Abstract
Objective: Ivacaftor was approved in 2012 to treat patients with cystic fibrosis (CF) with specific CFTR gene mutations. The objective of this analysis was to analyze the impact of ivacaftor on health resource utilization through analysis of claims data.
Methods: Patients diagnosed with CF aged ≥6 years prescribed ivacaftor between January 1, 2012 and July 31, 2014 with ≥12 months of continuous insurance coverage prior to and following the prescription were identified. All-cause and CF-specific healthcare resource utilization during the pre- and post-prescription periods and ivacaftor adherence levels were studied.
Results: The 79 identified patients had a mean age of 20.8 years, and 54% were female. The proportion of patients with inpatient admissions (all-cause and CF-related) was significantly higher in the pre index compared to post index period (p ≤ 0.05). Mean ivacaftor medication possession ratio was 0.8 (SD = 0.3), and 73% of patients had a medication possession ratio >0.80.
Limitations: Only a small number of patients met the inclusion criteria. Additionally, claims data may contain errors or inconsistencies and cannot be used to determine if medications were taken as prescribed.
Conclusions: Ivacaftor therapy was associated with significant reductions in hospitalizations along with high rates of adherence to treatment over 12 months.
Introduction
Cystic fibrosis (CF) is a progressive, genetic disorder affecting nearly 70 000 children and adults across the globeCitation1,Citation2. Disease progression, in the form of lung disease and repeated infections, is mainly the result of two mutations in the gene that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein, which controls electrolyte transport across cells in the lungs, sweat glands, pancreas, and other tissues. Standard therapies, focused on treating symptoms of the disease and associated with significant treatment burden, include chest physiotherapy, chronic antibiotics, pancreatic enzyme replacement, and nutritional supplementsCitation3. Ivacaftor was the first drug approved to treat the abnormality in CF caused by CFTR mutations.
Ivacaftor potentiates the activity of the CFTR protein, thus addressing the underlying cause of CFCitation4–6. Two 48-week randomized, placebo-controlled studies reported improvements in lung function and measures of nutritional health in patients receiving ivacaftor as compared with placeboCitation1,Citation2. In the study involving adolescent and adult patients, there was a significant decrease in the number and severity of pulmonary exacerbations (PEx), with patients receiving ivacaftor 55% less likely to have a PExCitation1. This finding is meaningful due to the importance and impact of PEx on patients’ quality-of-life, as well as on the decline in pulmonary functionCitation7–9. Additionally, patients receiving ivacaftor also experienced fewer hospital days due to PEx as compared to patients receiving placeboCitation1. In response to these promising outcomes, the US Food and Drug Administration approved ivacaftor for treatment of patients with CF and a G551D-CFTR mutation. Ivacaftor was initially approved for use in the US in 2012, and, as of 2013, ivacaftor has been added to the guidelines for care in patients with CF who have at least one copy of the G551D-CFTR mutation, which accounts for ∼4% of patients with CFCitation10,Citation11. Following additional successful clinical trials in the relevant patient populations, ivacaftor’s approval was expanded to include nine other CFTR gene mutations and for use in children aged 2–5; however, the full population indicated to receive treatment with ivacaftor remains a small proportion of patients with CF.
Although the results of the clinical trials are robust, it is important to understand the impact of treatment and adherence in the real-world setting. Insurance claims databases can provide an abundance of data to assess the impact of treatments in clinical practice. Thus, this retrospective cohort study utilized data from a large US claims database to evaluate clinical outcomes among commercially insured patients with CF who were treated with ivacaftor. We sought to evaluate real-world healthcare resource utilization and adherence to ivacaftor during the time periods surrounding initiation of ivacaftor treatment.
Patients and methods
Data source
This retrospective study used data from the Truven Health MarketScan Commercial Claims and Encounters database (MSCCD) for the period January 1, 2012 to July 31, 2014. The MSCCD includes medical and pharmaceutical claims for ∼40 million individuals less than 65 years old from over 160 large employers and health plans in the US. The health plans include preferred provider organizations, health maintenance organizations, point-of-service plans, and indemnity plans. Medical claims are linked to retail and mail-order prescription drug claims and person-level enrollment data through the use of unique enrollee identifiers. The database is fully de-identified and complies with the Health Insurance Portability and Accountability Act so that approval from institutional review boards is not considered necessary.
Patient selection
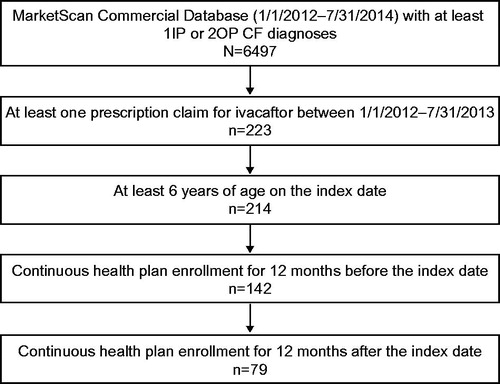
Patients diagnosed with CF who are at least 6 years old with at least one new claim for ivacaftor (NDC: 51167-0200-02) after January 1, 2012 were eligible for inclusion in the study. Each patient’s first claim for ivacaftor between January 1, 2012 and July 31, 2013 was designated as their index date. All patients were required to have a CF diagnostic code (ICD9: 277.0x) on at least one inpatient or two outpatient (at least 30 days apart) claims, as well as continuous enrollment in the database with both medical and pharmaceutical benefits for 12 months before (pre index period) and 12 months after (post index period) the index date.
Outcome measures
The following patient demographic characteristics were captured at the index date: age, gender, health plan type, and geographic region. Clinical characteristics measured during the 12-month pre index period included specific comorbidities defined by primary or secondary ICD-9 diagnosis codes on any claim generated during the pre-index period and concomitant medications on any claim generated during the pre-index period. Healthcare resource utilization outcomes pre- and post-index date were evaluated on all inpatient admissions, outpatient clinic visits, and outpatient claims, with a sub-set of claims identified as CF-related. CF-related healthcare resource utilization was defined by the presence of a diagnosis code for CF (ICD-9: 277.0x) in the primary position on inpatient claims or in any position on emergency room or outpatient claims. The specific healthcare resource utilization outcome measures included the number and proportion of patients with an inpatient admission, mean number of admissions among patients with an inpatient admission, the average length of inpatient stay among patients with at least one admission, the mean number of outpatient office visits, and the mean number of outpatient claims. Ivacaftor adherence was assessed in the post period with: the mean number of ivacaftor claims per patient during the post index period, the medication possession ratio (MPR; total days supplied from all ivacaftor refills divided by 365 days of the post-index period), and the overall proportion with a high adherence rate (defined as the proportion of patients with an MPR ≥0.80)Citation12,Citation13.
Statistical analysis
All study outcome measures were summarized using descriptive statistics. Categorical measures were presented as counts and percentages. Continuous measures were presented as means and standard deviations (SD). As this analysis is a comparison between pre- and post-period for the same patient cohort, the two samples are not independent samples. To account for this non-independence, statistical tests of significance for differences across pre- and post-index periods included McNemar’s tests for categorical variables and paired t tests for continuous variables. A p-value <0.05 was considered significant. All analyses were performed using Treatment Pathways (Truven Health Analytics, Cambridge, MA) and Stata version 12.1 (StataCorp, College Station, TX).
Results
Patient identification
A total of 223 patients with at least one prescription claim for ivacaftor between January 1, 2012 and July 31, 2013 were identified. After inclusion criteria were applied, the final study cohort consisted of 79 patients who were at least 6 years old at the index date and with 12 months of pre index and 12 months of post index continuous health plan enrollment ().
Demographic and clinical characteristics
The average age of the study patients was 20.8 (SD =11.8) years, 54% were female, and 36.7%, 25.3%, and 20.3% were from the Northeast, North Central, and Western US Census regions (). The majority of patients were insured by a preferred provider organization (PPO) (61%), followed by a health maintenance organization (HMO) (15%) and point-of-service (POS) (10%) plan.
Table 1. Baseline demographic characteristics.
The most prevalent clinical characteristics (identified by primary or secondary ICD-9 diagnosis code) on any claim during the pre-index period were sinusitis (35%), non-Pseudomonas pulmonary infection (25%), cough (24%), asthma (22%), bronchiectasis (22%), malabsorption (19%), and gastroesophageal reflux disease (18%) (). The most prevalent concomitant medications identified on claims during the pre-index period were antibiotics (95%), anti-asthmatics (84%), pancreatic enzymes (84%), airway clearance medications (77%), gastrointestinal medications (49%), tobramycin inhalation solution (43%), and nutrition medications (27%) ( and Supplementary Table S1).
Table 2. Clinical characteristics and medications within the 12-month pre-index period.
Healthcare resource utilization and medication adherence
A significant decrease was observed in the proportion of patients with at least one CF-related inpatient admission during the 12 months after ivacaftor initiation compared to the 12 months prior (19.0% pre index vs 6.3% post index; p = 0.041), corresponding to a 66.9% reduction (). While the percentage of patients with a CF-related admission decreased following ivacaftor initiation, among those patients with at least one hospital admission there were no significant differences pre- vs post-initiation of ivacaftor in either average number of admissions with an admitting diagnosis of CF per patient (1.6 pre index vs 1.2 post index) or average total hospital days (13.8 days pre index vs 7.8 days post index) (). A significant decrease was also observed in the proportion of patients with an all-cause inpatient admission (32.9% pre index vs 16.5% post index; p = 0.021). This corresponds to a 49.9% reduction in the proportion of patients with an inpatient admission. Finally, 21 patients had a decrease in all-cause inpatient admissions, while 11 patients had an increase, and one patient had no change in the 12 months after initiation of ivacaftor. For CF-related inpatient admissions, 15 patients had a decrease, while five had an increase in the 12 months after initiation of ivacaftor. A sensitivity analysis was conducted using 6-month pre- and post-index periods, which resulted in a larger sample size (n = 106), and the results found were consistent.
Table 3. Healthcare resource utilization.
Table 4. Ivacaftor treatment characteristics during the post-index period.
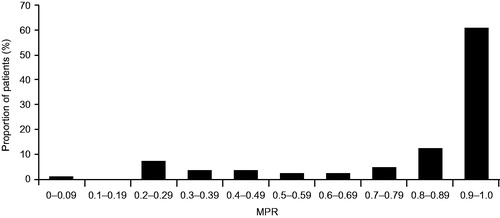
The average number of ivacaftor claims per patient in the 12-month post index period was 8.8 (SD =3.6) (). The majority of patients (73%) were adherent to ivacaftor therapy (MPR >0.80), and the average MPR of all patients in the study was 0.8 (SD =0.3). Patients with single-month supply claims for ivacaftor (n = 63) had an average MPR of 0.8 (SD =0.3), while those with multi-month claims for ivacaftor (n = 16) had an average MPR of 0.9 (SD =0.2). The distribution of MPR deciles can be seen in .
Discussion
This retrospective, observational study demonstrates that real-world ivacaftor use from January 2012 through July 2014 is associated with adherence higher than that seen with other chronic CF medications and reduced healthcare resource utilization. Using administrative claims data from commercially insured patients with CF in the US, this study demonstrates statistically significant lower rates of all-cause and CF-related inpatient hospitalizations in the 12 months following the initiation of ivacaftor therapy compared with the 12 months prior. In total, following ivacaftor initiation there was a 50% reduction in the proportion of patients with an all-cause inpatient admission and a 67% reduction in the proportion of inpatient admissions where CF was listed as the admitting diagnosis. It is important to note that a CF diagnosis code was listed as a primary or secondary diagnosis code on all all-cause inpatient admissions, and a respiratory-related code (ICD9: 460-519) was the primary diagnosis in 64% of instances where CF was a secondary diagnosis on the inpatient admission.
The pre-index hospitalization rates and lengths of stay observed in this analysis are consistent with rates reported in the general CF literature from studies of multiple CF populationsCitation7,Citation11,Citation14,Citation15. A UK study of resource utilization rates reported that 57% of patients with CF had at least one inpatient stay for a PEx, with an average length of stay of 9.2 days; one-half of those patients required continuation of intravenous antibiotic after discharge for an additional mean of 4.9 daysCitation7. Inpatient hospitalizations in a French study were reported among 26% and 30% for patients during 2000 and 2003, respectivelyCitation14. Another resource utilization study including US and Canadian patients with CF reported that nearly a quarter (22.4%) of patients required at least one hospitalization over the 48-week study period, of which 85.8% were related to CFCitation15. Although these are lower than the 35% rate of PEx reported in the 2013 US Cystic Fibrosis Foundation Patient Registry (CFFPR)Citation11, the US registry does not distinguish between PEx that require hospitalization and those that do not. Further, while these studies may have had differing criteria for identification of inpatient stays and for PEx analyzed, the similarity in results reported suggests consistency in CF care. The average number of days of hospitalizations for PEx per year was 19.1 days, with an additional 11.9 days of home intravenous treatment annually.
Certain categories of healthcare resource utilization were not statistically significantly different in the post index period: for instance, while the mean number of CF-related outpatient claims trended lower than in the pre-index period (5.4 pre-index vs 4.7 post-index; p = 0.111), there was no statistical significance observed in this small sample size study. Larger sample sizes and longer observation time may be needed to confirm or refute this trend. CF Foundation clinical care guidelines recommend that patients aged 6 or older visit their care center at least 4 times per year, which is reflected in the CF-related outpatient visit resultsCitation16. These results reflect continued best practices for specialized CF care.
Overall, clinical characteristics reported in this analysis are consistent with those reported in the CFFPR; this includes similar rates of sinusitis (35% vs 30%), asthma (22% vs 26%), diabetes (16% vs 20%), and depression (1% vs 4%). Medication use is also comparable between this analysis and the CFFPR: for example, 84% of patients received pancreatic enzymes compared with 87% of patients in the CFFPR, and dornase alfa usage was 73% compared with 85% in the CFFPRCitation11. Drug classification for concomitant medications was conducted based on therapeutic class, which may be different from the intended use in patients with CF.
Pulmonary exacerbations often require hospitalization, are associated with loss of lung function, markedly impact quality-of-life, and are associated with increased mortalityCitation7–9,Citation17. In a randomized, double-blind, placebo-controlled phase 3 study, ivacaftor taken orally twice a day showed a 55% reduction in the risk of PEx (p = 0.001) over a 48-week period as compared with placebo. Furthermore, patients treated with ivacaftor experienced significantly fewer hospitalization days due to a PEx (3.92 vs 4.15; p = 0.028)Citation1. A separate longitudinal observational cohort study by Rowe et al.Citation18 assessed hospitalizations before and after a group of patients with CF initiated ivacaftor treatment. During the 6 months prior to starting ivacaftor, 27% of patients had an inpatient hospitalization, whereas only 8% had a hospitalization during the 6 months after ivacaftor initiation (difference =19.1%; 95% confidence interval [CI] = 10.8–27.5; p < 0.001). Additionally, the total number of hospitalizations in the 6 months following ivacaftor initiation declined by 16.3% (95% CI =8.1–24.4; p < 0.001), as compared with the 6 months prior. Consistent with these findings, results of our current analysis also show that initiation of ivacaftor therapy is associated with lower rates of healthcare resource utilization, specifically inpatient hospitalizations, in patients with CF.
Poor adherence to standard-of-care regimens may play a major role in increasing the cost of healthcare in patients with CF, and thus ensuring high rates of adherence is a goal of therapyCitation19. Aggressive CF treatment plans that incorporate increasingly complicated, time-consuming daily regimens have led to challenges to adherence and disease self-managementCitation20,Citation21. Multiple studies have documented low adherence to treatment routines in CFCitation21–31. Barriers to adherence include perceived lack of efficacy, time management, and developmental challenges during the transition into adulthoodCitation21–24. Among adolescents and young adults with CF, adherence to chest airway clearance has been estimated to be 40–47%, while adherence to dietary recommendations is even lower, at ∼20%Citation25. Additionally, a study assessing the impact of medication adherence on lung function among patients with CF reported that adherence to chronic pulmonary medication averages close to 50%, with a range from 35–75%Citation26. Furthermore, the authors concluded that patients with CF with low adherence (MPR <50%) had the highest probability of suffering a PEx eventCitation27. These low levels of adherence among patients with CF are similar to those reported for patients suffering from other chronic diseases where numerous reviews have found adherence rates to cluster around 50%Citation28–31. In our study, adherence to ivacaftor exceeded reported adherence estimates from other studies of other chronic CF therapies, potentially due to several factors, including oral administration and high perceived treatment benefit among patients. Future work directed to those with low adherence is needed to understand drivers.
This analysis is limited by the small number of patients who met the inclusion criteria and initiated treatment with ivacaftor during the study period. At that time, ivacaftor was approved for treatment in patients with at least one G551D-CFTR mutation, which had a prevalence in the US of 4.4% according to the 2013 CFFPRCitation11. However, the sensitivity analysis which assessed a sample with shorter follow-up period found consistent results. Additionally, some patients included in the analysis may have received ivacaftor during clinical trials prior to January 1, 2012, since the use of ivacaftor in a clinical trial would not appear in an insurance claims database. As a result, some patients may have received ivacaftor during the pre-index period, potentially biasing their pre-index healthcare resource utilization. Despite this possibility of pre-index ivacaftor use, the analysis shows significant reductions in all-cause and CF-related inpatient admissions. Further, the decision to hospitalize a patient is driven by the patient’s clinician and may vary by center and region as well as other factors. Second, the analysis was conducted using healthcare claims data, which are collected for billing purposes and may be prone to data coding limitations and data entry error. The prevalence of co-morbid conditions, for instance, may differ from clinical trial or registry analyses due to differences in disease coding and/or reporting. Additionally, claims data do not include certain clinical characteristics such as lung function measurements, limiting the ability to assess impact on such physiologic outcomes. Third, adherence with therapy was estimated using outpatient pharmacy claims during the study period. Although office-administered medications indicate that therapy is actually given, it cannot be assumed that medications obtained in the outpatient pharmacy are taken by patients as prescribed. Additionally, this data does not capture written prescriptions that went unfilled. Finally, the claims data only included patients with commercial insurance in the US who received ivacaftor during the study period, thus the results may not be generalizable to individuals without insurance coverage or those with government subsidized insurance plans, which tend to include individuals in the US with lower socioeconomic status. Additionally, the results may not be generalizable to individuals who received ivacaftor after the study period, as a result of the expanded indication into patients with additional CFTR mutations.
Ivacaftor, a precision medicine for an orphan disease, is mutation-specific and indicated for a small percentage of patients with CF (∼5% of patients with CF). The objective of this study was to evaluate the clinical outcomes among commercially insured patients with CF who were treated with ivacaftor to assess the real-world treatment benefits in healthcare resource utilization, and was not designed to analyze the costs of medical care. Improvements in clinical outcomes, such as the reductions in hospitalizations demonstrated in this study, are important for assessment of therapeutic value along with the wider social value and other considerations, particularly in the case of orphan diseases like CF where the overall cost of treatment regimens can be highCitation32,Citation33. Future studies could expand the value assessment of ivacaftor therapy in several ways, including longer term clinical outcomes, health-related quality-of-life, impact on the patients’ families and broader society, along with the associated costs.
Conclusions
In this real-world, US-based claims database, ivacaftor therapy was associated with significant reductions in hospitalizations among a geographically diverse group of patients with CF. A majority of patients demonstrated adherence rates >80% to ivacaftor therapy. Although these findings should be interpreted with the knowledge that certain clinical parameters and biometric information are not captured in insurance claims data, the presented findings are consistent with previously reported results from clinical trials and observational studies, and suggest that ivacaftor treatment has benefits to patients outside the clinical trial settingCitation1–3,Citation12,Citation13.
Transparency
Declaration of funding
This study was funded by Vertex Pharmaceuticals Incorporated, and conducted by Truven Health Analytics, Cambridge, MA, USA.
Declaration of financial/other relationships
MB and BL are employees of Truven Health Analytics, who were paid by Vertex Pharmaceuticals Incorporated for this study and for the development of this manuscript. ES, LO, and DP are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. JSW was a former employee of Vertex Pharmaceuticals Incorporated. GS and JSW have received consultant fees and their institutions have received financial support from Vertex Pharmaceuticals Incorporated for study participation. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial co-ordination and support was provided by Dhrupad Patel, PharmD.
References
- Ramsey BW, Davies J, McElvaney NG, et al.; VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72
- Davies JC, Wainwright CE, Canny GJ, et al.; Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013;187:1219-25
- Whiting P, Al M, Burgers L, et al. Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2014;18:1-106
- Pedemonte N, Sonawane ND, Taddei A, et al. Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol 2005;67:1797-807
- MacDonald KD, McKenzie KR, Zeitlin PL. Cystic fibrosis transmembrane regulator protein mutations: 'class' opportunity for novel drug innovation. Paediatr Drugs 2007;9:1-10
- Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009;106:18825-30
- Bradley JM, Blume SW, Balp MM, et al. Quality of life and healthcare utilisation in cystic fibrosis: a multicentre study. Eur Respir J 2013;41:571-7
- de Boer K, Vandemheen KL, Tullis E, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011;66:680-5
- Britto MT, Kotagal UR, Hornung RW, et al. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002;121:64-72
- Mogayzel PJ, Jr., Naureckas ET, Robinson KA, et al.; Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013;187:680-9
- Cystic Fibrosis Foundation. Patient Registry Annual Data Report, 2013. Bethesda, MD: Cystic Fibrosis Foundation, 2014. https://www.cff.org/Our-Research/CF-Patient-Registry/. Accessed 1, February 2016
- Ziller V, Zimmermann SP, Kalder M, et al. Adherence and persistence in patients with severe osteoporosis treated with teriparatide. Curr Med Res Opin 2010;26:675-81
- Ziller V, Wetzel K, Kyvernitakis I, et al. Adherence and persistence in patients with postmenopausal osteoporosis treated with raloxifene. Climacteric 2011;14:228-35
- Huot L, Durieu I, Bourdy S, et al.; REMU study. Evolution of costs of care for cystic fibrosis patients after clinical guidelines implementation in a French network. J Cyst Fibros 2008;7:403-8
- Dewitt EM, Grussemeyer CA, Friedman JY, et al. Resource use, costs, and utility estimates for patients with cystic fibrosis with mild impairment in lung function: analysis of data collected alongside a 48-week multicenter clinical trial. Value Health 2012;15:277-83
- CF Care Center Visits. Bethesda, MD: CF Foundation; 2014. https://www.cff.org/Living-with-CF/Treatments-and-Therapies/The-Treatment-Plan/CF-Care-Center-Visits/. Accessed 18, March 2016
- Baumann U, Stocklossa C, Greiner W, et al. Cost of care and clinical condition in paediatric cystic fibrosis patients. J Cyst Fibros 2003;2:84-90
- Rowe SM, Heltshe SL, Gonska T, et al.; GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014;190:175-84
- Sawicki GS, Goss CH. Tackling the increasing complexity of CF care. Pediatr Pulmonol 2015;50(40 Suppl):S74-9
- Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009;8:91-6
- Ziaian T, Sawyer MG, Reynolds KE, et al. Treatment burden and health-related quality of life of children with diabetes, cystic fibrosis and asthma. J Paediatr Child Health 2006;42:596-600
- Kettler LJ, Sawyer SM, Winefield HR, et al. Determinants of adherence in adults with cystic fibrosis. Thorax 2002;57:459-64
- Myers LB, Horn SA. Adherence to chest physiotherapy in adults with cystic fibrosis. J Health Psychol 2006;11:915-26
- Sawicki GS, Tiddens H. Managing treatment complexity in cystic fibrosis: challenges and opportunities. Pediatr Pulmonol 2012;47:523-33
- Bregnballe V, Schiøtz PO, Boisen KA, et al. Barriers to adherence in adolescents and young adults with cystic fibrosis: a questionnaire study in young patients and their parents. Patient Prefer Adherence 2011;5:507-15
- Modi AC, Lim CS, Yu N, et al. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros 2006;5:177-85
- Eakin MN, Riekert KA. The impact of medication adherence on lung health outcomes in cystic fibrosis. Curr Opin Pulm Med 2013;19:687-91
- Eakin MN, Bilderback A, Boyle MP, et al. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros 2011;10:258-64
- Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh 2003;35:207
- Haynes RB, McDonald H, Garg AX, et al. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2002;CD000011:1-50
- Sackett DL, Haynes RB, Gibson ES, et al. Patient compliance with antihypertensive regimens. Patient Couns Health Educ 1978;1:18-21
- O’Sullivan BP, Orenstein DM, Milla CE. Pricing for orphan drugs: will the market bear what society cannot? JAMA 2013;310:1343-4
- Silverman E. Tiger in the fiscal room: beware the increasing cost and number of orphan drugs. Manag Care 2013;22:10-14