Abstract
Purpose
This study investigates how the COVID-19 pandemic (CP) impacted the timeline between initial diagnosis (ID) of prostate carcinoma and subsequent therapy consultation (TC) or radical prostatectomy (RP) due to the implementation of a “minimal contact concept,” which postponed clinical examinations until the day of admission.
Methods
We analyzed patient data from a tertiary care center from 2018 to September 2021. The focus was on comparing the time intervals from ID to TC and from ID to RP before and during the CP.
Results
Of 12,255 patients, 6,073 (61.6%) were treated before and 3,791 (38.4%) during the CP. The median time from ID to TC reduced from 37 days (IQR: 21 – 58d) pre-CP to 32 days (IQR: 20 – 50d) during CP (p < 0.001). Similarly, the time from ID to RP decreased from 98 days (IQR: 70 – 141d) to 75 days (IQR: 55 – 108d; p < 0.001) during the CP. There was a significant decrease in low-risk tumor cases at ID (18.9% vs. 21.4%; p = 0.003) and post-RP (4% vs. 6.7%; p < 0.001) during the CP.
Conclusion
Our findings suggest that the COVID-19 pandemic facilitated more timely treatment of prostate cancer, suggesting potential benefits for both low-risk and aggressive tumor management through expedited clinical procedures.
Introduction
The COVID-19 pandemic (CP) resulted in a major impact on the screening, clinical management, and treatment of oncological patients [Citation1]. Prostate cancer (PCa) is one of the most common malignancies in men in the Western world and is more common in older people. Radical prostatectomy (RP) represents one of the most frequently used curative treatment options in localized PCa in patients with a longer life expectancy [Citation2]. Even without the influence of COVID-19, RP represents a major surgical intervention in the pelvis with corresponding perioperative risks for morbidity and mortality [Citation3, Citation4].
Since the recognition of SARS-CoV-2 in December 2019 in China, COVID-19 has rapidly spread worldwide, causing widespread disease and mortality. Patients with cancer are disproportionately affected by severe outcomes from COVID-19 infection [Citation5]. Risk factors associated with worse outcomes include advanced age, poorer Eastern Cooperative Oncology Group (ECOG), and active cancer compared to patients in remission [Citation5–7]. Male (versus (vs.) female) sex is associated with higher rates of hospitalization and admission to intensive care units from COVID-19 infection [Citation8]. In order to contain the uncontrolled spread of COVID-19 and to provide sufficient capacity in the intensive care unit, medical disciplines had to develop new routines and risk strategy protocols [Citation9]. On the other hand, a potentially life-threatening tumor should be treated at an early stage. Additionally, outpatient urologists had to adapt their hygiene protocols to COVID-19 regulations while keeping cancer screening services regularly available.
Therefore, we analyzed how our COVID-19-related measures affected the prostate cancer patients’ temporal therapy from initial PCa diagnosis (ID) to management and outcomes. We hypothesized that the implementation of COVID-19-related measures, specifically the “minimal contact concept,” would not delay but rather expedite the timeline from initial prostate cancer diagnosis to subsequent therapy consultation, contrary to initial expectations.
Materials and methods
Patient population
We identified 12,255 patients who had a therapy consultation (TC) after ID and/or underwent radical prostatectomy (RP) between 2018 and September 2021 in our tertiary care center. The period before 02.28.2020 (the first COVID-19 case in Hamburg) was defined as before the pandemic [Citation10]. 2,391 patients were excluded from our cohort. These exclusions were primarily due to incomplete data sets and indeterminate pre- or post-pandemic treatment timelines.
Data assessment was performed using our prospective institutional database (FileMaker Pro 10; FileMaker, Inc.). Our institutional review board approved the study, and all patients agreed on data collection by informed consent. All pre- and postoperative data were collected at our prospective, review-board-approved institutional database. Our goals were to evaluate the time difference between ID and TC before versus (vs.) during the CP and to evaluate the time difference between ID and RP before vs. during the CP.
Procedure
Procedure before the CP: The ID was made mainly by an outpatient urological colleague or in our clinic. Appropriate staging was initiated if necessary, and the patient presented personally to our clinic to undergo TC. After the joint determination of the therapy procedure, the elective planning and implementation of the surgical therapy took place.
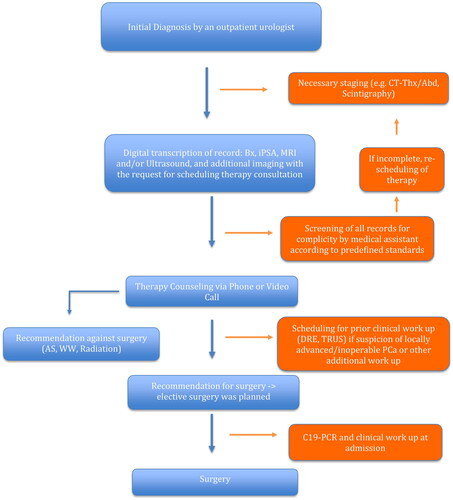
Procedure during CP: Due to the limited hospital capacities, the general hospital policy was only to perform the oncological intervention and postpone elective interventions (e.g. benign prostatic obstruction surgery). Where possible, personal contact was kept to a minimum [Citation9]. The ID was made mainly by an outpatient urological colleague or in our clinic. TC took place via telephone/video consultation. A personal presentation was made during the clinic consultation hour only in exceptional cases with suspected locally advanced findings. After consultation and determination of the procedure, elective surgery was planned and performed. If necessary, further examinations were carried out. Based on the national recommendations, no patient underwent surgery in direct temporal relation to the vaccination [Citation11]. In case of COVID-19 infection, patients were postponed to a date minimum six weeks past the acute phase. Surgery was only performed in case of a negative polymerase chain reaction test at admission () [Citation12, Citation13].
Figure 1. Adapted prostate cancer management protocol during the COVID-19 pandemic for minimized patient contact.
CT Thx/Abd: CT Chest and Abdomen; Bx: Prostate Biopsy; iPSA: initial Prostate Specific Antigen; AS: Active Surveillance; WW: Watchful Waiting; C19-PCR: Covid-19 Polymerase Chain Reaction Test.
Statistical analyses
Descriptive statistics included frequencies and proportions for categorical variables (pathological T-stage, N-stage, Gleason score). Means, medians, and ranges were reported for continuously coded variables (age, PSA, number of biopsy cores, and number of dissected lymph nodes). The Chi-square tested the statistical significance of the difference in proportions. The t-test and Kruskal-Wallis test examined the statistical significance of the difference in means and median. R software environment for statistical computing (version 3.4.3) was used for all statistical analyses.
Results
Overall, 9,864 consecutive patients were analyzed. Of those, 6,073 (61.6%) were treated before and 3,791 (38.4%) during CP (NRP: 2018 = 2542; 2019 = 2660; 2020 = 2510; 2021 = 2152). Patient characteristics are shown in . Due to PCa being an oncological disease, a minimum of 80% of the surgical capacity was maintained under strict hygiene measures compared to before CP [Citation9].
Table 1. Basic patient characteristics of prostate cancer patients during and before the COVID-19 pandemic.
The median age was 64 years (interquartile range (IQR): 59 – 69 years), and the median PSA was 6.8 ng/ml (IQR: 4.6 − 10.5 ng/ml). The median time interval between ID and TC was 37 days (IQR: 21 – 58d) before vs. 32 days (IQR: 20 – 50d) during CP (p < 0.001). The median time interval between ID and RP was 98 days (IQR: 70 – 141d) before vs. 75 days (IQR: 55 – 108d; p < 0.001) during CP. We saw fewer patients with low-risk tumors in biopsy (Gleason score 6 tumors in biopsy; 1,302 (21.4%) vs. 715 (18.9%); p = 0.003) and post-RP (Gleason score 6 tumors post-RP 404 (6.7%) vs. 151 (4%); p < 0.001) before then during CP. Fewer patients received neoadjuvant hormone therapy during CP (339 (8.9%) vs. 756 (12.4%), p < 0.001). The median number of lymph nodes dissected before CP was 14 (IQR 8–20) vs. 10 (IQR: 1–17) during CP (p < 0.001). The clinical stage of the patients after digital-rectal examination was worse during than before CP (p < 0.001; ). Pathological T, N-status, and the rate of positive margins showed no significant difference (). During CP, we had more patients with pNX status (p < 0.001; ).
Table 2. Outcomes after radical prostatectomy of patients during and before the COVID-19 pandemic.
Discussion
The CP has presented incredible stress on international healthcare systems. Due to the pandemic, clinical practice worldwide had to adapt to new conditions. Of course, this also applies to the care of urological cancer patients. According to current literature, in the specific case of PCa, the CP has had a negative impact on both early diagnosis through reduced participation in screening programs and on the time from diagnosis to surgery/radiotherapy, which could translate into higher prostate cancer-specific mortality in the years to come [Citation1, Citation14–17].
Our current analyses demonstrate several noteworthy findings. Firstly, the time interval between ID and TC has shortened (37 vs. 32 days; p < 0.001) during the CP. Secondly, the time interval between ID and RP also shortened (98 vs. 75 days; p < 0.001) during the CP. Ultimately, the causes are certainly multifactorial. Some patients will have pushed for rapid treatment due to the CP and the tumor disease. While others may have postponed their cancer screening or opted for medical management [Citation1]. Additionally, the uptake of telephone and video consultation has contributed to fast-tracking outpatient pipelines. Redundant consultations with missing clinical information were reduced due to the optimization of workflow algorithms. Whereas patients with long travel times were lifted of the burden of inpatient consultation, allowing more flexibility in scheduling follow-up appointments or procedures. In contrast to current literature, we could not detect any delay in cancer therapy at our clinic, despite recurrent therapy postponements in the case of COVID-19 infections of planned patients [Citation15, Citation17]. Garcia-Rojo et al. described an exceeded waiting time for surgery in their cohort [Citation15]. Our clinic, a high-volume tertiary prostate cancer center in Germany, maintained its operational volume during the COVID-19 pandemic. Given these unique circumstances, the findings related to the time from ID to RP may not be applicable to other centers or countries. Additionally, our ability to receive patient referrals from ambulatory urologists for treatment consultation and potential RP without significant delays should be considered when interpreting our results. While our findings are directly applicable to high-volume tertiary centers, variations in facility resources and patient demographics may influence outcomes in other settings.
Interestingly, the rate of low-risk tumor patients who presented in our clinic decreased during the COVID-19 pandemic (Gleason score 6 tumors in biopsy; 715 (18.9%) vs. 1,302 (21.4%); p = 0.003) and post-radical prostatectomy (RP; Gleason score 6 tumors post-RP; 151 (4%) vs. 404 (6.7%); p < 0.001)). This shift towards fewer low-risk presentations and more high-risk cases at diagnosis is consistent with broader trends observed in the literature. Beatrici et al. reported a continuous stage and grade migration towards more aggressive prostate cancer (PCa) at diagnosis, likely exacerbated by the pandemic’s impact on healthcare access and cancer screening practices. Their findings suggest a significant drop in low-risk PCa diagnosis from 34.9% in 2010 to 17.7% in 2020, affirming a trend towards more aggressive disease at diagnosis [Citation18]. Similarly, Ward et al. used a microsimulation model to estimate the impact of COVID-19 on cancer diagnosis delays in Chile, projecting increased excess cancer deaths due to worse stage distribution at diagnosis, which aligns with our observations of more aggressive tumors being treated during the pandemic [Citation17]. These trends confirm that the pandemic may have influenced the stage at which patients present, underscoring the necessity to evaluate the long-term impact on cancer-specific mortality in the years to come. Possible explanations could be that healthy patients were more prone to choose surveillance or not attend screening appointments at all, so earlier stages were not currently diagnosed. Ferrari et al. have demonstrated that the lockdown period established during the first peak of the COVID-19 outbreak in Italy’s Verona province was associated with a dramatic decrease in routine prostate cancer screenings [Citation16]. It is also possible that low-risk patients initially discussed active surveillance with outpatient urologists and did not present to our clinic. This would correspond to the recommendations of Wallis et al. [Citation19].
The fact that we found fewer patients with neoadjuvant hormone therapy during CP may be due to more efficient therapy delivery. Another reason may have been the desire to treat the solid tumor as quickly as possible, as active cancer is a risk factor for a worse COVID-19 infection outcome [Citation5].
In our discussion on the role of telephone and telemedicine consultations, it’s important to also consider broader implementation challenges and best practices as described by Smith et al. Their work outlines a comprehensive guide for rapidly integrating telemedicine into practice during the COVID-19 pandemic, emphasizing the necessity of several key components, including provider and staff training, patient education, and robust information technology support. This framework highlights the potential of telemedicine to maintain continuity of care while adhering to social distancing guidelines. However, as noted in their study, while telemedicine can substantially reduce the need for in-person visits, it also underscores that certain clinical situations, such as the evaluation of patients with complex comorbidities or suspected advanced disease, still necessitate face-to-face interactions to ensure comprehensive assessment and management [Citation20]. Therefore, our experience aligns with the findings of Smith et al. supporting the use of telemedicine as an effective adjunct to traditional care methods rather than a complete substitute, particularly in complex clinical scenarios.
From our current clinical experience, we can say that telephone counseling of patients is a viable alternative in current patient care. However, as we have pointed out, face-to-face presentation remains essential, especially in suspected locally advanced disease cases or patients with multiple comorbidities.
Additionally, one reason for the higher pNX rate described is that we are currently conducting a prospective randomized multicenter study (PREDICT trial; NCT04269512) that was also recruited during the pandemic. It assesses the prognostic role of pelvic lymph node dissection in intermediate-risk PCa patients (extended vs. no lymph node dissection). The increased performance of extended lymph node dissection in the PREDICT trial probably explains the lower number of dissected lymph nodes during CP.
Savin et al. describe that selectively delaying image-guided prostate biopsies during the pandemic is unlikely to result in a higher rate of significant cancer [Citation21]. However, no one can predict whether we will see patients with more aggressive tumors in the following years.
Our study has several limitations that need to be considered when interpreting its results. Firstly, its retrospective design limits our ability to establish causality between observed trends and the interventions implemented during the COVID-19 pandemic. Additionally, this design precluded the evaluation of aspects of quality of life, particularly regarding telephone consultations. The generalizability of our results may be constrained by the unique operational capabilities of our high-volume center, which may not reflect conditions at smaller or differently resourced facilities. Furthermore, some patients might have opted for radiation therapy outside of our data collection reach, leading to potential underreporting of the time to definitive treatment. We also lack longitudinal follow-up data to assess long-term impacts and data on specific changes in the outpatient sector or therapy recommendations made by outpatient colleagues. Lastly, our analysis did not account for potential confounders, such as variations in patient demographics or healthcare access, which could influence the outcomes.
Conclusion
Contrary to expectations, we could not prove a delay from diagnosis to TC and RP. Counseling patients by telephone or virtual seems to be a tested and efficient counseling solution during the pandemic. The adaptation of telemedicine is beneficial for declustering and optimizing the in- and outpatient structures of prostate cancer patients. Further investigations might be the foundation for policy adaptation.
Compliance with ethical standards
Disclosure of potential conflict of interest: The authors declare that they have no conflict of interest related to this work. In accordance with federal and institutional guidelines, all men signed an institutional review board-approved, protocol-specific informed consent form before study entry. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Anonymized data were used.
Informed consent
All patients agreed on prospective data collection by informed consent.
Research involving human participants and/or animals
This study did not involve human or animal subjects. Data was collected prospectively.
Author contributions
DKF: Protocol/project development, Data analysis, Data collection or management, Manuscript writing/editing, Data analysis. RMP: Protocol/project development, Data analysis, Manuscript writing/editing, Data analysis. PM: Data collection or management, Data analysis, Manuscript writing/editing. PT: Data analysis, Data collection or management, Data analysis. BB: Data analysis, Manuscript writing/editing. DT: Protocol/project development, Manuscript writing/editing, Data analysis. HI: Data collection or management, Manuscript writing/editing. TM: Manuscript writing/editing. TAL: Manuscript writing/editing. HH: Manuscript writing/editing. TS: Protocol/project development, Manuscript writing/editing.
Acknowledgments
There was no external financial support for this study.
Disclosure statement
No potential competing interest was reported by the authors.
Additional information
Funding
References
- Bakouny Z, Paciotti M, Schmidt AL, et al. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458–460. doi: 10.1001/jamaoncol.2020.7600.
- Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–282. doi: 10.1016/j.eururo.2020.09.046.
- Pompe RS, Beyer B, Haese A, et al. Postoperative complications of contemporary open and robot-assisted laparoscopic radical prostatectomy using standardised reporting systems. BJU Int. 2018;122(5):801–807. doi: 10.1111/bju.14369.
- Filipas DK, Labban M, Beatrici E, et al. Association of urinary incontinence and depression: findings from the national health and nutrition examination survey. Urology. 2023;181:11–17. doi: 10.1016/j.urology.2023.08.008.
- Schmidt AL, Tucker MD, Bakouny Z, et al. Association Between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19. JAMA Netw Open. 2021;4(11):e2134330. doi: 10.1001/jamanetworkopen.2021.34330.
- Aboueshia M, Hussein MH, Attia AS, et al. Cancer and COVID-19: analysis of patient outcomes. Future Oncol. 2021;17(26):3499–3510. doi: 10.2217/fon-2021-0121.
- Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet. 2020;396(10250):532–533. doi: 10.1016/S0140-6736(20)31748-7.
- Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6.
- Würnschimmel C, Maurer T, Knipper S, et al. Martini-Klinik experience of prostate cancer surgery during the early phase of the COVID-19 pandemic. BJU Int. 2020;126(2):252–255. doi: 10.1111/bju.15115.
- Unternehmenskomunikation UHE. Bestätigter Corona Fall am UKE Pressemitteillung UKE, 2020.
- Stellungnahme zu Operation und Intervall zu COVID19 Impfung oder Infektion_12.05.2021. [press release]. DGAI, 12.05.2021, 2021.
- Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76(6):748–758.
- Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X.
- Crocetto F, Buonerba L, Scafuri L, et al. COVID-19 and prostate cancer: a complex scenario with multiple facets. Future Sci OA. 2021;8(1).
- García-Rojo E, Manfredi C, Santos-Pérez-de-la-Blanca R, et al. Impact of COVID-19 outbreak on urology surgical waiting lists and waiting lists prioritization strategies in the post-COVID-19 era. Actas Urol Esp (Engl Ed). 2021;45(3):207–214. doi: 10.1016/j.acuro.2020.11.001.
- Ferrari A, Sanchis-Gomar F, Mattiuzzi C, et al. Is COVID-19 impacting prostate cancer screening? A survey of prostate-specific antigen test requests during a local outbreak. Ejifcc. 2021;32(1):69–77.
- Ward ZJ, Walbaum M, Walbaum B, et al. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: a simulation-based analysis. Lancet Oncol. 2021;22(10):1427–1437. doi: 10.1016/S1470-2045(21)00426-5.
- Beatrici E, Filipas DK, Stone BV, et al. Clinical stage and grade migration of localized prostate cancer at diagnosis during the past decade. Urol Oncol. 2023;41(12):483.e11–483.e19. doi: 10.1016/j.urolonc.2023.09.012.
- Wallis CJD, Novara G, Marandino L, et al. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management During the COVID-19 pandemic. Eur Urol. 2020;78(1):29–42. doi: 10.1016/j.eururo.2020.04.063.
- Smith WR, Atala AJ, Terlecki RP, et al. Implementation guide for rapid integration of an outpatient telemedicine program During the COVID-19 pandemic. J Am Coll Surg. 2020;231(2):216–222.e2. doi: 10.1016/j.jamcollsurg.2020.04.030.
- Savin Z, Dekalo S, Marom R, et al. The effect of delaying transperineal fusion biopsy of the prostate for patients with suspicious MRI findings-implications for the COVID-19 era. Urol Oncol. 2021;39(1):73.e1–e8. doi: 10.1016/j.urolonc.2020.07.009.

