Abstract
Background
Whether erectile dysfunction (ED) leads to considerable stress for affected men remains unclear? In this study, we investigated whether organic ED (OED) is associated with increased risks of herpes zoster (HZ) and postherpetic neuralgia (PHN).
Methods
A representative subset of Taiwan’s National Health Insurance Research Database was employed for this study. Enrollees with OED from the years 2000 to 2018 were selected. To ensure comparability between the case and control groups, we implemented 1:1 propensity score matching based on age, index year, comorbidities, and medications.
Results
The case group included 20,808 patients with OED, while the control group consisted of 20,808 individuals without OED. The OED group exhibited a significantly elevated risk of HZ (adjusted hazard ratio [aHR] = 1.74) and PHN (aHR = 1.56) compared to the non-OED group.
Conclusions
Men experiencing OED seem to face elevated risks of HZ and PHN compared to those without OED. ED may serve as a warning sign for individuals at HZ risk.
1. Introduction
Erectile dysfunction (ED) is characterized by the difficulty of achieving and maintaining an erection. The reported global prevalence of ED ranges from 3% to 76.5%; this discrepancy can be primarily attributed to the differences in screening tools used for ED assessment in population-based studies [Citation1]. ED has traditionally been associated with older age. A meta-analysis of six studies involving 8,653 Asian individuals estimated age-specific ED prevalence to be: 15.1% for individuals aged 20–29 years, 29.6% for those aged 30–39 years, 40.6% for those aged 40–49 years, 54.3% for those aged 50–59 years, and 70.0% for those aged 60–69 years [Citation2]. In a meta-analysis of 25 studies with 48,254 Chinese participants, Wang et al. [Citation3] investigated ED prevalence in mainland China and reported the overall prevalence of ED was 49.69%; however, the prevalence was estimated to be 14.19%, 44.60% and 49.91% on the basis of self-reports, the Chinese Index of Erectile Function, and the International Index of Erectile Function-5, respectively. Furthermore, 32.54% of all individuals with ED had mild ED, 9.86% had moderate ED, and 13.97% had severe ED. Li et al. [Citation4] conducted a self-reported internet survey among the general population in the United Kingdom and revealed that 41.5% of the 12,490 men surveyed reported experiencing ED, with 7.5% having severe ED. The authors also discovered that men with ED were more likely than men without ED to have one or more comorbid chronic conditions (73.7% vs. 47.7%), including diabetes mellitus (DM) (15.9% vs. 6.1%), hypertension (31.8% vs. 16.3%), and depression (24.3% vs. 14.6%). In a preceding investigation, Naya et al. examined the relationship between ED and not only DM and hypertension but also cardiac disease and chronic renal failure. The study revealed associations of these chronic diseases with both the incidence and severity of ED [Citation5].
Herpes zoster (HZ) caused by the reactivation of the varicella-zoster virus (VZV); this reactivation is typically triggered by factors such as advanced age [Citation6] or compromised immune system [Citation7,Citation8]. Moreover, it has been demonstrated that stress induced by specific medical conditions is associated with an increased incidence of HZ [Citation9–13]. Upon reactivation, VZV spreads along the nerve, leading to the development of painful rashes. Postherpetic neuralgia (PHN) is a common complication of HZ characterized by nerve pain in the area of the rash which can persist for weeks, months, or even years after the resolution of the rash.
Does ED influence the incidence of HZ? In a recent investigation conducted by Pozzi et al. 86.2% and 13.8% of a cohort comprising 2009 consecutive patients seeking treatment for ED were categorized as presenting with organic ED (OED) and psychogenic ED, respectively [Citation14]. Given that the predominant etiology among patients with ED is organic in nature, our study specifically focuses on the organic subtype. The objective of our study is to explore the hypothesis that OED exerts stress on affected individuals, potentially precipitating the onset of HZ and subsequent PHN.
2. Materials and methods
2.1. Data source
Taiwan’s National Health Insurance Research Database (NHIRD) was established in 1995 and encompasses approximately 99% of the Taiwanese population. The NHIRD contains comprehensive medical records, which include information on insurance beneficiaries’ outpatient visits, admissions, diagnoses, and prescription medications. In this databases, disease diagnoses are coded according to the diagnostic codes outlined in the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). For this study, we used data from a representative subset of the NHIRD, the Longitudinal Generation Tracking Database (LGTD 2000). This database contains the data of two million randomly sampled beneficiaries who enrolled in Taiwan’s National Health Insurance (NHI) system in the year 2000. The present study was approval by the Research Ethics Committee of China Medical University Hospital (CMUH111-REC2-109-CR-1). Given that the patient data utilized in this investigation were de-identified and derived from the NHIRD, the Institutional Review Board of China Medical University Hospital in Taichung, Taiwan, granted a waiver for the requirement of informed consent.
2.2. Study population
The study period was from January 1, 2000, to December 31, 2018. Patients with diagnostic code (ICD-9-CM: 607.84; ICD-10-CM: N52) of OED were enrolled. For the case group, the index date was determined as the date of the first diagnosis of OED. Random dates within the study period were selected as the index date for the control group. We excluded individuals aged <20 years and those who had been diagnosed as having HZ before the index date and those with psychogenic ED. To ensure comparability between the case and control groups, we conducted 1:1 propensity scores matching for age (with a 5-year interval), index year, comorbidities, and medications.
2.3. Main outcome and confounders
ICD-9-CM and ICD-10-CM diagnostic codes were used to identify patients with newly diagnosed HZ (ICD-9-CM: 053; ICD-10-CM: B02) and PHN (ICD-9-CM: 053.12, 053.13, and 053.19; ICD-10-CM; B02.0, B02.22, B02.23, B02.24, and B02.29). To be eligible for inclusion, a patient had to have ≥2 outpatient visits or ≥1 hospitalization related to these conditions. The end date of the follow-up period was determined by the date of HZ or PHN diagnosis, withdrawal from the NHIRD, death, or December 31, 2018. We included several comorbidities as covariates in the analysis: DM (ICD-9-CM: 250; ICD-10-CM: E08-E13), chronic kidney disease (CKD) (ICD-9-CM: 585; ICD-10-CM: N18), coronary artery disease (CAD) (ICD-9-CM: 410–414; ICD-10-CM: I20-I25), cancer (ICD-9-CM: 140–208; ICD-10-CM: C00-C99, C30-C34, C37-C41, C43-C50, C53-C55, C4A, C7A, D03, and Z51.12), and depression (ICD-9-CM: 296.2, 296.3, 300.4, and 311; ICD-10-CM: F32, F33.0, F33.1, F33.2, F33.3, F33.4, F33.9, and F34.1). These comorbidities were assessed prior to the index date. We also considered the following medications as potential confounding factors: metformin (Anatomical Therapeutic Chemical [ATC] code: A10BA02, A10BD02, A10BD03, A10BD05, A10BD07, A10BD08, A10BD10, A10BD11, A10BD13-A10BD15, and A10BD20), beta-blockers (ATC code: C07), and selective serotonin reuptake inhibitors (SSRIs) (ATC code: N06AB).
2.4. Statistical analysis
Baseline demographic data for categorical variables are presented in terms of the number and percentage values, whereas continuous variables are presented in term of the mean and standard deviation value. Between-group comparisons were performed using the SMD (standardized mean difference). When SMD <0.1, there was no significant difference between the two groups. The Kaplan-Meier survival analysis was performed to plot the cumulative incidence and survival curves. Cox proportional hazards regression models were used to calculate the hazard ratios (HR) and 95% confidence intervals (CI). In the multivariable Cox regression analysis, age, sex, comorbidities, and medications were included as covariates. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA). A p value of <0.05 was considered to be statistically significant.
3. Results
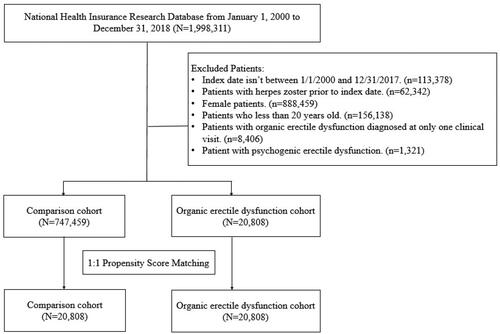
presents the flowchart for patient selection. presents the baseline characteristics of the study cohort. The case group comprised 20808 patients with OED (mean age: 52.96 years), and the control group comprised 20808 individuals without OED (mean age: 53.42 years). Propensity score matching ensured no significant between-group differences in the matched variables.
Table 1. Baseline characteristics for organic erectile dysfunction cohort and control cohort.
Individuals diagnosed with OED exhibited a notably elevated risk of developing HZ compared to those without OED, with an adjusted HR (aHR) of 1.74 (95% CI = 1.62–1.88) (). The log-rank test further affirmed an increased risk of HZ among patients with OED in comparison to those without OED (). In contrast to the 20–39 age group, the likelihood of developing HZ escalated with advancing age, reaching an aHR of 1.88 for individuals aged 40–59 and an aHR of 2.75 for those aged 60 and above. An increased HZ risk was also observed in patients with CAD (aHR = 1.21, 95% CI = 1.10–1.32) and those taking beta-blockers (aHR = 1.19, 95% CI = 1.09–1.29).
Figure 2. (a) Illustrates the cumulative incidence of herpes zoster among patients diagnosed with organic erectile dysfunction compared to those without the condition, where the X-axis denotes time elapsed since initial diagnosis in years, and the Y-axis represents the probability of developing herpes zoster over time. Conversely, (b) presents a similar comparative analysis for the cumulative incidence of postherpetic neuralgia, offering insights into the progression of this complication following herpes zoster.
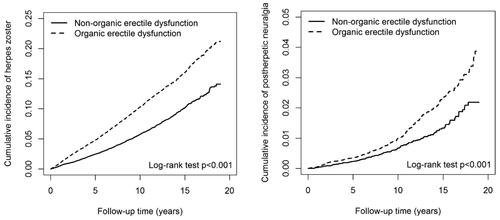
Table 2a. Incidences and hazard ratios of herpes zoster for individuals with and without organic erectile dysfunction by age, comorbidities and medication.
displayed the risk of PHN. Individuals with OED faced a significantly higher risk of PHN compared to those without OED, with an aHR of 1.56 (95% CI = 1.27–1.92). Compared with the 20–39 age group, the risk of developing HZ increased with age progression, reaching an aHR of 2.20 for individuals aged 40–59 and an aHR of 4.31 for those aged 60 and above. Patients taking beta-blockers (aHR = 1.52, 95% CI = 1.20–1.93) also exhibited significantly higher risks of PHN compared to those not taking them. The Kaplan-Meier curves illustrating the cumulative risk of incident PHN revealed a significantly elevated risk among OED patients compared to non-OED patients (log-rank test, p < 0.001; ).
Table 2b. Incidences and hazard ratios of postherpetic neuralgia for individuals with and without organic erectile dysfunction by age, comorbidities and medication.
presents the results of the stratified analysis for HZ. When stratifying by age, comorbidity, and medication, a significant association was noted between an increased HZ risk and OED across all age groups. This association remained significant in patients with (aHR = 1.81, 95% CI = 1.62–2.01) or without (aHR = 1.69, 95% CI = 1.53–1.87) any comorbidity and those with (aHR = 1.69, 95% CI = 1.50–1.91) or without (aHR = 1.78, 95% CI = 1.62–1.95) medication use. This observation suggests that, notwithstanding the presence of comorbidities, individuals with OED exhibit an elevated risk of developing HZ. Furthermore, even in the absence of any comorbid conditions, the risk of HZ remains significantly higher in patients with OED compared to those without such a dysfunction.
Table 3a. Cox proportional hazards regression analysis for the risk of herpes zoster.
displays the outcomes of the stratified analysis for PHN. Within the subgroup of individuals aged 40–59 (aHR = 1.42, 95% CI = 1.04–1.96) and those aged ≥60 years (aHR = 1.75, 95% CI = 1.31–2.33), patients with OED exhibited a higher risk of PHN compared to individuals without OED. The association between OED and PHN risk remained significant in patients with (aHR = 1.52, 95% CI = 1.14–2.02) or without (aHR = 1.62, 95% CI = 1.21–2.17) any comorbidity, and those not taking any medication (aHR = 1.80, 95% CI = 1.36–2.36). This analysis implies that, regardless of comorbid conditions, individuals diagnosed with OED are at an increased risk for the development of PHN. Moreover, this heightened risk persists even in the absence of any comorbidities, indicating that patients with OED are significantly more susceptible to PHN when compared to individuals without such dysfunction.
Table 3b. Cox proportional hazards regression analysis for the risk of postherpetic neuralgia.
4. Discussion
To the best of our knowledge, this study is the first to present evidence for the association of OED with HZ and PHN risks. Our findings indicate that patients with OED are more likely to develop HZ and PHN than are individuals without OED.
ED has been associated with certain medical conditions, including physical (such as DM and CKD) and psychological (such as depression) diseases, which have also been linked to HZ susceptibility. In a comprehensive meta-analysis of 145 studies involving 88577 men, Kouidrat et al. [Citation15] discovered that the overall ED prevalence among men with DM was 52.5% and these men have a 3.62 times higher risk of ED than do their healthy counterparts. Shiferaw et al. [Citation16] conducted a meta-analysis involving 17 studies with 6,002 participants and concluded that prolong DM (>10 years) was associated with a significantly higher risk of ED in individuals with DM (odds ratio [OR] of 2.63). Fan et al. [Citation17] reported that patients with DM for ≥49 months are 3.86 times more likely to develop ED than are those with a relative short DM duration, reflecting a significant association between DM duration and ED. Considering these results, Shiferaw et al. [Citation16] recommended the development of preventive strategies and interventions aimed at mitigating the risk factors associated with ED in individuals with DM to reduce the incidence and effect of ED in this specific population. In a meta-analysis of 4 case-control and 12 cohort studies, Huang et al. [Citation18] stated that patients with DM have a significantly higher HZ risk than does the general population (relative risk: 1.38). Together, these findings suggest associations between ED, DM, and HZ.
ED is also prevalent among men with CKD. This may be because both conditions have similar underlying causes, such as vascular and hormonal abnormalities. In fact, the estimated prevalence of ED among patients with end-stage renal disease can be as high as 70% [Citation19]. Pizzol et al. [Citation20] conducted a meta-analysis of 34 articles involving a total of 5,986 men with CKD; their results indicated a higher ED prevalence in patients with CKD (78%) than those undergoing hemodialysis (77%) or kidney transplantation (64%). These findings highlight the substantial burden of ED among men with CKD. Lai et al. [Citation21] conducted a national retrospective cohort study involving 16,655 individuals with newly diagnosed predialysis CKD and 33310 control individuals to investigate the association between predialysis CKD and HZ occurrence. The authors reported that the overall incidence rate of HZ was 1.4-fold higher in the predialysis CKD group than in the non-CKD group (8.76 and 6.27 cases per 1000 person-years, respectively). The study highlights the importance of considering the effect of CKD on HZ risk and suggests the need for appropriate preventive measures and clinical management for individuals with predialysis CKD. Together, these findings indicate associations between ED, CKD, and HZ.
Yang et al. [Citation22] observed that 79.56% of their 768 patients with ED had depression and patients aged ≤35 years, and those with a prolonged ED (duration >12 months) had a relatively high incidence and severity of depression. In their meta-analysis, Liu et al. [Citation23] revealed an OR of 1.39 for studies analyzing the association between depression and ED risk and an OR of 2.92 for those analyzing the association between ED and depression risk. The authors proposed the implementation of regular screening for depression in patients with ED as well as routine assessment for ED in patients with depression symptoms. By using a rat model of depression, Hong et al. [Citation24] elucidated the mechanisms underlying the association between ED and depression. Their findings indicated that reduced erectile function was associated with dysfunctional dopamine system and dopaminergic synapse signaling pathway. Two nation-wide cohort studies were conducted to investigate the potential association between depression and HZ [Citation25,Citation26]. Liao et al. [Citation25] reported that the incidence of HZ was 1.3-fold higher in individuals with depression than in those without it (4.58 and 3.54 cases per 1,000 person-years, respectively). Similarly, Choi et al. [Citation26] reported that the incidence of HZ was 6.8% in the individuals with depression, and 6.3% in those without it. Both studies also reported an aHR for HZ of approximately 1.1 in individuals with depression, indicating a higher risk of HZ in these individuals than in those without depression. Accordingly, depression is regarded as a risk factor for HZ. Taken together, these findings imply associations between ED, depression and increased HZ risk.
Mori et al. [Citation27] conducted an investigation to delineate the pathophysiological underpinnings of stress-induced ED, employing a rat model for their study. Their research demonstrated that exposure to water immersion stress markedly augmented the contractile reaction of the corpus cavernosum to noradrenaline. Furthermore, it was observed that stress substantially diminished erectile functionality, as evidenced by alterations in intracavernous pressure. The authors posited that the genesis of stress-induced ED can be attributed to the contraction of the corpus cavernosum, which is mediated through the RhoA/Rho kinase signaling pathway. ED serves as a significant source of stress among affected individuals, particularly manifesting in psychological stress. Wang et al.’s [Citation28] research revealed that individuals diagnosed with posttraumatic stress disorder exhibited a significantly elevated risk of ED compared to those without posttraumatic stress disorder, with incidence rates of 47.58 versus 9.03 per 100,000 person-years, respectively. This study underscores the critical link between psychological stress and ED. Rahangdale et al. [Citation29] elucidated the biological mechanisms underpinning the relationship between stress and HZ. They highlighted that psychological stress is implicated in the downregulation of natural killer cells, neutrophils, and lymphocytes, alongside a suppression of Th1 cytokine-mediated cellular immunity, thereby elevating the risk of infection. Further, it posited psychological stress as a significant risk factor for HZ infections. The stress-associated reactivation of the VZV is mediated through the activation of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system pathway, culminating in the release of stress hormones such as cortisol and catecholamines. These hormones contribute to a diminished immune response, thereby facilitating the reactivation of VZV. Consequently, an association between ED and HZ development has been established.
Typically, retrospective cohort studies are considered to provide lower levels of evidence compared to randomized controlled trials due to the potential presence of numerous unknown or uncontrolled confounding factors. Consequently, this study did not definitively establish a causal link between OED and HZ. Nevertheless, within the cohort devoid of these comorbidities, individuals with OED demonstrated an increased susceptibility to developing HZ relative to their counterparts without OED. These observations suggest that OED alone may significantly impact the health of affected individuals.
In this study, the inclusion criteria of requiring patients to have had two or more outpatient visits or at least one hospitalization with the same diagnosis are designed to enhance the accuracy, significance, and reliability of the data used in research. This approach ensures that the diagnosis is not only accurate but also of sufficient severity and importance to warrant study, thus reducing the risk of misclassification and improving the consistency and quality of the research data. Such criteria help in focusing on patients who truly have the condition of interest, thereby facilitating more reliable and reproducible research findings. This study is not without its limitations. These limitations are primarily related to the use of the NHIRD data. First, the use of ICD-9-CM codes precluded the determination of OED severity, which might have influenced the development of HZ and, thus, affected our findings. Second, some patients with ED might not have sought medical treatment; this might have led to under-diagnosis and consequently introduced bias in our results, affecting the generalizability of our findings. Third, the NHIRD does not provide information on lifestyle factors, such as smoking habit, which has been well established to be association with ED [Citation30]. Finally, the diagnoses in this study were made by physicians from various medical specialties; this raises concerns regarding the accuracy of the diagnoses. However, NHI Administration implements a strict evaluation system, conducts random audits, and imposes penalties for inappropriate prescriptions or medication, thus ensuring a certain level of accuracy. Despite these limitations, the large sample size of our study can offer valuable insights. In addition, this study may serve as a reference for clinicians. Future studies should be conducted to address the aforementioned limitations and validate our findings.
5. Conclusions
Men with OED appear to be at higher risks of HZ and PHN than are those without OED. ED may serve as a warning sign for individuals at HZ risk.
Authors’ contributions
K-HW was responsible for conceiving and designing the study, as well as writing the initial draft of the manuscript. W-CH contributed to the study design and provided critical revisions to the manuscript. H-JL conducted the statistical analyses, created figures and tables, and contributed to the interpretation of the results. F-JT offered valuable insights to the idea and project administration. C-YH supervised the study, provided guidance throughout the research process, and approved the manuscript for publication. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
This study received partial support from the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW112-TDU-B-212-144004) and the China Medical University Hospital (DMR-112-087). The authors would like to express their gratitude to the Health Data Science Center, China Medical University Hospital, for providing valuable administrative and technical assistance throughout the research process.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The study’s data were sourced from the National Health Insurance Research Database, facilitated by the Taiwan National Health Insurance Administration. Because of the Personal Data Protection Act, public disclosure of the data is restricted. Nevertheless, researchers interested in utilizing the data for research can submit applications for access via the Taiwan National Health Insurance Administration’s website: http://nhird.nhri.org.tw.
Additional information
Funding
References
- Kessler A, Sollie S, Challacombe B, et al. The global prevalence of erectile dysfunction: a review. BJU Int. 2019;124(4):587–599. doi:10.1111/bju.14813.
- Cheng JY, Ng EM, Chen RY, et al. Prevalence of erectile dysfunction in Asian populations: a meta-analysis. Int J Impot Res. 2007;19(3):229–244. doi:10.1038/sj.ijir.3901517.
- Wang W, Fan J, Huang G, et al. Meta-analysis of prevalence of erectile dysfunction in mainland China: evidence based on epidemiological surveys. Sex Med. 2017;5(1):e19–e30. doi:10.1016/j.esxm.2016.10.001.
- Li JZ, Maguire TA, Zou KH, et al. Prevalence, comorbidities, and risk factors of erectile dysfunction: results from a prospective real-world study in the United Kingdom. Int J Clin Pract. 2022;2022:5229702.
- Naya Y, Mizutani Y, Ochiai A, et al. Preliminary report of association of chronic diseases and erectile dysfunction in Middle-aged men in Japan. Urology. 2003;62(3):532–536. doi:10.1016/s0090-4295(03)00383-2.
- Shiraki K, Toyama N, Shiraki A, et al. Age-dependent trigeminal and female-specific lumbosacral increase in herpes zoster distribution in the elderly. J Dermatol Sci. 2018;90(2):166–171. doi:10.1016/j.jdermsci.2018.01.009.
- Muñoz-Quiles C, López-Lacort M, Díez-Domingo J, et al. Herpes zoster risk and burden of disease in immunocompromised populations: a population-based study using health system integrated databases, 2009-2014. BMC Infect Dis. 2020;20(1):905. doi:10.1186/s12879-020-05648-6.
- McKay SL, Guo A, Pergam SA, et al. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125-134–e134. doi:10.1093/cid/ciz1090.
- Hsu CY, Ke DS, Lin CL, et al. Association between de quervain syndrome and herpes zoster: a population-based cohort study. BMJ Open. 2021;11(12):e046891. doi:10.1136/bmjopen-2020-046891.
- Hsu CY, Ke DS, Lin CL, et al. Plantar fascial fibromatosis and herpes zoster. PLoS One. 2021;16(11):e0259942. doi:10.1371/journal.pone.0259942.
- Hsu CY, Ke DS, Lin CL, et al. Association between lateral epicondylitis and the risk of herpes zoster development. Postgrad Med. 2021;133(1):96–101. doi:10.1080/00325481.2020.1816713.
- Hsu CY, Ke DS, Lin CL, et al. To investigate the risk of herpes zoster in women with endometriosis: a Taiwan national population-based cohort study. Front Med (Lausanne). 2021;8:584322. doi:10.3389/fmed.2021.584322.
- Hsu CY, Ke DS, Lin CL, et al. Risk of herpes zoster infection in men with varicocele. Postgrad Med. 2021;133(6):599–603. doi:10.1080/00325481.2021.1893066.
- Pozzi E, Fallara G, Capogrosso P, et al. Primary organic versus primary psychogenic erectile dysfunction: findings from a real-life cross-sectional study. Andrology. 2022;10(7):1302–1309. doi:10.1111/andr.13212.
- Kouidrat Y, Pizzol D, Cosco T, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34(9):1185–1192. doi:10.1111/dme.13403.
- Shiferaw WS, Akalu TY, Petrucka PM, et al. Risk factors of erectile dysfunction among diabetes patients in africa: a systematic review and meta-analysis. J Clin Transl Endocrinol. 2020;21:100232. doi:10.1016/j.jcte.2020.100232.
- Fan J, Peng T, Hui J, et al. Erectile dysfunction in type 2 diabetes mellitus patients: predictors of early detection and treatment. Urol Int. 2021;105(11-12):986–992. doi:10.1159/000514700.
- Huang CT, Lee CY, Sung HY, et al. Association between diabetes mellitus and the risk of herpes zoster: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107(2):586–597. doi:10.1210/clinem/dgab675.
- Papadopoulou E, Varouktsi A, Lazaridis A, et al. Erectile dysfunction in chronic kidney disease: from pathophysiology to management. World J Nephrol. 2015;4(3):379–387. doi:10.5527/wjn.v4.i3.379.
- Pizzol D, Xiao T, Yang L, et al. Prevalence of erectile dysfunction in patients with chronic kidney disease: a systematic review and meta-analysis. Int J Impot Res. 2021;33(5):508–515. doi:10.1038/s41443-020-0295-8.
- Lai SW, Kuo YH, Lin CL, et al. Risk of herpes zoster among patients with predialysis chronic kidney disease in a cohort study in Taiwan. Int J Clin Pract. 2020;74(10):e13566. doi:10.1111/ijcp.13566.
- Yang Y, Song Y, Lu Y, et al. Associations between erectile dysfunction and psychological disorders (depression and anxiety): a cross-sectional study in a Chinese population. Andrologia. 2019;51(10):e13395. doi:10.1111/and.13395.
- Liu Q, Zhang Y, Wang J, et al. Erectile dysfunction and depression: a systematic review and meta-analysis. J Sex Med. 2018;15(8):1073–1082. doi:10.1016/j.jsxm.2018.05.016.
- Hong ZM, Chen ZL, Feng JL, et al. Mechanistic analysis of erectile dysfunction in a depression rat model. J Int Med Res. 2022;50(5):3000605221100334. doi:10.1177/03000605221100334.
- Liao CH, Chang CS, Muo CH, et al. High prevalence of herpes zoster in patients with depression. J Clin Psychiatry. 2015;76(9):e1099-1104–e1104. doi:10.4088/JCP.14m09311.
- Choi HG, Kim EJ, Lee YK, et al. The risk of herpes zoster virus infection in patients with depression: a longitudinal follow-up study using a national sample cohort. Medicine (Baltimore). 2019;98(40):e17430. doi:10.1097/MD.0000000000017430.
- Mori T, Hotta Y, Nakamura D, et al. Enhancement of the RhoA/rho kinase pathway is associated with stress-realted erectile dysfunction in a restraint water immersion stress model. Physiol Rep. 2021;9(20):e15064. doi:10.14814/phy2.15064.
- Wang SC, Chien WC, Chung CH, et al. Posttraumatic stress disorder and the risk of erectile dysfunction: a nationwide cohort study in Taiwan: PTSD and erectile dysfunction. Ann Gen Psychiatry. 2021;20(1):48. doi:10.1186/s12991-021-00368-w.
- Rahangdale RR, Tender T, Balireddy S, et al. Interplay between stress and immunity triggers herpes zoster infection in COVID-19 patients: a review. Can J Microbiol. 2022;68(5):303–314. doi:10.1139/cjm-2021-0242.
- Verze P, Margreiter M, Esposito K, et al. The link between cigarette smoking and erectile dysfunction: a systematic review. Eur Urol Focus. 2015;1(1):39–46. doi:10.1016/j.euf.2015.01.003.