analysis. Sex Med Rev. 2016;4(3):257–269. doi:10.1016/j.sxmr.2016.02.005.
- Schmidt SAJ, Lo S, Hollestein LM. Research techniques made simple: sample size estimation and power calculation. J Invest Dermatol. 2018;138(8):1678–1682. doi:10.1016/j.jid.2018.06.165.
- Xuan XJ, Sun P, Teng JB, et al. [Value of degree diagnosis with color doppler flow imaging in the treatment of male erectile dysfunction]. Zhonghua Yi Xue Za Zhi. 2009;89(40):2835–2838.
- Lue TF, Hricak H, Marich KW, et al. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed doppler spectrum analysis. Radiology. 1985;155(3):777–781. doi:10.1148/radiology.155.3.3890009.
- Butaney M, Thirumavalavan N, Hockenberry MS, et al. Variability in penile duplex ultrasound international practice patterns, technique, and interpretation: an anonymous survey of ISSM members. Int J Impot Res. 2018;30(5):237–242. doi:10.1038/s41443-018-0061-3.
- Teloken PE, Park K, Parker M, et al. The false diagnosis of venous leak: prevalence and predictors. J Sex Med. 2011;8(8):2344–2349. doi:10.1111/j.1743-6109.2011.02298.x.
- Bassiem MA, Ismail IY, Salem TA, et al. Effect of intracavernosal injection of prostaglandin E1 on duration and rigidity of erection in patients with vasculogenic erectile dysfunction: is it dose dependent? Urology. 2021;148:173–178. doi:10.1016/j.urology.2020.09.030.
- Wang J, Wang J, Liu Q, et al. Time-effect of penile color duplex doppler ultrasound for diagnosing vascular erectile dysfunction. Med Ultrason. 2020;22(1):37–42. doi:10.11152/mu-2059.
- Park K, Kwon DD, Oh BR, et al. Efficacy of virtual glasses in audio-visual sexual stimulation during penile color duplex doppler ultrasonography [comparative study]. Eur Urol. 2002;41(1):62–65. doi:10.1016/s0302-2838(01)00012-4.
- Lei Q, Wang D, Liu C, et al. Comparison of the efficacy and safety of low-intensity extracorporeal shock wave therapy versus on-demand sildenafil for erectile dysfunction. Transl Androl Urol. 2021;10(2):860–868. doi:10.21037/tau-20-1069.
- Montorsi F, Guazzoni G, Barbieri L, et al. The effect of intracorporeal injection plus genital and audiovisual sexual stimulation versus second injection on penile color doppler sonography parameters [clinical trial comparative study]. J Urol. 1996;155(2):536–540. doi:10.1016/S0022-5347(01)66443-7.
- Pescatori ES, Silingardi V, Galeazzi GM, et al. Audiovisual sexual stimulation by virtual glasses is effective in inducing complete cavernosal smooth muscle relaxation: a pharmacocavernosometric study [clinical trial comparative study randomized controlled trial. Int J Impot Res. 2000;12(2):83–88. doi:10.1038/sj.ijir.3900458z.
- Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200(3):633–641. doi:10.1016/j.juro.2018.05.004.
- De Rocco Ponce M, Vecchiato M, Neunhaeuserer D, et al. Association between penile color doppler ultrasonography and cardiorespiratory fitness in patients with vascular erectile dysfunction. Sex Med. 2021;9(3):100347–100347. doi:10.1016/j.esxm.2021.100347.
- Corona G, Fagioli G, Mannucci E, et al. Penile doppler ultrasound in patients with erectile dysfunction (ED): role of peak systolic velocity measured in the flaccid state in predicting arteriogenic ED and silent coronary artery disease. J Sex Med. 2008;5(11):2623–2634. doi:10.1111/j.1743-6109.2008.00982.x.
- Ioakeimidis N, Vlachopoulos C, Rokkas K, et al. Dynamic penile peak systolic velocity predicts major adverse cardiovascular events in hypertensive patients with erectile dysfunction [evaluation study]. J Hypertens. 2016;34(5):860–868. doi:10.1097/HJH.0000000000000877.
- Zou Z, Lin H, Zhang Y, et al. The role of nocturnal penile tumescence and rigidity (NPTR) monitoring in the diagnosis of psychogenic erectile dysfunction: a review. Sex Med Rev. 2019;7(3):442–454. doi:10.1016/j.sxmr.2018.10.005.
- Elhanbly S, Elkholy A. Nocturnal penile erections: the role of RigiScan in the diagnosis of vascular erectile dysfunction. J Sex Med. 2012;9(12):3219–3226. doi:10.1111/j.1743-6109.2012.02954.x.
- Zhang Y, Zhang Z, Zhang N. Role of RigiScan parameters in differentiation of vascular erectile dysfunction. Andrologia. 2020;52(10):e13620. doi:10.1111/and.13620.
- Morgado A, Diniz P, Silva CM. Is there a point to performing a penile duplex ultrasound? J Sex Med. 2019;16(10):1574–1580. doi:10.1016/j.jsxm.2019.07.010.
- Christiansen E, Guirguis WR, Cox D, et al. Long-term efficacy and safety of oral viagra (sildenafil citrate) in men with erectile dysfunction and the effect of randomised treatment withdrawal [clinical trial randomized controlled trial research support, non-U S gov’t]. Int J Impot Res. 2000;12(3):177–182. doi:10.1038/sj.ijir.3900527.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32(4):379–395. doi:10.1016/j.ucl.2005.08.007.
- Andersson KE. PDE5 inhibitors – pharmacology and clinical applications 20 years after sildenafil discovery [review]. Br J Pharmacol. 2018;175(13):2554–2565. doi:10.1111/bph.14205.
- Porst H. The rationale for prostaglandin E1 in erectile failure: a survey of worldwide experience. J Urol. 1996;155(3):802–815. doi:10.1016/S0022-5347(01)66315-8.
- Park JK, Park JS, Jeon SB, et al. Why a combined intracavernosal injection with trimix and oral sildenafil is reliable therapy in the ultrasonographic evaluation of erectile dysfunction [randomized controlled trial research support, non-U S gov’t]. BJU Int. 2008;102(8):993–997. doi:10.1111/j.1464-410X.2008.07712.x.

