Abstract
Objective: One quit attempt with varenicline has been found to be a cost-effective smoking cessation intervention. The purpose of this study was to analyze varenicline’s cost-effectiveness in patients who relapse during or after the first treatment. A comparison was made between re-treatment schema with varenicline and re-treatment schema with bupropion, NRT and unaided cessation, and treatment once with varenicline in a Finnish context.
Methods: The two-quit version of BENESCO Markov model was used to follow a cohort of smokers making up to two quit attempts over a lifetime. The abstinence rates of the interventions were derived from a Cochrane review. Gender- and age-specific data on the incidence and prevalence of five smoking-related diseases were included in the model. Quality-adjusted life-years, total expected costs, and the lifetime cumulative incidence of smoking-related morbidities and mortality were the primary outcomes evaluated.
Results: The study cohort comprised 116,533 smokers who were willing to make a quit attempt. In the lifetime simulation, re-treatment with varenicline yielded 6,150–20,250 extra quitters, depending on the comparator. Among these quitters it was possible to prevent 899–2,972 additional cases of smoking-related diseases, and 395–1,307 deaths attributable to smoking. Re-treatment with varenicline resulted in cost savings of up to 54.9 million Euros. Re-treatment with varenicline dominated all the other smoking cessation interventions used in the analysis. Sensitivity analysis supported the robustness of the base case results.
Limitations: The analysis did not consider adverse events, and included only five major smoking-related diseases, which is a conservative approach, and probably leads to under-estimation of cost-effectiveness of cessation interventions. Furthermore, assumptions of constant relative risks for smoking-related diseases for each smoking status and the proxy values used as efficacy estimates of second quit attempts for other interventions than varenicline are limitations.
Conclusions: A second quitting effort with varenicline is economically justifiable.
Introduction
Although smoking is commonly decreasing in Western countries, it still causes vast economic and quality-of-life impacts in terms of morbidity, disability, and mortality associated to itCitation1. Healthcare costs and the number of premature deaths due to smoking-related diseases, like lung cancer, respiratory diseases, stroke and coronary heart disease, are significant. The burden of smoking-related morbidity in terms of direct and indirect costs can be significantly reduced by investing in effective smoking cessation. In 2012, 21% of men and 14% of women aged 15–64 years smoked daily in Finland. Despite the prevalence of smoking declining over time, tobacco use remains a major public health issue. According to Finnish statistics, more than half of the smokers would like to quit smoking and, of smokers, 38% of men and 46% of women have tried to quit during the last 12 monthsCitation2.
Because of the addictive nature of nicotine, the quit attempt often fails. Typically, 3–4 quit attempts are required for long-term abstinence, even with the aid of effective pharmacological agents and supportCitation3. Subsequent quit attempts increase the probability of successCitation4. In addition to efficacy and related clinical value, it is important to know the economic value of smoking cessation re-treatment. Varenicline has been found to be the most effective pharmacologic treatment in terms of smoking abstinenceCitation5,Citation6. It has also been found to be a cost-saving or cost-effective alternative compared to other commonly used smoking cessation interventions when considering only one quit attemptCitation7,Citation8. Only one cost effectiveness study related to re-treatment with varenicline has been published so farCitation9. The study evaluated the cost-effectiveness of re-treatment with varenicline compared with other smoking cessation interventions in Belgium, comparing one quit attempt of varenicline followed by re-treatment to treatment and re-treatment with nicotine replacement therapy, bupropion, or placebo, and with one quit attempt of varenicline. For 1,000 smokers willing to quit, varenicline re-treatment saved 275,000€, 118,000€, 316,000€, and 237,000€ compared to NRT, bupropion, placebo, or one varenicline quit attempt, respectively, in lifetime analysis from the healthcare payer perspective. The number of quality adjusted life years gained was 74, 63, 193, and 111 QALYs, respectively. In the long-term, varenicline re-treatment was a dominant intervention, meaning better effectiveness and less costs than with other interventions.
The purpose of this pharmacoeconomic analysis was to extend the cost effectiveness analysis to two quit attempts with varenicline using an adaptation of the Benefits of Smoking Cessation on Outcomes (BENESCO) modelCitation10 in a healthcare environment of Finland. Most smokers need more than one quit attempt to successfully quit smoking and, hence, this analysis represented real life more accurately compared to the previous analyses.
In this analysis the smoking cessation (SC) intervention of one quit attempt with varenicline (CHAMPIX) followed by a re-treatment with varenicline in case the patient fails with or relapses after initial treatment (two quit attempts with Varenicline = 2QA Varenicline) was compared with the other following SC interventions:
One quit attempt with bupropion followed by re-treatment bupropion in case of failure or relapse (= 2QA Bupropion);
One quit attempt with nicotine replacement therapy (NRT) followed by re-treatment NRT in case of failure or relapse (= 2QA NRT);
One quit attempt with unaided cessation followed by a 2nd quit attempt with unaided cessation (= 2QA Unaided); and
One quit attempt with varenicline followed by an attempt with unaided cessation (no medical re-treatment; 1QA Varenicline).
The patient population of this analysis is the Finnish smokers who may need up to two quit attempts to effectively stop smoking.
Patients and methods
Study population
The modeled population was the Finnish smokers aged 18 years and over who were motivated to quit smoking (in total 116,533 persons). The size and demography of this starting population is based on the 2013 census figuresCitation11, published smoking ratesCitation12, and quit attempt ratesCitation13. The smoking and quit attempt rates and baseline utility valuesCitation14 are presented in . The population was divided into age groups (18–34, 35–64, and 65+) by gender and the baseline smoking status (current smoker, former smoker, and never smoker). Therefore, the risks of developing smoking-related morbidities and death, as well as baseline utility values, differ between men and women and different age groups.
Table 1. Baseline information of the study population.
The BENESCO-model
Health economics modeling is a tool for estimating costs and health benefits related to a phenomenon of interest combining information from different sources. Modeling is necessary when there is no real-life data available, which is the case in this analysis. Because of the often complex nature of different phenomena, the models are not able to capture all aspects perfectly accurately, but the aim is to imitate real-life as well as possible, based on published data.
The Two-quit BENESCO (Benefit of Smoking Cessation on Outcomes) model is based on an adaptation of the original BENESCO model, a Markov model that was developed to assess the cost-effectiveness of one quit attempt with smoking cessation interventionsCitation10. The BENESCO model simulates the incidence of smoking-related morbidity and mortality over time and is an extension of the HECOS (Health Economic Consequences of Smoking) model that was used by the World Health Organization European Partnership Project to reduce tobacco dependence. The BENESCO model has also been reviewed in various health technology assessmentsCitation15. This model has been customized for various countries, including FinlandCitation7,Citation8,Citation16, and the results have been widely published. Quality assurance has been conducted for all structural changes made to the original BENESCO model, as well as for scenario testing and face validity checks of model results.
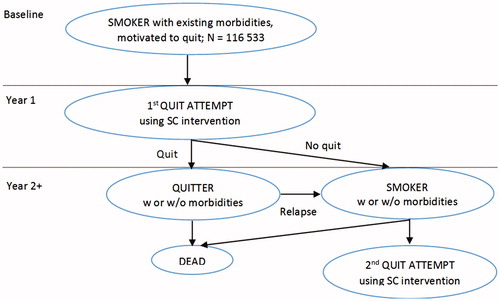
The BENESCO-model follows a hypothetical cohort of smokers from the time they have started their first quit attempt, until all members of the cohort have either died or reached the age of 100. The baseline smokers were simulated through the model such that their smoking, morbidity, and mortality status was evaluated after each year (cycle length = 1 year). The smoking statuses were: smoker due to failure or relapse, recent quitter (abstinent for 2–5 years after a successful quit attempt), and long-term quitter (abstinent for more than 5 years after successful quit attempt). Acute smoking-related morbidities which were taken into account were acute coronary syndromes (ACS [unstable angina and ST-segment and non-ST-segment elevation myocardial infarction (STEMI and NSTEMI)]), stroke, and asthma exacerbation (only those requiring hospitalization in an emergency), and chronic morbidities were lung cancer and COPD. The smokers could die at whichever state of the model either because of smoking related diseases or other causes. The simulation process is presented in Citation10.
Each health state was associated with utility: 0.76 for COPD for first and subsequent yearsCitation17,Citation18; 0.61 for first year and 0.50 for subsequent years for lung cancerCitation19; 0.76 for ACS for first and subsequent eventsCitation20; 0.74 for first event and 0.15 for subsequent events for strokeCitation21–23; and 0.52 for asthma exacerbationCitation24. The utility scores were used to adjust the time in a specific health state for the quality-of-life during this time, in order to calculate the quality-adjusted life-years (QALYs).
The model derived its transition probabilities from three factors: (1) the smoking quit rate (the efficacy of smoking cessation intervention), (2) time since quitting (the risks of relapsing to smoking and developing smoking-related morbidities markedly reduce with increasing duration of abstinence), and (3) the relative risk of developing one of the smoking-related morbidities (depending on the smoking state; does not differ between treatment groups)Citation10.
The annual relapse rate for abstinent quitters is 6%Citation25, and this rate is applied to recent quitters in the period of 2–5 years. After 5 years the risk of relapsing is reduced to 2% annually for the years 6–10Citation26. After 10 years the annual relapse rate is reduced to 1%. The hazard ratios of dying from smoking-related diseases have been used as a proxy for the relative risk (RR) of the incidence and prevalence of smoking-related diseasesCitation10. The risk for lung cancer equals the risk in recent quitters over a lifetime. The transition probabilities to smoking-related diseases are based on these RRs and the prevalence, incidence, and mortality cases of each smoking-related disease by age and gender.
The relative risks (RR) of smoking related morbidities and mortality were applied as in Howard et al.Citation10 separately to the baseline current, former, and never smokers. The RR for never smokers was 1.0, and the risks for current and former smokers were assessed relative to never smokers. It was assumed that there was no smoking related morbidity or mortality in the under 35 age group (except for asthma exacerbations) and, hence, the RR at the age group 18–34 was 1.0 for all the baseline smoking groups. Risk of COPD, lung cancer, ACS, and stroke increased with age independent to smoking status. For former smokers, the RR of these smoking-related morbidities was reduced over time compared to current smokers of the same age. The impact of abstinence time was taken into account by setting recent quitter’s RR to the level of former smoker and long-term quitter’s RR to the level of never smokerCitation9. The baseline percentages of COPD, lung cancer, ACS events, and stroke events were derived from Finnish prevalence and population figures entered into the model. The prevalence and incidence data for smoking-related morbidities were from Finnish population studies and registries. Mortality data for lung cancer were from the Finnish Cancer register and for COPD, ACS, and stroke from Statistics Finland.
As in any Markov model, the health states were mutually exclusive, which means that a subject could not have two different morbidities at the same time. Moreover, if a subject had one of the two acute morbidities (ACS or stroke) they could not develop the other; the same applied to the two chronic morbidities (lung cancer or COPD). Subjects could progress from an acute morbidity to a chronic, but not from a chronic to an acute. If a subject did progress from an acute morbidity to a chronic one, their acute morbidity was ignored from that point forward. This was a conservative approach as it excluded significant amount of costs and QALYs gained from the analysisCitation10.
As an extension to Howard et al.Citation10, in this two-quit model the cohort was allowed to have a second quit attempt after the first quit attempt which was unsuccessful due to treatment failure or relapse after a successful quit. For varenicline, the abstinence rates were derived from the recent Cochrane systematic review on the current smoking cessation strategies (21.1% for first quit attempt with varenicline)Citation6, as well as on the results of the new randomized clinical trial of varenicline in re-treatment (20.1% for second quit attempt)Citation27. This randomized, double-blind, placebo-controlled, multi-centre trial evaluated the efficacy and safety of re-treatment with varenicline. Trial participants were healthy adult smokers (≥10 cigarettes/day) with one or more prior quit attempts using varenicline and no quit attempts in the last 3 months. Patients were randomized to 12 weeks’ varenicline (n = 251) or placebo (n = 247) treatment, with individual counseling, plus 40 weeks’ non-treatment follow-up. The primary efficacy end-point was the carbon monoxide–confirmed continuous abstinence rate for weeks 9–12, which was 45.0% for varenicline and 11.8% for placebo (OR = 7.08; 95% CI = 4.34–11.55; p < 0.0001). Abstinence rates were comparable with rates reported for varenicline-naive smokers.
In the absence of efficacy data of bupropion and NRT at 52 weeks in re-treatment trials, we conservatively considered similar efficacy in initial treatment and re-treatment for these two SC interventions (15.7% for Bupropion and 14.9% for NRT)Citation6. Abstinence rate for unaided cessation was assumed to be 5.0% for first and second quit attemptCitation10. The annual relapse rate for years 2–5 after successful quit attempt (recent quitters) was 6%Citation25, for years 6–10 it was 2%, and after 10 years until death it was 1%Citation26.
The analysis was undertaken from the healthcare payer perspective. Only direct healthcare costs were taken into account, even though smoking-related morbidities, particularly stroke and COPD, may lead to important disability and significant indirect costs. The costs due to smoking-related morbidities were defined based on Finnish treatment practices and unit costs and represented the 2013/2014 costs. The costs of medicinal products (bupropion, varenicline, and NRT) were defined in retail prices without VAT. The cost estimates used in the model are presented in . Because smoking dependence was considered as a chronic condition with shift between remissions and relapses, and because smoking cessation strategies have long-term benefits, a lifetime horizon was considered. A discount rate of 3% was used for both costs and utilities, which represents the commonly used rate in Finland.
Table 2. Cost estimates (€) used in the model.
Limitations
Only the efficacy of the smoking cessation interventions is considered, with no consideration of adverse events. However, meta-analysisCitation6 shows that the probability of adverse events associated with varenicline, NRT, and bupropion is low and their impact on costs and effectiveness estimates, and, thus, the analysis, is minimal. Also, the model only includes five major smoking-related diseases, keeping co-morbidities to a minimum to keep the model as simple and illustrative as possible but to capture the main health and economic consequences at the same time. This simplified approach is conservative, and highly likely leads to under-estimation of cost-effectiveness of cessation interventions. Furthermore, relative risks for smoking-related diseases are assumed constant for each smoking status, which may be considered as a simplification of reality. Lastly, the assumption on equal efficacy in 1st and 2nd quit attempt with NRT, bupropion, or unaided cessation, or the fact that varenicline’s re-treatment efficacy estimate is based on only one clinical trial is a limitation. However, this approach is again conservative; the efficacy of varenicline re-treatment is slightly lower than in the 1st quit attempt, whereas equal efficacy is assumed for other interventions. The approach probably leads to under-estimation of varenicline’s cost-effectiveness in re-treatment.
Sensitivity analysis
To account for variability in outcomes due to statistical uncertainty in inputs, one-way deterministic sensitivity analyses (DSA) and a probabilistic sensitivity analysis (PSA) were performed. In PSA, the simulation was replicated for 1,000 times to generate incremental cost-effectiveness ratios (ICERs) by varying the effectiveness of SC interventions (beta distribution), treatment costs for smoking-related morbidities (log-normal distribution), and utilities (beta distribution) simultaneously.
Results
Base case
If 116,533 smokers motivated to quit smoking (current smokers who make a quit attempt in ) initiate SC treatment with varenicline and are re-treated with it in the case of failure or relapse (2QA Varenicline), 27,104 smokers will have successful quit at lifetime; equivalent figures for SC intervention 2QA Bupropion is 20,954, for 2QA NRT 19,882, for 2QA Unaided 6,854, and for 1QA Varenicline 17,482. This means that 6,150–20,250 extra smokers would be able to quit at lifetime if they were treated twice with varenicline instead of twice with bupropion, NRT, or unaided, or only once with varenicline.
The additional quitters from 2QA Varenicline consequently results in a decrease in the number of smoking-related morbidities and deaths. summarizes the number of COPD, lung cancer, ACS, and stroke cases and deaths that could be avoided by using 2QA Varenicline instead of the other SC interventions. Most morbidities and especially deaths can be prevented if they are caused by lung cancer, followed by stroke. Among the 116,533 smokers who are willing to make a quit attempt, a total of 2,519–8,372 life years could be saved if re-treatment with varenicline was used instead of the comparators.
Table 3. Numbers of smoking-related morbidities and mortalities avoided if using 2QA varenicline instead of other CS interventions.
The total lifetime costs, including medicinal costs and the treatment cost of smoking-related morbidities, and quality adjusted life years (QALYs) gained related to each SC intervention among the 116,533 smokers are presented in . 2QA Varenicline is more effective and less costly compared to all other SC interventions, i.e. it dominates all its comparators.
Table 4. The incremental costs, effectiveness, and cost effectiveness ratios (ICER) of SC intervention 2QA Varenicline compared to the other SC interventions (incremental costs represent the additional costs, and incremental QALYs the lower effectiveness, compared to 2QA Varenicline).
Sensitivity analysis
Similar to a previously reported re-treatment analysisCitation9, discount rates of both costs and effects, cost of cessation methods, and relative risks for smoking related diseases were the parameters the model is most sensitive to in one-way deterministic sensitivity analysis (DSA). However, changes of these parameter values within a plausible range did not change the conclusion drawn from the analysis.
The base case point estimate of varenicline’s re-treatment efficacy (20.1%) is based on one published clinical trial. To assess the significance of the related uncertainty, parameter value was varied by ±20% around the base case estimate in the DSA, from 16.1–24.1%. At the lower limit the dominance of 2QA Varenicline remained in comparisons vs 1QA Varenicline and Q2A Unaided. In comparisons vs 2QA Bupropion the ICER with was 4,550€/QALY and vs 2QA NRT it was 1,584€/QALY. At the upper limit dominance prevailed across the range of comparisons, similarly to the base case analysis.
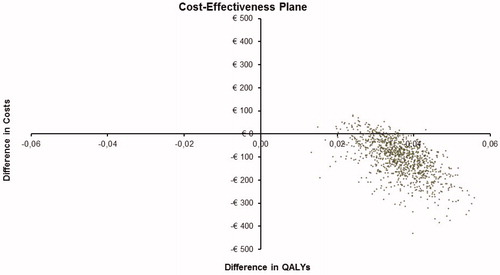
The probabilistic sensitivity analysis (PSA) revealed the robustness of the model results. Compared to the SC interventions 2QA Bupropion, 2QA Unaided, and 1QA Varenicline, the costs remain systematically lower and the effects higher with 2QA Varenicline. Compared to the SC interventions 2QA NRT, smoking cessation twice with varenicline is 99.9% cost effective at a willingness-to-pay threshold of 5,000€/QALY. Scatter plot from PSA vs 2QA NRT () shows that the vast majority of simulated observations locate in the 1st (low-right) quarter of the cost-effectiveness plane and 99.9% of all cases fulfil the criterion of being less than 5,000€/QALY. In analyses vs 2QA NRT, 2QA Bupropion, 2QA Unaided, and 1QA Varenicline, all simulated observations locate in the 1st quarter. This also means that cost-effectiveness acceptability curves (not shown) are flat, i.e. the probability of cost-effectiveness is at 100% throughout the range of all positive willingness to pay (WTP) threshold values.
Discussion
Our analysis shows that re-treatment with varenicline is therapeutically and economically worthwhile, even if the first smoking cessation attempt fails. It is a cost-saving smoking cessation intervention as compared to re-treatment with bupropion and nicotine replacement therapy, no re-treatment after one quit attempt with varenicline, and no treatment. Successful smoking cessation may be difficult in one attempt. Our results suggest that re-treatment with varenicline decreases mortality and morbidity due to smoking-related diseases and saves costs in a Finnish healthcare environment.
The original BENESCO-model considering only one quit attempt with varenicline has been widely used in many countriesCitation7,Citation8,Citation16, reviewed in health technology assessmentsCitation15, and the results have given strong evidence on the cost effectiveness of varenicline compared to all other commonly used SC interventions. In this analysis we have used the extended two-quit version of the BENESCO-model, which takes into account two quit attempts with varenicline and, hence, represents real life more accurately. Our results are in line with the previous analyses of one quit attempt: two quit attempts with varenicline also dominates by being more effective and less costly compared to all its comparators used in this analysisCitation7,Citation16.
Smoking cessation is one of the most significant targets in healthcare because it decreases morbidity risks markedly. In Finland, there is an ongoing project in which a smoking prevalence target of 3–5% by 2030 has been set (Non-smoking Finland by 2040–project; http://savutonsuomi.fi/). The target is ambitious since, currently, every fifth Finn still continues smokingCitation2. Our results demonstrate that investment in medical smoking cessation supports this target and even second attempts should be encouraged and assisted with effective drugs. By offering smokers two quit attempts with varenicline instead of a single one, it would be possible to achieve nearly 10,000 extra quitters, resulting in more than 1,400 cases avoided of COPD, lung cancer, myocardial infarction, and stroke, and 600 premature deaths. Additionally, this would save costs of 27.6 million euros in the long-term. Compared to unaided smoking cessation, re-treatment with varenicline would yield more than 20,000 extra quitters, and would avoid nearly 3,000 morbidity cases and ∼1,300 premature deaths. This would save treatment costs in the long-term by 54.9 million euros. From the healthcare perspective, effective smoking cessation is very important as it releases scarce healthcare resources into other use over the long-term. For therapeutic and economic value, varenicline and other smoking cessation medicines have become reimbursed in several countries or covered by insurance or other healthcare systemsCitation3,Citation28. For example, in Finland, one quit attempt with varenicline is reimbursed.
In this analysis we have concentrated solely on the health benefits achieved by effective smoking cessation and on the possibility of avoiding treatment costs related to smoking-related diseases. However, as a consequence of smoking abstinence people have a longer lifespan and, hence, time to cause costs to the society in terms of pensions and treatment of many age-related diseases. Tiihonen et al.Citation29 have explored the net effect of smoking by taking into account also these costs of increased life expectancy. According to their results, smoking causes savings to society only if no value is given for improved quality-of-life caused by smoking cessation. However, if each lost quality adjusted life year is considered to be worth 22,200€, the net effect is reversed to be 70,200€ per individual in favor of non-smoking. Proportioning this to our results of the amount of successful quitters, the possible savings would be even greater than those we have presented.
As all health economic models, the BENESCO-model is a simplification of real world. It is not able to take into account the complex nature of smoking and increased morbidity and mortality risks associated to it completely accurately. For simplicity the model has concentrated on the five most significant smoking-related diseases or events. The main shortcoming of the model is its inability to take two or more of these morbidities into account simultaneously, which might be the case with a real smoker. However, this does not favor varenicline relative to other SC interventions and, hence, can be considered as a conservative approach.
The assumption on equal efficacy in 1st and 2nd quit attempt with NRT, bupropion or unaided cessation, or the fact that varenicline’s re-treatment efficacy estimate is based on only one clinical trial is a limitation. However, this approach is again conservative; the efficacy of varenicline re-treatment is slightly lower than in the 1st quit attempt, whereas equal efficacy is assumed for other interventions. The approach probably leads to under-estimation of varenicline’s cost-effectiveness in re-treatment.
In conclusion, tobacco dependence is a chronic disease and success in quitting smoking may require more than one quit attempt. Our analysis show that re-treatment with varenicline in the case of failure with or relapse after initial treatment is a cost saving SC intervention compared to re-treatment with bupropion and NRT, no re-treatment after one quit attempt with varenicline, and no treatment. Supporting smokers in their attempts to quit smoking is also economically highly justifiable.
Transparency
Declaration of funding
This work was funded by Pfizer, Inc.
Declaration of financial/other interests
At the time of manuscript preparation, KK was an employee of a consulting company Medaffcon Oy which offers medical and health economic expert services to pharmaceutical companies. JH is CEO and partner at Medaffcon Oy. The personnel of the company have completed, and at the moment are completing, assignments for many pharmaceutical companies, including Pfizer Oy, Finland. JL and KL are employees of Pfizer Oy, Finland. HE has received fees for speaking or consultancy from Pfizer. MP has received fees for speaking or consultancy from Pfizer and Johnson & Johnson. HL has received fees for speaking or consultancy from Pfizer and Lundbeck. This study was sponsored by Pfizer. KK and JH, both at Medaffcon Oy, were paid consultants to Pfizer in connection with the development of this manuscript.
Acknowledgments
Triin Männik provided valuable assistance in revising the manuscript.
References
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf. Accessed June 10, 2015. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health 2014
- National Institute for Health and Welfare. Tobacco statistics 2012. http://www.julkari.fi/bitstream/handle/10024/110551/Tr27_13.pdf?sequence =4. Accessed January 19, 2015. Helsinki: THL 2013
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence: 2008 Update. Clinical Practice Guideline. http://www.ncbi.nlm.nih.gov/books/NBK63952/. Accessed 26, March 2015. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service 2008
- Getsios D, Marton JP, Revankar N, et al. Smoking cessation treatment and outcomes patterns simulation: a new framework for evaluating the potential health and economic impact of smoking cessation interventions. PharmacoEconomics 2013;31:767-80
- Mills EJ, Wu P, Lockhart I, et al. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med 2012;44:588-97
- Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013;5
- Linden K, Jormanainen V, Linna M, et al. Cost effectiveness of varenicline versus bupropion and unaided cessation for smoking cessation in a cohort of Finnish adult smokers. Cur Med Res Opin 2010;26:549-60
- Zimovetz EA, Wilson K, Samuel M, et al. A review of cost-effectiveness of varenicline and comparison of cost-effectiveness of treatments for major smoking-related morbidities. J Ev Clin Pract 2011;17:288-97
- Annemans L, Marbaix S, Nackaerts K, et al. Cost-effectiveness of retreatment with varenicline after failure with or relapse after initial treatment for smoking cessation. Prev Med Reports 2015;2:189-95
- Howard P, Knight C, Boler A, et al. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO simulation model: application to a population of US Adult Smokers. PharmacoEconomics 2008;26:497-511
- Statistics Finland. Population by age (1-v), gender and area 1980–2013. http://193.166.171.75/Dialog/varval.asp?ma=050_vaerak_tau_104&ti=V%E4est%F6+i%E4n+%281%2Dv%2E%29+ja+sukupuolen+mukaan+alueittain+1980+%2D+2013&path=./Database/StatFin/vrm/vaerak/&lang=3&multilang=fi. Accessed January 19, 2015
- Borodulin K, Levälahti E, Saarikoski L, et al. Kansallinen FINRISKI 2012 –terveystutkimus. Osa 2: tutkimuksen taulukkoliite. http://www.julkari.fi/bitstream/handle/10024/110912/URN_ISBN_978-952-302-053-5.pdf?sequence=. Accessed January 9, 2015. Helsinki: THL 2014
- Helldán A, Helakorpi S, Virtanen S, et al. Health behaviour and health among the Finnish Adult Population, Spring 2013. http://www.julkari.fi/bitstream/handle/10024/110841/URN_ISBN_978-952-302-051-1.pdf?sequence =1. Accessed January 19, 2015. Helsinki: THL 2013
- Koskinen S, Lundqvist A, Ristiluoma N, eds. Health, functional capacity and welfare in Finland in 2011. http://www.julkari.fi/bitstream/handle/10024/90832/Rap068_2012_netti.pdf?sequence=1. Accessed February 2, 2015. Helsinki: THL 2012
- Hind D, Tappenden P, Peters J, et al. Varenicline in the management of smoking cessation: a single technology appraisal. Health Technol Assess 2009;13(2 Suppl)
- Annemans L, Nackaerts K, Bartsch P, et al. Cost-effectiveness of varenicline in Belgium, compared with bupropion, nicotine replacement therapy, brief counselling and unaided smoking cessation: a BENESCO Markov cost-effectiveness analysis. Clin Drug Investiq 2009;29:655-65
- Mannino DM, Buist AS, Petty TL, et al. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003;58:388-93
- Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. PharmacoEconomics 2005;23:619-37
- Trippoli S, Vaiani M, Lucioni C, et al. Quality of life and utility in patients with non-small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. PharmacoEconomics. 2001;19:855-63
- Hay JW, Sterling KL. Cost effectiveness of treating low HDL-cholesterol in the primary prevention of coronary heart disease. PharmacoEconomics 2005;23:133-41
- Gage BF, Cardinalli AB, Owens DK. Cost-effectiveness of preference-based antithrombotic therapy for patients with nonvalvular atrial fibrillation. Stroke 1998;29:1083-91
- Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacol 2000;39:835-41
- Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. PharmacoEconomics 2003;21:191-200
- Szende A, Svensson K, Stahl E, et al. Psychometric and utilitybased measures of health status of asthmatic patients with different disease control level. PharmacoEconomics 2004;22:537-47
- Wetter DW, Cofta-Gunn L, Fouladi RT, et al. Late relapse/sustained abstinence among former smokers: a longitudinal study. Prev Med 2004;39:1156-63
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res 2002;4:95-100
- Gonzales D, Hajek P, Pliamm L, et al. Retreatment With Varenicline for Smoking Cessation in Smokers Who Have Previously Taken Varenicline: A Randomized, Placebo-Controlled Trial. Clin PharmTher. 2014;96:390-396.
- Reda AA, Kotz D, Evers SMAA, et al. Healthcare financing systems for increasing the use of tobacco dependence treatment (Review). The Cochrane Collaboration 2012;Issue 6
- Tiihonen J, Ronkainen K, Kangasharju A, et al. The net effect of smoking on healthcare and welfare costs. A cohort study. BMJ Open 2012;2:e001678
- Kapiainen S, Väisänen A, Haula T. Terveyden- ja sosiaalihuollon yksikkökustannukset Suomessa vuonna 2011. https://www.julkari.fi/bitstream/handle/10024/114683/THL_RAPO3_2014_web.pdf?sequence =1. Accessed March 9, 2015. Helsinki: THL 2014