Abstract
Objective
To assess various management options for renal angiomyolipoma (AML) to guide clinical practice.
Methods
A single center retrospectively reviewed an AML series from 2002 to 2022. The image reports and chart reviews of patients who received two abdominal scans at least 6 months between the first and last scans were assessed.
Results
A total of 203 patients with 209 tumors were identified and followed up for a median of 42.6 months. Active surveillance (AS) was the most frequently selected option (70.9% of cases). Interventions were required for 59 AMLs, of which 20 were treated with embolization, 29 with partial nephrectomy, 9 with radical nephrectomy, and 1 with radiofrequency (RF) ablation. The median size of the lesions at intervention was 5 cm. The average growth rate of the lesions was 0.12 cm/year, and there was a significant difference in the average growth rate of lesions ≤4 cm and those >4 cm (0.11 vs. 0.24 cm/year; p = 0.0046).
Conclusion
This series on AMLs confirms that lesions >4 cm do not require early intervention based on size alone. Appropriately selected cases of renal AML can be managed by AS.
KEYWORDS: Angiomyolipoma; active surveillance; embolization; nephrectomy; nephron-sparing surgery
Introduction
Renal angiomyolipoma (AML) is a benign kidney tumor belonging to the PEComa family. It is formed by perivascular epithelioid cells composed of mature adipose tissue, poorly developed vessels, and smooth muscle [Citation1,Citation2]. Most AMLs are sporadic and approximately 20% of them are hereditary problems caused by alterations in TSC1 or TSC2 [Citation2]. Clinical presentation ranges from incidental detection to severe conditions such as spontaneous non-traumatic bleeding leading to shock and hypotension (also known as Wunderlich’s syndrome) [Citation2,Citation3].
Currently, AML’s natural history is largely unknown. Randomized controlled trials are not available in the decision-making process for optimal AML management, and only a few case series and comparative studies exist. Various clinics and doctors utilize distinct follow-up regimens and treatment protocols to handle renal AML [Citation4]. The widely acknowledged treatment threshold remains controversial, with previous recommendations suggesting a 3–4 cm threshold [Citation5]. Advances in minimally invasive therapies have increased the number of options for AML management. Over the past few years, the changing trend in AML management has gained knowledge from follow-up studies. In most cases of AML, the European Association of Urology (EAU) recommends surveillance, and treatment is only recommended for large tumors, women of childbearing age, and those who may lack access to emergency care [Citation6,Citation7]. However, further research on larger AMLs is necessary to refine active surveillance (AS) strategies for these lesions.
This study aimed to guide follow-up and treatment protocols by analyzing the long-term natural history and changes in AML trends over the last 20 years of diagnosis at our institution. Furthermore, we sought to delineate the clinical presentation, patient characteristics, and treatment outcomes using single-center experience in AML management.
Materials and methods
Study design, patient selection, and image analysis
We conducted a retrospective single-institution study. From March 2001 to February 2023, the radiology record screening system identified patients with renal AML using keyword scanning. The search terms “angiomyolipoma” or “AML,” and “kidney” were used in patients who underwent abdominal imaging (computed tomography [CT], ultrasonography [US], and magnetic resonance imaging [MRI]). Two authors worked separately to extract the data analyzed by an independent statistician for possible bias. Patients who underwent two or more abdominal imaging tests over a minimum of 6 months between the first and last scans were included in the detailed image report review. The chart review also included patients who underwent follow-up imaging at outside of our institution. We obtained complete medical records to extract the clinical, radiographic, and historical details.
Furthermore, we examined interventions such as selective arterial embolization (SAE), radiofrequency (RF) ablation, and surgery that included open and laparoscopic partial or radical nephrectomies and the reasons for such interventions. Patients were excluded from our study if they had a follow-up period of less than 6 months, incomplete follow-up data, or were lost to follow-up. We defined follow-up time per lesion as the number of months between the date of the initial report of the lesion and the date of the last imaging scan. To determine the average growth per year, the growth difference between the last and first scans was divided by the number of years between the scans (Supplementary Figure 1).
Ethics
The Institutional Review Board (IRB) approved this study and the Ethics Committee (Approval number: 2022/0755 dated 21 December 2022).
Statistical analysis
We categorized the size of lesions by assessing their maximal axial diameter, specifically as follows: ≤2 cm, >2–4 cm, >4–6 cm, and >6 cm. We used the Mann-Whitney U test to compare continuous data (for the greatest lesion per patient) and the Fisher exact or Chi-square test to compare categorical data. The number of years between the date of the earliest available scan that reported the mass and the date of the last scan was used to compute the follow-up duration per lesion. We divided the difference in growth from the final scan to the first scan by the number of years between scans. This was done to calculate the annual growth rate. For assessing statistical significance, a p value of <0.05 was considered significant. The analysis was performed using SPSS version 23.0 (SPSS Inc., Chicago, IL).
Results
Demographics
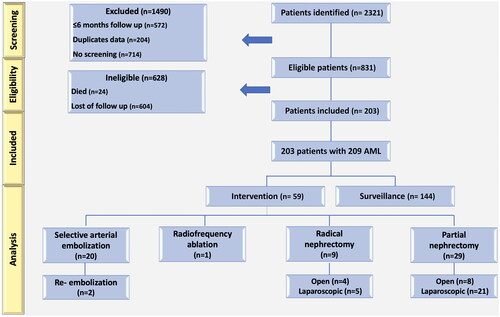
We identified 2321 patients with radiology reports of renal AML. Of these, 2118 were excluded as duplicates, deceased, lost to follow-up, or had a follow-up of fewer than 6 months. A total of 203 patients with 209 AMLs with a median follow-up of 46.3 months (range 6–163.7 mo) were included in our study (Supplementary Table 1). shows the entire cohort represented by a flow diagram. 156 (76.8%) patients were females and 47 (23.2%) were males. A total of 144 (70.9%) and 59 (29.1%) of the patients underwent AS and intervention, respectively. presents a comparison of demographic data between patients who received surveillance and those who received intervention.
Table 1. The comparison of patients demographics between surveillance and intervention groups.
Interventions and surveillance
The primary treatment included 38 surgical procedures, of which 29 (14.3%) were (6 on-clamp, 23 off-clamp) nephron-sparing (8 open, 21 laparoscopic) and 9 (4.4%) were nephrectomies (4 open, 5 laparoscopic). The median patient age was 59 (25–83), and the median tumor size was 4.7 cm (range 1.1–23 cm). Nineteen (50%) patients underwent surgery due to a size >4 cm, whereas 10 (26.3%) patients were suspected of malignancy based on preoperative imaging.
Twenty (33.9%) with sporadic AML underwent SAE. A median age of 62.5 years was observed among patients receiving SAE (range 31–81 years) and a median tumor size of 6.8 cm (range 1.1–15.6). SAE was indicated in three (15%) patients with bleeding, and four (20%) patients with pain. Two patients presented with acute hematuria and underwent a successful emergency SAE. The median follow-up for patients was 7.8 months (range 6–149 months), and the median size of AMLs decreased from 6.8 (1.1–15.6) to 5.7 (0.6–13) cm by 15.4% at the latest follow-up (p = 0.003). Only one of the patients was a known tuberous sclerosis complex (TSC) and polycystic kidney patient, who was being followed by nephrology for multiple small AMLs and treated with SAE (Supplementary Figure 2).
In addition, there was no significant change between the serum creatinine before and after all actively treated patients (0.78 ± 0.9 mg/dL vs. 0.88 ± 0.7 mg/dL, p = 0.224). There was a significant difference in intervention rates between those presenting with lesions measuring ≤4 cm and those presenting with lesions measuring >4 cm (33.8%, n:20/59 vs. 66.1%, n:39/59; p < 0.001). Upon comparing AMLs across all size groups requiring intervention, we found that AMLs measuring ≤2 cm necessitated fewer interventions than AMLs measuring >2–4 cm, >4–6 cm, and >6 cm (p ≤ 0.001). There was no significant difference between AMLs measuring >2–4 cm and >4–6 cm (p = 0.269). Nevertheless, when comparing AMLs measuring >4–6 cm with those >6 cm, we observed that AMLs >6 cm required a greater number of interventions (p = 0.037). Patients treated surgically or by embolization included more symptomatic patients at presentation (54.2%, n:32/59 vs. 15.9%, n:23/144; p < 0.001) than those treated conservatively. We also found 26 (12.8%) >4–6 cm and 35 (17.2%) ≥6 cm AMLs, and the symptomatic rates of these AMLs were 4.4% (n = 9) and 10.3% (n = 21), respectively. Patients with sporadic AML were analyzed using logistic regression to identify factors predicting intervention needs ().
Table 2. The coefficients of the significant variables in the multivariate logistic regression analysis for the prediction of the intervention.
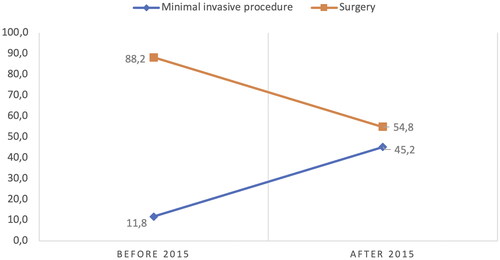
We analyzed the treatment approaches in AML management after stratifying the interventions by date (the year the interventional radiology clinic opened) and found that surgery was the most common AML management intervention, with 15 (88.2%) of the 17 interventions performed before 2015. After 2015, we observed a shift towards predominantly minimally invasive interventions, with 18 (42.8%) SAE and 1 RF (2.8%). Since then, several advances have been made in minimally invasive methods for managing AML. In our center, SAE has become the first-line interventional method since 2015 ().
Our series reported that AS was also the most common management option for 70.9% (n = 144) of the patients. The patients’ mean age was 63 years (range, 14–90 years), and the median tumor size was 1.5 cm (0.5–19). The natural history of the disease for each patient was followed to identify patients during the surveillance period. A total of 121 patients (84%) remained asymptomatic. The mean follow-up durations were 39.3 months. Of the total patients with AML, 1159 scans were performed during the follow-up period at the end of the study. Most of these were US (40.8%), and the rest (60.2%) were CT or MRI. The median number of scans per patient was determined to be 3.7 (ranging between 2 and 15). The median screening frequency was 0.78/year (ranging from 1 to 5).
Size and growth rate
We analyzed all untreated AMLs to measure their natural growth rates. In addition, we included patients who underwent the intervention and those whose follow-up dimensions were measured. In our series, the median growth rate from the first to the last scan was 0.12 cm (0–2.42). As a result, the median growth rate for patients with AMLs larger than 4 cm was 0.24 cm/year, significantly higher than the median growth rate for patients with AMLs smaller than 4 cm of 0.11 cm/year (p = 0.046). Sex (p = 0.673), age (p = 0.081), and BMI (p = 0.498) did not affect the growth rate.
Discussion
Current guidelines recommend monitoring smaller tumors, but there is not any size threshold to suggest intervention. Though a lack of the existence of a common consensus is still a problem, there is limited data on the long-term outcomes of AS for larger renal AMLs. Most studies on AMLs have focused on surgical management, and there is a lack of well-designed studies investigating the safety and efficacy of AS.
Surveillance
AS has emerged as an approach that allows careful monitoring of lesions over time, to avoid unnecessary surgery. During the follow-up period, we reported growth rates according to AML size: AMLs <4 cm had a growth rate of 0.11 cm/year, and AMLs >4 cm had a growth rate of 0.24 cm/year. Many other studies, however, have reported growth rates comparable to ours, such as those reported by Chan et al. (0.1 cm/year), MacLean et al. (0.015 cm/year), Bhatt et al. (0.02 cm/year) as well as Mues et al. (0.08 cm/year) [Citation4,Citation8–10].
Several studies have revealed that a larger initial tumor size, similar to ours, is linked to a faster growth rate [Citation9,Citation11]. This suggests that larger AMLs may require a more aggressive follow-up protocol than smaller AMLs. Although there is no consensus on the ideal scanning modality or interval according to our series, AS is the preferred option for the management of renal AML in 71% of cases. This means that nearly two-thirds of patients diagnosed with AML prefer to undergo regular monitoring instead of immediate treatment.
Intervention
The concern of urologists is that AMLs larger than 4 cm are more likely to rupture and cause life-threatening bleeding, so most of the studies conducted to date have compared AML classifications of ≤4 and ≥4 cm and reported results according to this classification [Citation10]. However, many studies have reported that this traditional 4 cm size cannot be the threshold value [Citation12]. Nelson and Sanda reviewed 13 series of symptomatic AMLs >4 cm and found that the treatment rate ranged from 52% to 94% [Citation13]. In our study, we stratified the patients according to the size of renal AML. There were 26 patients (12.8%) >4–6 cm and 35 patients (17.2%) ≥6 cm AML, and intervention required to 6.8% (n = 14) for 4–6 cm and 12.3% (n = 25) for ≥6 cm. The majority of patients with larger AMLs (>6 cm) require treatment within two years of presentation, either because of symptomatology or patient preference. Similarly, Ouzaid’s study identified an increased risk of bleeding in lesions of >6 cm in diameter and reported that historical criteria based on size less than or larger than 4 cm may lead to overtreatment by up to 65% [Citation5]. Despite the small number of cases and wide range of AML discrimination of 4–8 and ≥8 cm, Dickinson et al. advocate treatment of AML >8 cm electively before symptoms or complications occur [Citation14]. We advocate that changing the threshold size from 4 to 6 cm allows for a more precise outcome and may decrease morbidity. Additionally, despite the lack of consensus, patients with larger AML lesions are required to reinforce AS strategies.
Changing spectrum, strengths, and limitations
The intervention trend in renal AML management has changed over the years with the exploration of safer, less invasive approaches, and follow-up experiences. Historically, surgery has been the primary treatment option for high-risk AML patients. De Luca identified 53 kidneys with AML; all lesions >4 cm were surgically treated and 30% underwent total nephrectomy [Citation15]. In our series, in the early years of AML treatment before 2015, the fear of malignancy continued to be a major concern for some patients with uncertain imaging findings. This has led to the development of many surgical interventions. Thus, while partial/radical nephrectomy was dominant in the initial years of AML management due to the development of imaging methods and the proven effectiveness and safety of minimally invasive treatments, minimally invasive interventions were the first in line in the last decade. Furthermore, off-clamp partial nephrectomy appears to preserve renal function at long-term follow-up for patients with complex masses, whereas clamping may result in impaired renal function over time [Citation16–18].
According to Sookorman et al. SAE was associated with a size reduction rate of 28%; however, the number of secondary interventions was higher and elective SAEs were lower than in our study (20 cases vs. 12 cases) [Citation19]. This has led to a significant decrease in the number of surgical interventions, as well as a decrease in recovery times and hospital stays. As with previous studies, our study demonstrated that AML size was significantly reduced after SAE and kidney function was not significantly altered [Citation20,Citation21].
Our study’s retrospective design, absence of histopathological data, and variability in radiological reports were its limitations. In addition, US measurements cannot be standardized. Case identification with search terms may have missed fat-poor AMLs. To measurement of growth, we excluded cases with fewer than two images this could be interpreted as a bias against cases with fewer imaging sessions. Acknowledging the restrictions of our study, we believe the outcomes are significant in regard to AML management and its course. The exploration of a single institution’s experience with renal AMLs supplied a greater understanding of the native chronology and rate of growth. This extensive series, with a follow-up of in more than 3 years, demonstrates that earlier intervention for renal AML is superfluous, disregarding the traditional limit of 4 cm.
Conclusion
Given the increasing number of renal mass lesions being discovered incidentally due to the widespread use of axial imaging, we suggest that for patients with asymptomatic or mildly symptomatic AMLs, even if they are larger than 4 cm, AS should remain the initial treatment option for such lesions, even if they become larger than 4 cm, as it allows for monitoring potential changes without the need for immediate intervention.
Informed consent
Informed consent could not be taken from the patients due to the retrospective design of the study.
Author contributions
A. K. Project development, data collection, data analysis, interpretation of data, manuscript writing. A. Y. Project development, manuscript writing, manuscript editing, supervision. A. I. Study concept and design, data collection, manuscript editing. F. K. Administrative, technical, or material support, data collection. K. N. B. Statistical analysis, interpretation of data M. C. Draft of manuscript writing, critical revision of the manuscript. Ö. A. Manuscript editing, interpretation of data. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download Zip (8.8 MB)Acknowledgment
None.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The datasets are available from the corresponding author.
Additional information
Funding
Notes
* The preliminary results of this study were presented as a podium presentation at the 38th Annual European Association of Urology (EAU 23) Congress in Milan.
References
- Kapoor A, Girard L, Lattouf JB, et al. Evolving strategies in the treatment of tuberous sclerosis complex-associated angiomyolipomas (TSC-AML). Urology. 2016;89:19–26. Mar doi: 10.1016/j.urology.2015.12.009.
- Kim JW, Kim JY, Ahn ST, et al. Spontaneous perirenal hemorrhage (wunderlich syndrome): an analysis of 28 cases. Am J Emerg Med. 2019;37(1):45–47. doi: 10.1016/j.ajem.2018.04.045.
- Nason GJ, Morris J, Bhatt JR, et al. Natural history of renal angiomyolipoma favors surveillance as an initial approach. Eur Urol Focus. 2021;7(3):582–588. doi: 10.1016/j.euf.2020.06.004.
- Mues AC, Palacios JM, Haramis G, et al. Contemporary experience in the management of angiomyolipoma. J Endourol. 2010;24(11):1883–1886. doi: 10.1089/end.2010.0223.
- Ouzaid I, Autorino R, Fatica R, et al. Active surveillance for renal angiomyolipoma: outcomes and factors predictive of delayed intervention. BJU Int. 2014;114(3):412–417. doi: 10.1111/bju.12604.
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810. doi: 10.1016/j.eururo.2019.02.011.
- Oesterling JE, Fishman EK, Goldman SM, et al. The management of renal angiomyolipoma. J Urol. 1986;135(6):1121–1124. Jun doi: 10.1016/s0022-5347(17)46013-7.
- Chan KE, Chedgy E, Bent CL, et al. Surveillance imaging for sporadic renal angiomyolipoma less than 40 mm: lessons learnt and recommendations from the experience of a large district general hospital. Ann R Coll Surg Engl. 2018;100(6):480–484. doi: 10.1308/rcsann.2018.0040.
- Maclean DF, Sultana R, Radwan R, et al. Is the follow-up of small renal angiomyolipomas a necessary precaution? Clin Radiol. 2014;69(8):822–826. doi: 10.1016/j.crad.2014.03.016.
- Bhatt JR, Richard PO, Kim NS, et al. Natural history of renal angiomyolipoma (AML): most patients with large AMLs >4cm can be offered active surveillance as an initial management strategy. Eur Urol. 2016;70(1):85–90. doi: 10.1016/j.eururo.2016.01.048.
- Swärd J, Henrikson O, Lyrdal D, et al. Renal angiomyolipoma-patient characteristics and treatment with focus on active surveillance. Scand J Urol. 2020;54(2):141–146. doi: 10.1080/21681805.2020.1716066.
- Fernández-Pello S, Hora M, Kuusk T, et al. Management of sporadic renal angiomyolipomas: a systematic review of available evidence to guide recommendations from the european association of urology renal cell carcinoma guidelines panel. Eur Urol Oncol. 2020;3(1):57–72. doi: 10.1016/j.euo.2019.04.005.
- Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002;168(4 Pt 1):1315–1325. doi: 10.1016/S0022-5347(05)64440-0.
- Dickinson M, Ruckle H, Beaghler M, et al. Renal angiomyolipoma: optimal treatment based on size and symptoms. Clin Nephrol. 1998;49(5):281–286.
- De Luca S, Terrone C, Rossetti SR. Management of renal angiomyolipoma: a report of 53 cases. BJU Int. 1999;83(3):215–218. doi: 10.1046/j.1464-410x.1999.00932.x.
- Simone G, Capitanio U, Tuderti G, et al. On-clamp versus off-clamp partial nephrectomy: propensity score-matched comparison of long-term functional outcomes. Int J Urol. 2019;26(10):985–991. doi: 10.1111/iju.14079.
- Tuderti G, Brassetti A, Mastroianni R, et al. Expanding the limits of nephron-sparing surgery: surgical technique and mid-term outcomes of purely off-clamp robotic partial nephrectomy for totally endophytic renal tumors. Int J Urol. 2022;29(4):282–288. doi: 10.1111/iju.14763.
- Bertolo R, Simone G, Garisto J, et al. Off-clamp vs on-clamp robotic partial nephrectomy: perioperative, functional and oncological outcomes from a propensity-score matching between two high-volume centers. Eur J Surg Oncol. 2019;45(7):1232–1237. doi: 10.1016/j.ejso.2018.12.005.
- Sooriakumaran P, Gibbs P, Coughlin G, et al. Angiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treated. BJU Int. 2010;105(1):101–106. doi: 10.1111/j.1464-410X.2009.08649.x.
- Lin L, Wang C, Pei R, et al. Prophylactic selective arterial embolization for renal angiomyolipomas: efficacy and evaluation of predictive factors of significant shrinkage. Int Urol Nephrol. 2018;50(10):1765–1770. doi: 10.1007/s11255-018-1953-3.
- Ramon J, Rimon U, Garniek A, et al. Renal angiomyolipoma: long-term results following selective arterial embolization. Eur Urol. 2009;55(5):1155–1161. doi: 10.1016/j.eururo.2008.04.025.