Abstract
Background
Previous research has shown that testosterone deficiency (TD) increases the risk of anemia, but it is unclear whether anemia affects testosterone levels. This study investigated the influence of anemia on testosterone levels.
Methods
Utilizing data from six NHANES cycles, including demographic, testosterone levels, and hemoglobin concentrations, we employed multivariable-adjusted logistic regression to investigate the relationship between anemia and testosterone levels. Moreover, a two-sample Mendelian randomization (MR) study employing genome-wide association study (GWAS) data examined the causal relationship. Kaplan–Meier survival estimation was used to compared the overall survival (OS) of anemic and nonanemic patients with low testosterone and normal testosterone levels.
Results
The inclusion of 21,786 participants (2318 with anemia and19,468 without anemia) revealed that nonanemic patients exhibited higher testosterone levels than did anemic patients (β = 22.616, 95% CI: 3.873–41.359, p = 0.01807). MR analysis confirmed anemia as a cause of TD (OR = 1.045, 95% CI: 1.020–1.071, p < 0.001). Anemic males with low testosterone had reduced OS compared to those with normal levels (p < 0.001).
Conclusions
Anemia emerged as a potential risk factor for TD, highlighting a bidirectional relationship between these conditions. Additional prospective investigations are essential for the validation and reinforcement of our findings.
1. Introduction
Anemia is a medical condition characterized by a decrease in red blood cells or hemoglobin levels, resulting in reduced oxygen transport in the body [Citation1]. This decrease in oxygen-carrying capacity can result in symptoms such as fatigue, weakness, shortness of breath, pale skin, and various other complications. Approximately, one-third of the world’s population suffers from anemia [Citation2], in older men, unexplained anemia is often associated with low testosterone levels [Citation3].
Testosterone is a primary sex hormone produced by the testes or ovaries and adrenal glands. It plays a crucial role in maintaining normal bodily functions in both men and women [Citation4]. Testosterone is the most abundant endogenous and physiologically important androgen in males and plays a key role in promoting the development of the male reproductive system and maintaining sexual function [Citation4,Citation5]. Although females have much lower serum testosterone levels than males, normal testosterone levels are equally important for female health, contributing to the maintenance of normal libido and sexual function [Citation6]. Low testosterone levels can negatively impact muscle growth, bone density, fat distribution, lipid metabolism, cardiovascular health, cognitive function, and psychological well-being [Citation7]. Testosterone replacement therapy (TRT) has been shown to improve sexual activity, bone density, muscle strength, cognitive function, and depressive symptoms in males with testosterone deficiency (TD) [Citation8–10]. TRT can also improve unexplained anemia [Citation11].
Anemia and TD are two closely related health issues that often coexist, but their causal relationship has not been fully elucidated [Citation3]. Previous research has largely supported the role of testosterone in increasing hemoglobin levels. For example, males generally have higher hemoglobin concentrations than females, which may be attributed to higher circulating testosterone levels in males [Citation12]. Additionally, elderly men have a significantly greater prevalence of anemia than young men, which is associated with an age-related decrease in testosterone levels [Citation13]. This evidence suggests that TD is a risk factor for anemia [Citation3,Citation14]. On the other hand, there is limited research on whether anemia affects testosterone levels. This study aimed to capitalize on the advantages of utilizing a large sample dataset from the National Health and Nutrition Examination Survey (NHANES) to investigate the impact of anemia on testosterone levels. Furthermore, the use of Mendelian randomization (MR) analysis will help validate the causal relationship between these two conditions, providing valuable insights into the reciprocal influence between anemia and TD.
2. Materials and methods
2.1. Study population in the NHANES
The NHANES is a research program that assesses the health and nutritional status of individuals in the United States. The study included approximately 5000 individuals each year, sampled from various states and visiting 15 counties annually. The NHANES database consists of demographics data, dietary data, examination data, laboratory data, and questionnaire data. This study utilized the NHANES database from 1999 to 2004 and 2011 to 2016, excluding data from 2005 to 2010 due to no data on testosterone. The data for this research were sourced from the NHANES database, and its survey protocol received approval from the National Center for Health Statistics (NCHS) Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm). All participants had provided written informed consent.
We included blood testosterone levels as an outcome variable, hemoglobin concentration as an exposure variable, and the following variables as covariates in our analysis: age, sex, race, BMI, waist circumference, marital status, education, hypertension status, diabetes status, low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and MCH concentration (MCHC). Patients with missing values for testosterone and hemoglobin concentrations were excluded, and multiple imputation was employed to fill in missing values for covariates, such as marital status, TC, HDL, LDL, hypertension status, and diabetes status. Anemia was diagnosed according to the World Health Organization (WHO) criteria [Citation1]: hemoglobin < 120 g/L in adult males and <120 g/L in nonpregnant adult females. TD was defined according to the International Society for Sexual Medicine (ISSM) and International Society for the Study of the Ageing Male (ISSAM) criteria, which define men with a serum total testosterone level of less than 350 ng/dL as testosterone deficient [Citation15,Citation16].
2.2. Genetic instruments for anemia and testosterone deficiency in Mendelian randomization
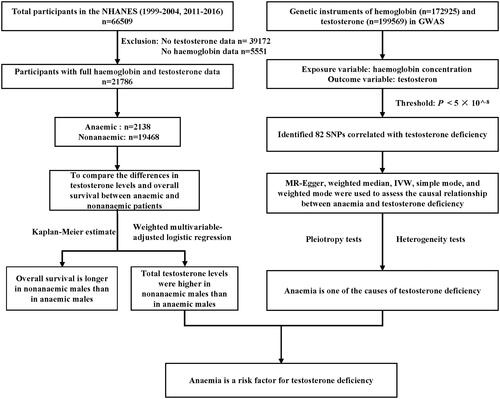
MR is an analytical method that utilizes genetic variation as an instrumental variable to estimate causal relationships between exposures and outcomes. MR analysis is akin to a randomized controlled trial and is considered the “gold standard” for providing medical evidence [Citation17]. In this study, we utilized genome-wide association study (GWAS) data from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/). The genetic instruments for hemoglobin concentration were derived from a meta-analysis of GWAS on human erythrocyte traits, encompassing a total of 172,925 individuals of European ancestry after excluding sample overlaps. For the GWAS analysis of bioavailable testosterone and total testosterone, we recruited 184,205 and 199,569 individuals of European ancestry, respectively. Based on the principles of inferring causal relationships through MR, which requires a strong correlation between single nucleotide polymorphisms (SNPs) and the exposure, as well as independence between SNPs and the outcome and confounding factors, we set a threshold of p < 5 × 10−8 to identify SNPs strongly associated with either testosterone or hemoglobin concentrations, separately. shows the approximate process of inclusion and analysis in this study.
Figure 1. General flow chart of the study.
2.3. Statistical analysis
During the NHANES observational study, categorical variables were represented numerically (%), while nonnormally distributed continuous variables were described by the median (interquartile range [IQR]). Chi-square tests were used to analyse p values for categorical variables, and the Mann–Whitney U test was used for comparing nonnormally distributed continuous variables between groups. To address the issue of partial missing data in the covariates, we employed a method of multiple imputation for filling in these gaps, accomplished using the Multivariate Imputation by Chained Equations (MICE) package. Weighted multivariable-adjusted logistic regression was used to assess the association between anemia and testosterone levels. Kaplan–Meier survival curves were used to visualize overall patient survival.
F-statistics were calculated to assess the strength of SNPs, with F > 10 indicating strong instruments. Robustness checks were conducted using the MR-PRESSO test and MR–Egger intercept. Five methods, including inverse–variance weighting (IVW), MR–Egger, weighted median, simple mode, and weighted mode, were employed to evaluate the causal relationship between exposure and outcome. Heterogeneity and directional pleiotropy were assessed using the Cochrane Q test and Egger’s intercept test, respectively. Sensitivity analysis was performed using the leave-one-out approach. Statistical analysis was conducted using R software (version 4.2.3, The R Foundation, Lanzhou, China) and Empower RCH software (version 4.1, Wuhan, China). A significance level of p < 0.05 was considered to indicate statistically significance.
3. Results
3.1. Demographic and clinical characteristics of participants included in the NHANES
This study analyzed data from six cycles of the NHANES (1999–2004 and 2011–2016), including a total of 21,786 participants after excluding cases with missing values for testosterone or hemoglobin concentrations. According to the WHO diagnostic criteria for anemia, 10.6% of participants were diagnosed with anemia, with a higher prevalence in females (64.2% vs. 35.8%). Compared with nonanemia individuals, anemic individuals had significantly lower hemoglobin (115 g/L vs. 143 g/L) and testosterone levels (27.4 ng/dL vs. 42.3 ng/dL). Anemic individuals were generally older and had higher BMI values. Regarding lipid profiles, anemic individuals had lower LDL and TC levels, but higher HDL levels. Anemic individuals also had lower MCV, MCH, and MCHC values. Furthermore, the prevalence of hypertension (47.5% vs. 32.8%) and diabetes (29.8% vs. 17.5%) was significantly greater in the anemic group than in the nonanemic group ().
Table 1. Demographic and clinical characteristics of participants included in the NHANES.
3.2. Observational associations between anemia and testosterone deficiency in the NHANES
According to logistic regression analysis using NHANES data, nonanemic patients exhibited significantly greater testosterone levels than did anemic patients (β = 93.723, 95% CI: 81.996–105.449, p < 0.00001). This association remained consistent even after adjusting for age, sex, and race (Adjusted Model I, β = 17.936, 95% CI: 10.359–25.513, p < 0.00001). The adjusted model with all variables also demonstrated the same trend (Adjusted Model II, β = 22.616, 95% CI: 3.873–41.359, p = 0.01807) (). To address missing data, multiple imputation was utilized, and the regression analysis results after five rounds of imputation (Adjusted Model II) consistently indicated that nonanemic patients had higher testosterone levels than did anemic patients (). Furthermore, the results from the unadjusted model and Adjusted Model I models remained consistent with the original findings, as these models did not incorporate any missing variables. Subgroup analysis revealed that the difference in testosterone levels between female anemic and nonanemic patients was not statistically significant (p > 0.05). However, in other stratified analyses, anemic patients exhibited lower testosterone levels than did nonanemic patients (p < 0.05) ().
Table 2. Causal relationships between anemia and testosterone deficiency: logistic regression.
Table 3. Results of regression analysis for anemia and testosterone levels after 5 imputations.
Table 4. Stratified analysis of testosterone levels in anemic and nonanemic patients.
3.3. Causal relationships between anemia and testosterone deficiency in MR
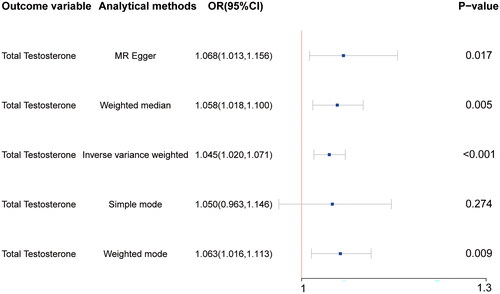
A total of 82 SNPs were associated with both hemoglobin (exposure variable) and testosterone (outcome variable) levels (Supplementary Table 1). The results of the IVW, MR Egger, weighted median, and weighted mode analyses consistently demonstrated a causal relationship between hemoglobin concentration and testosterone levels (). These findings are consistent with the results of our previous observational study using NHANES data, indicating that anemia is one of the aetiological factors for TD. Subsequently, heterogeneity and pleiotropy tests were performed to assess the reliability of the results, and the findings indicated non significant heterogeneity or pleiotropy (p > 0.05) (Supplementary Table 2). This confirms the reliability of the results obtained in this study, thereby supporting its validity.
Figure 2. Mendelian randomization reveals a causal relationship between anemia and testosterone deficiency. Hemoglobin as the exposure variable and total testosterone was used as the outcome variable. The results revealed greater testosterone levels in nonanemic individuals than in anemic individuals (p < 0.05 for MR–Egger, weighted median, inverse–variance weighting, and weighted mode).
3.4. Correlation between serum testosterone levels and overall survival in patients with and without anemia
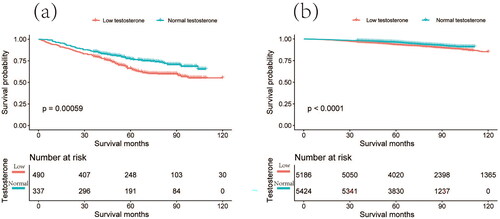
We are aware that the hemoglobin concentration in anemia patients has an impact on the supply of oxygen to organs and tissues, ultimately affecting the overall survival (OS) of these patients [Citation18–20]. However, another crucial aspect we are investigated in our study was the potential correlation between serum testosterone levels in anemia patients and their OS. The Kaplan–Meier survival curves revealed that both anemic and nonanemic patients with TD had shorter OS (). Importantly, TD has a greater impact on the OS of anemia patients than that of nonanemic patients. This suggests that TD not only increases the risk of anemia but also influences its prognosis.
Figure 3. Kaplan–Meier survival analysis of testosterone levels differences between anemic and nonanemic patients. The red line represents individuals with testosterone deficiency, while the green line represents individuals with normal testosterone levels. (a) Kaplan–Meier survival plot comparing anemic patients with testosterone deficiency to anemic patients with normal testosterone levels. (b) Kaplan–Meier survival plot comparing nonanemic patients with testosterone deficiency to nonanemic patients with normal testosterone levels.
4. Discussion
In this study, we analyzed on the association between hemoglobin concentrations and testosterone levels among participants from six cycles in the NHANES database. Our findings revealed that individuals with anemia had lower testosterone levels than did those without anemia. Furthermore, utilizing MR analysis, we confirmed that anemia is indeed one of the contributing factors to TD. Building upon the existing evidence from previous studies regarding TD as a risk factor for anemia, we propose that anemia and TD are interrelated conditions that mutually influence each other. Additionally, our research revealed that TD not only impacts hemoglobin concentrations but also potentially affects patient prognosis. Notably, the impact of TD on OS was found to be more pronounced among individuals with anemia than among those without anemia.
Previous studies have shown that men with TD due to androgen deprivation therapy or hypogonadism often have a concomitant decrease in hemoglobin concentration, while TRT can improve hemoglobin concentration [Citation21,Citation22]. Approximately, one-third of anemia in elderly patients has no clear cause. For elderly male anemia patients with TD, testosterone supplementation can improve anemia [Citation11,Citation23]. These lines of evidence suggest that TD reduces hemoglobin concentrations and that testosterone supplementation may improve the resulting anemia to some extent.
In contrast, there is limited research reporting the effects of anemia on testosterone levels, which is also the focus and highlight of this study. Our cross-sectional analysis of a large dataset revealed that testosterone levels in anemic patients were significantly lower than those nonanemic patients, with a β value of 22.616 (95% CI: 3.873–41.359, p = 0.01807). This indicates that anemia is an independent risk factor for TD. Furthermore, we employed MR analysis to validate this causal relationship (IVW, OR = 1.045, 95% CI: 1.020–1.071, p < 0.001). The exact mechanisms underlying the testosterone-lowering effects of anemia remain unclear, as both clinical and basic research in this area is scarce. However, based on the pathophysiology of anemia, it can be speculated that the decreased testosterone levels in anemic patients may be attributed to insufficient red blood cell count and/or hemoglobin content in the blood, leading to reduced oxygen-carrying capacity of red blood cells, thereby causing systemic tissue and organ hypoxia [Citation24]. Under hypoxic conditions, the activity of testosterone synthesis enzymes is known to decrease, thus inhibiting testosterone synthesis and secretion and ultimately resulting in lower testosterone levels [Citation25]. Further exploration in this direction is warranted to gain a better understanding of the relationship between anemia and testosterone levels.
However, the question of whether all patients with low testosterone levels require testosterone supplementation therapy remains controversial, especially considered age-associated decreases in testosterone levels [Citation26]. Carolyn A. Salter and Mulhall reviewed treatment guidelines for TD provided by multiple international medical associations – including the American Urological Association, the European Association of Urology, the American Association of Clinical Endocrinologists, the Endocrine Society, and the ISSM. They concluded that testosterone therapy should only be administered to males diagnosed with TD and that the diagnosis should take into account both serum testosterone levels and clinical symptoms or physical signs [Citation27]. There is currently no consensus on the indications for testosterone supplementation in women; it is primarily used in women who have undergone oophorectomy or are experiencing premature ovarian failure [Citation6,Citation28,Citation29]. For patients with anemia who also have TD, testosterone supplementation can not only improve the anemia but also provide a multitude of other benefits. These include improved cognitive function, alleviation of depressive symptoms and mood, increased bone density, delayed progression of coronary artery plaque volume, and enhancement of erectile function in men [Citation10,Citation30].
Admittedly, this study has some limitations. First, this was a cross-sectional study, which affects our ability to establish temporal relationships and make causal inferences. Although we have employed MR analysis to validate our findings, prospective studies are still needed to further confirm our conclusions. Second, the patients included in this study were exclusively from the United States. There may be variations in hemoglobin and testosterone levels among different regions and ethnicities, necessitating the inclusion of data from diverse populations of various countries for further analysis. Finally, due to the limitations of the NHANES database, we did not categorize anemia or explore the effects of different types and severities of anemia on testosterone. These areas represent the focus of our future research endeavors.
5. Conclusion
In general, our study presents a new perspective indicating that anemia may contribute to TD, and vice versa. Anemia and TD have a mutually causal relationship. Thus, when managing patients with unexplained anemia or TD in clinical practice, it is essential to consider the potential causal connection between these two conditions and ensure their simultaneous treatment to achieve optimal therapeutic outcomes.
Ethical statement
The data for this study were sourced from the NHANES database, which operates under a survey protocol approved by the NCHS Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm). Consequently, no additional ethical review is required.
Consent to participate
The data for this study was obtained from the NHANES public database, and participants in the survey had already received and signed an informed consent form. Therefore, this study is exempt from the need for consent to participate.
Consent to publish
Not applicable, as this study does not include any personal identifiers or private information.
Figures raw data
The data for this study were sourced from the public databases NHANES and GWAS, with a portion of the raw data uploaded in Supplementary Tables 1 and 2. More comprehensive raw data can be accessed and downloaded from the official websites of the databases at NHANES (https://www.cdc.gov/nchs/nhanes/index.htm) and GWAS (https://gwas.mrcieu.ac.uk/).
Author contributions
ZZ, JP, and XZ conceptualized and designed the study; ML, ZC, JG, and LZ collected and analyzed the data; JP and ML interpreted the results; ZZ drafted the manuscript; and PG and XZ critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript for submission.
Supplemental Material
Download Zip (33.1 KB)Acknowledgments
We gratefully acknowledge the NHANES and GWAS project participants for providing valuable data and information. We also thank the staff members involved in database maintenance and management. Our appreciation extends to all the contributors who provided valuable insights and feedback on this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used in this study are available at the official websites of the NHANES (https://www.cdc.gov/nchs/nhanes/index.htm) and GWAS (https://gwas.mrcieu.ac.uk/) projects.
Additional information
Funding
References
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011 (WHO/NMH/NHD/MNM/11.1). https://iris.who.int/handle/10665/85839.
- Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anaemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. doi: 10.1182/blood-2013-06-508325.
- Ferrucci L, Maggio M, Bandinelli S, et al. Low testosterone levels and the risk of anaemia in older men and women. Arch Intern Med. 2006;166(13):1380–1388. doi: 10.1001/archinte.166.13.1380.
- Naamneh Elzenaty R, Du Toit T, Flück CE. Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab. 2022;36(4):101665. doi: 10.1016/j.beem.2022.101665.
- Alwani M, Yassin A, Talib R, et al. Cardiovascular disease, hypogonadism and erectile dysfunction: early detection, prevention and the positive effects of Long-Term testosterone treatment: prospective observational, Real-Life data. Vasc Health Risk Manag. 2021;17:497–508. doi: 10.2147/VHRM.S309714.
- Davis SR, Wahlin-Jacobsen S. Testosterone in women–the clinical significance. Lancet Diabetes Endocrinol. 2015;3(12):980–992. doi: 10.1016/S2213-8587(15)00284-3.
- Traish AM, Miner MM, Morgentaler A, et al. Testosterone deficiency. Am J Med. 2011;124(7):578–587. doi: 10.1016/j.amjmed.2010.12.027.
- Tajar A, Forti G, O'Neill T, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European male ageing study. J Clin Endocrinol Metab. 2010;95(4):1810–1818. doi: 10.1210/jc.2009-1796.
- Rodrigues Dos Santos M, Bhasin S. Benefits and risks of testosterone treatment in men with age-related decline in testosterone. Annu Rev Med. 2021;72(1):75–91. doi: 10.1146/annurev-med-050219-034711.
- Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39(3):369–386. doi: 10.1210/er.2017-00234.
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anaemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177(4):480–490. doi: 10.1001/jamainternmed.2016.9540.
- Murphy WG. The sex difference in haemoglobin levels in adults - mechanisms, causes, and consequences. Blood Rev. 2014;28(2):41–47. doi: 10.1016/j.blre.2013.12.003.
- Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the framingham heart study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439. doi: 10.1210/jc.2010-3012.
- Lee JH, Choi JD, Kang JY, et al. Testosterone deficiency and the risk of anaemia: a propensity score-matched analysis. Am J Hum Biol. 2022;34(8):e23751. doi: 10.1002/ajhb.23751.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18(1):5–15. doi: 10.3109/13685538.2015.1004049.
- Khera M, Adaikan G, Buvat J, et al. Diagnosis and treatment of testosterone deficiency: recommendations from the fourth international consultation for sexual medicine (ICSM 2015). J Sex Med. 2016;13(12):1787–1804. doi: 10.1016/j.jsxm.2016.10.009.
- Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–1621. doi: 10.1001/jama.2021.18236.
- Vu C, Bush A, Choi S, et al. Reduced global cerebral oxygen metabolic rate in sickle cell disease and chronic anaemias. Am J Hematol. 2021;96(8):901–913. doi: 10.1002/ajh.26203.
- Chaparro CM, Suchdev PS. Anaemia epidemiology, pathophysiology, and etiology in low- and Middle-income countries. Ann NY Acad Sci. 2019;1450(1):15–31. doi: 10.1111/nyas.14092.
- Carmel R. Anaemia and aging: an overview of clinical, diagnostic and biological issues. Blood Rev. 2001;15(1):9–18. doi: 10.1054/blre.2001.0146.
- Grossmann M, Zajac JD. Hematological changes during androgen deprivation therapy. Asian J Androl. 2012;14(2):187–192. doi: 10.1038/aja.2011.102.
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. doi: 10.1210/jc.2018-00229.
- Ershler WB. Unexplained anaemia in the elderly. Clin Geriatr Med. 2019;35(3):295–305. doi: 10.1016/j.cger.2019.03.002.
- Sankaran VG, Weiss MJ. Anaemia: progress in molecular mechanisms and therapies. Nat Med. 2015;21(3):221–230. doi: 10.1038/nm.3814.
- Oyedokun PA, Akhigbe RE, Ajayi LO, et al. Impact of hypoxia on male reproductive functions. Mol Cell Biochem. 2023;478(4):875–885. doi: 10.1007/s11010-022-04559-1.
- Yeap BB, Page ST, Grossmann M. Testosterone treatment in older men: clinical implications and unresolved questions from the testosterone trials. Lancet Diabetes Endocrinol. 2018;6(8):659–672. doi: 10.1016/S2213-8587(17)30416-3.
- Salter CA, Mulhall JP. Guideline of guidelines: testosterone therapy for testosterone deficiency. BJU Int. 2019;124(5):722–729. doi: 10.1111/bju.14899.
- Mathur R, Braunstein GD. Androgen deficiency and therapy in women. Curr Opin Endocrinol Diabetes Obes. 2010;17(4):342–349. doi: 10.1097/MED.0b013e32833ab083.
- Yialamas MA, Hayes FJ. Androgens and the ageing male and female. Best Pract Res Clin Endocrinol Metab. 2003;17(2):223–236. doi: 10.1016/s1521-690x(03)00018-6.
- Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708–716. doi: 10.1001/jama.2016.21043.

