Abstract
Aims: This study assessed the cost-effectiveness of the subcutaneous RANKL inhibitor, denosumab, vs the intravenous bisphosphonate, zoledronic acid, for the prevention of skeletal-related events (SREs) in patients with prostate cancer, breast cancer, and other solid tumors (OST) in the Czech Republic.
Materials and methods: A lifetime Markov model was developed to compare the effects of denosumab and zoledronic acid on costs (including drug costs and administration, patient management, SREs, and adverse events), quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios from a national payer perspective. Different discount rates, time horizons, SRE rates, distributions, and nature (asymptomatic vs all SREs), and the inclusion of treatment discontinuation were considered in scenario analyses. The robustness of the model was tested using deterministic and probabilistic sensitivity analyses.
Results: Across tumor types, denosumab was associated with fewer SREs, improved QALYs, and higher total costs over a lifetime. The incremental cost per QALY gained for denosumab vs zoledronic acid was 382,673 CZK for prostate cancer, 408,450 CZK for breast cancer, and 608,133 CZK for OST. Incremental costs per SRE avoided for the same tumor type were 54,007 CZK, 51,765 CZK, and 94,426 CZK, respectively. In scenario analyses, the results remained similar to baseline, when different discount rates and time horizons were considered. At a non-official willingness-to-pay threshold of 1.2 million CZK, the probabilities of denosumab being cost-effective vs zoledronic acid were 0.64, 0.67, and 0.49 for prostate cancer, breast cancer, and OST, respectively.
Limitations: The SRE rates used were obtained from clinical trials; studies suggest rates may be higher in clinical practice. Additional evidence on real-world SRE rates could further improve the accuracy of the modeling.
Conclusions: Compared with zoledronic acid, denosumab provides a cost-effective treatment option for the prevention of SREs in patients with prostate cancer, breast cancer, and OST in the Czech Republic.
Introduction
Bone metastases are a common complication of advanced solid tumors, and induce bone destruction through increased osteoclast activityCitation1. Almost all patients with prostate cancer, the majority of patients with breast cancer, and a large proportion of patients with lung or kidney cancer, will develop bone metastases during the advanced stages of their diseaseCitation1,Citation2. Skeletal-related events (SREs; pathologic fracture, spinal cord compression, surgery to bone, and radiation to bone) are a common complication of bone metastases and are painful and debilitating. SREs also interfere with patients’ mobility, functional ability, and independence, and reduce overall quality-of-life (QoL)Citation1,Citation3–5. In addition, patients with SREs may require numerous hospitalizations, outpatient visits, and proceduresCitation6–8, all of which are associated with substantial costsCitation9,Citation10.
Bone-targeted agents such as bisphosphonates (predominantly intravenous [IV] zoledronic acid) and the RANK ligand inhibitor denosumab can prevent SREs in patients with bone metastasesCitation11,Citation12. Three large, active comparator controlled, double blind, double-dummy phase 3 clinical trials, with identical patient inclusion and exclusion criteria and study endpoints, have compared subcutaneous (SC) denosumab (120 mg every 4 weeks [Q4W]) with zoledronic acid (4 mg IV Q4W) in patients with solid tumors (OST) and bone metastasesCitation13–15. The three studies were run concurrently, and had identical study designs, except for tumor types and sample sizes. Denosumab was superior to zoledronic acid for preventing a first on-study SRE in patients with breast cancer or prostate cancer and was more effective than zoledronic acid in an ad hoc analysis of patients with OSTCitation13–15. Compared with zoledronic acid, denosumab also significantly reduced the risk of multiple (first and subsequent) SREs in all three tumor types (rate ratio [95% confidence interval (CI)] for breast cancer, 0.77 [0.66–0.89]; prostate cancer, 0.82 [0.71–0.95]; and OST, 0.85 [0.72–1.00])Citation13–15. Overall, patients randomized to denosumab experienced fewer SREs than those who received zoledronic acidCitation13–15. The proportions of patients who experienced adverse events (AEs) were similar with the two drugsCitation13–15. An integrated analysis of the three phase 3 trials reported that similar proportions of patients in the two groups experienced AEs and serious AEsCitation16. In general, the types of AEs experienced were similar, with the exceptions of hypocalcemia, which was more common in the denosumab group than in the zoledronic acid group, and acute phase reactions and renal AEs, which were more common in those receiving zoledronic acid than in those receiving denosumabCitation16.
Although denosumab has been shown to be clinically superior to zoledronic acid, the economic benefit of using denosumab rather than zoledronic acid in specific countries remains unclear. In this study, we assessed the cost-effectiveness of denosumab vs zoledronic acid in the prevention of SREs in adults with bone metastases from prostate cancer, breast cancer, and OST (excluding hematological malignancies) from a national payer perspective in the Czech Republic.
Methods
Model design
A Markov cohort model was constructed in Microsoft Excel® 2010. The model structure was identical for all tumors, but inputs for the analysis were specific to the tumor type. The model had three health states: “on treatment”, “off treatment”, and “death”. The “on treatment” state was associated with a risk for SREs, AEs, and death. Costs associated with treatment, administration, patient management, SREs, and AEs were calculatedCitation17–19. Declines in health utility, expressed as decrements in quality-adjusted life-years (QALYs) due to SREs, AEs, and mode of drug administration, were also estimated. The “off treatment” state was associated with the same risk of death as the “on treatment” state, but with a higher risk of SREs, resulting in higher costs and greater utility decrements.
A 28-day (4-week) cycle was applied to the model to correspond with the dosing schedule recommended for both productsCitation20,Citation21. The analysis considers a lifetime horizonCitation22; the model was run for 200 cycles (∼15.4 years), after which more than 99% of patients were expected to have died.
Model event parameters
Event probabilities for the following were derived from phase 3 trial dataCitation13–15,Citation23,Citation24: SRE distribution, AEs, treatment discontinuation, death, and relative risk of a SRE after treatment discontinuation. The model used a fixed (constant) rate over the modeling timeframe for all SREs and AEs, consistent with previously published dataCitation25–28, allowing the extrapolation of results beyond the timeframe of the clinical trials. All SRE rates (and all other annual rates in the model) were converted to 4-week probabilities, based on an exponential relationship between rates and probabilities, aligned with the assumption of a constant event rate over time.
Skeletal-related events
In the base case analysis, annualized SRE rates for patients treated with zoledronic acid were based on adjusted SRE rates observed in clinical trials. The adjustment factor was based on the rate ratio of real-world-based and clinical trial-based SRE ratesCitation13–15. Real-world SRE rates were based on an additional post hoc analysis of European data from the study by Hechmati et al.Citation9, a multi-center, prospective, observational study conducted in the US, Canada, Germany, Italy, Spain, and the UK. Based on a sample size of 188 patients, with an overall time at risk of 85.5 subject-years, the pooled yearly SRE rate observed was 3.8 across all tumors in patients treated with zoledronic acid and who had experienced at least one SRE prior to study enrollment. This rate was divided by 12 to estimate the monthly SRE rate, and was subsequently compared with the combined monthly SRE rate observed in the three clinical trials in patients receiving zoledronic acid and who had a history of at least one SRE at baseline. The rate ratio between the real-world data and trial results generated a real-world adjustment factor of 2.84 (0.3167 ÷ 0.1117). A scenario analysis was included with an adjustment factor of 2.01, based on results from a retrospective analysis of a US claims database (a nationally representative database of medical and pharmaceutical claims from 80 US health plans, including 55 million patients)Citation29, as described in Stopeck et al.Citation30. For denosumab, annualized SRE rates were estimated by applying a treatment effect rate ratio of denosumab vs zoledronic acid (). The SREs included in the model were pathologic fracture, radiation to bone, surgery to bone, and spinal cord compression (). The distributions of the different types of SRE applied in the base case are those observed in the three head-to-head clinical trials, by tumor type, pooled between the two treatment armsCitation13–15. The expected mean cost of a SRE, based on the proportion of each SRE and its associated cost, was calculated. Reductions in QALYs were estimated in the same manner. As a result, each SRE had an associated mean cost and QALY decrement, which were incorporated into the model explicitly.
Table 1. Inputs into the cost-effectiveness model: base case.
Adverse events
In a pre-specified integrated analysis of the three phase 3 trials assessing the efficacy of denosumab vs zoledronic acid in solid tumors, the overall proportions of patients experiencing any AE (96.2% for denosumab; 96.8% for zoledronic acid) or a serious AE (56.3% and 57.1%, respectively) were similar for the two drugsCitation16. Serious AEs from the integrated analysisCitation16 (i.e. positively adjudicated osteonecrosis of the jaw, hypocalcemia, renal toxicity, and acute phase reactions) were used to derive AE rates in the model, in order to reflect clinically and potentially economically important events that may be related to treatment. AEs are included in the base case analysis.
Discontinuation
Discontinuation of therapy was incorporated into the model based on treatment-specific clinical trial dataCitation30. For each tumor type, the number of patients who discontinued therapy for any reason (excluding death) was divided by the person-years of follow-up (the same value used to estimate annual SRE rates) and the resulting annual discontinuation rate () converted to a 4-week probability. The SRE rate for patients who discontinued treatment was based on a meta-analysis of clinical trials comparing zoledronic acid with placebo in patients with breast cancerCitation23, and a phase 3 randomized trial of zoledronic acid vs placebo in patients with prostate cancerCitation24. The relative SRE rate ratios for patients discontinuing placebo compared with those discontinuing zoledronic acid were 0.59 and 0.54, in breast and prostate cancer, respectively. The relative SRE rate for placebo vs zoledronic acid was calculated to be 1.75 (the mean of the two rates). To predict the SRE rate in patients discontinuing therapy, this relative rate was applied to the observed SRE rate for zoledronic acid in the phase 3 denosumab clinical trialsCitation13–15.
Overall mortality
Overall survival was not different between zoledronic acid and denosumab in the three phase 3 trialsCitation13–15. Therefore, overall mortality was estimated using data pooled across both treatment groups. A generalized gamma model was used to estimate the overall survival for the lifetime horizon of the cost-effectiveness analysis. The gamma distribution was selected based on a comparison against other parametric models including Weibull, log-logistic, generalized gamma, log-normal, exponential, normal, logistic, and extreme value. The mean overall survival based on the calculated survival functions was 2.24 years for prostate cancer, 3.73 years for breast cancer, and 1.73 years for OST.
Health-related quality-of-life
Baseline utility values (for patients with no SREs either on or off treatment) were derived from utility profile analyses of 5-dimension EuroQol questionnaire data measured every 4 weeks in the trial of denosumab vs zoledronic acid for patients who did not experience SREsCitation30. The QALY impact associated with having a SRE was obtained through an independent time trade-off (TTO) study in the general population, which showed that all SREs are associated with a QALY reduction, and suggested a decrease in QoL beyond that anticipated for cancer with bone metastasesCitation31. QALY decrements per SRE are presented in .
The impact of administration route (IV vs SC) on QoL was also assessed using a similar TTO approachCitation31. The utility decrement associated with a SC or IV infusion for SRE prevention therapy (i.e. denosumab or zoledronic acid) in addition to regular chemotherapy was estimated and applied in the model. The mean disutility for receiving monthly SC injections for 1 year (0.015) and the mean disutility for receiving monthly IV infusions for 1 year (0.027) were divided by 13, and applied in each cycle for patients who were on treatment with denosumab and zoledronic acid, respectively.
Costs
The national payer perspective was adopted for the analysis, and the cost inputs are presented in . SRE costs per SRE type were based on unit costs in the Czech RepublicCitation18,Citation19, applied to country-specific healthcare resource utilization (HRU) data obtained from a retrospective chart review of patients with bone metastases/lesions secondary to breast, lung, or prostate cancer or multiple myeloma conducted in eight European countries, including the Czech RepublicCitation6. The drug acquisition costs of denosumab 120 mg SC administration and zoledronic acid 4 mg IV administration were sourced from the State Institute for Drug Control (SÚKL) list of reimbursed medicines from December 2015Citation19. The costs of drug administration, AEs, and patient management were estimated based on a micro-costing approach. The resources associated with each type of cost were derived from a local expert panel and the unit costs from the SÚKL list of reimbursed medicines, list of diagnosis-related group (DRG) codes, and list of reimbursed health procedures in 2015 in the Czech RepublicCitation18,Citation19. The patient management costs reflect the expenses related with monitoring patients for renal toxicity as a consequence of being treated with denosumab or zoledronic acid, and included physician office visits and laboratory tests.
Both denosumab and zoledronic acid were assumed to be administered once every 4 weeks (or 13 times per year) while the patient is alive, and discontinued according to the methodology previously described.
Cost-effectiveness analyses
The cost-effectiveness analyses were performed in terms of incremental cost both per SRE avoided and per QALY gained. The base-case analysis considered cost-effectiveness over a lifetime, a discount rate of 3% per year for costs and health outcomes of interest from a payer’s perspective, in accordance with local guidelines for the economic evaluation of health technologiesCitation32.
Multiple additional scenario analyses were performed including: considering alternative discount rates (0% and 5% for both costs and health benefits); a shorter time horizon (39 cycles of 4 weeks, equivalent to 3 years); USA SRE rates for patients treated with zoledronic acid (reported in a retrospective analysis of a USA claims databaseCitation29); different distributions of SRE types (based on data from the Surveillance, Epidemiology, and End Results Program [SEER] databaseCitation33–35); no discontinuation of active treatment; and accounting for the fact that some SREs may be considered asymptomatic. Because limited information is available regarding the proportion of SREs that are asymptomatic, it was assumed in this latter analysis that all vertebral fractures were asymptomatic (their costs and QALY adjustments were set as 0, as these events would not be treated and there would be no utility decrement).
One-way deterministic sensitivity analyses
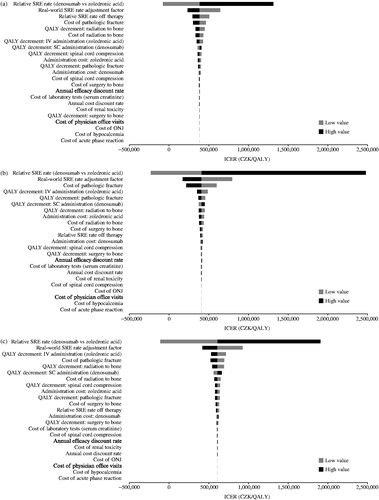
The robustness of the results was tested using one-way deterministic sensitivity analyses for key model probabilities, utilities, costs, treatment effect, and baseline risk of SREs (using the zoledronic acid SRE rate). A ±30% variation in the inputs was used to test the impact of each individual variable on the final results. Data are presented by tumor type in tornado diagrams.
Probabilistic sensitivity analyses
Probabilistic sensitivity analyses (PSAs) enable assessment of the uncertainty by sampling inputs according to distributions that reflect the range of likely values for each input, thus creating a distribution of possible outcomes. These were used to generate cost-effectiveness scatter plots (data not shown) and acceptability curves. The methodology used is similar to that described in Stopeck et al.Citation30, with specific parameters and distributions described in the Appendix.
Results
Cost-effectiveness analyses
Base case analysis
Using our model, the lifetime predicted SREs avoided per patient was 0.272 for OST, 0.628 for prostate cancer, and 0.731 for breast cancer (). It is likely that the lower number of predicted SREs avoided in patients with OST is owing to the shorter median overall survival compared with the other tumor typesCitation13–15. In this base case analysis, using denosumab was predicted to be associated with QALYs gained, additional total costs, costs avoided related to drug administration, and patient management of SREs and AEs (). The additional predicted QALYs gained were 0.0887 for prostate cancer, 0.0926 for breast cancer, and 0.0423 for OST. The costs avoided by using denosumab in the management of SREs were estimated at 41,525 Czech Republic Koruna (CZK) (prostate cancer), 74,440 CZK (breast cancer), and 21,400 CZK (OST). For administration costs, denosumab avoided an estimated 3084 CZK (prostate cancer), 5328 CZK (breast cancer), and 2240 CZK (OST). It was predicted that using denosumab would avoid AE-related costs: 522 CZK (prostate cancer), 1202 CZK (breast cancer), and 506 CZK (OST). Finally, for patient management, denosumab avoided 739 CZK (prostate cancer), 1711 CZK (breast cancer), and 721 CZK (OST). These savings were offset by the additional predicted overall costs associated with denosumab (33,934 CZK in prostate cancer; 37,838 CZK in breast cancer; 25,716 CZK in OST), in particular the drug costs of denosumab in comparison with zoledronic acid (79,805 CZK for prostate cancer, 120,518 CZK for breast cancer, and 50,584 CZK for OST). The resulting incremental cost-effectiveness ratios (ICERs) measured in incremental cost per incremental QALY were calculated to be 382,673, 408,450, and 608,133 CZK in prostate cancer, breast cancer, and OST, respectively. Considering the typical threshold in cost-effectiveness analysis in the Czech Republic (1.2 million CZK; 3 times gross domestic product per capita), the results indicate that denosumab provides a cost-effective treatment option in the Czech Republic vs zoledronic acid. When considering SREs avoided as the effectiveness measure, the ICERs predicted were 54,007 CZK in patients with prostate cancer, 51,765 CZK in patients with breast cancer, and 94,426 CZK in those with OST ().
Table 2. Base case analysis: costs, outcomes, and cost-effectiveness of denosumab vs zoledronic acid in patients with prostate cancer, breast cancer, or other solid tumors.
Scenario analysis
The results of the scenario analyses are summarized in . In all tumor types, the use of alternative SRE rates, based on the Hatoum et al.Citation29 study, and including asymptomatic fractures in the model, were the top two scenarios that substantially increased the predicted ICERs per QALY. Considering alternative discount rates for costs and health benefits (0% and 5%, respectively) and the alternative time horizon (39 cycles) impacted on the results only slightly. For breast and prostate cancer, using the SRE distribution derived from real-world dataCitation36 (SEER-Medicare population) did not affect the model results, but this SRE distribution was the third most influential scenario in patients with OST, increasing the incremental cost per QALY by 22%.
Table 3. Scenario analyses: effect of varying discount rate, time horizon, SRE rate, discontinuation of treatment, and asymptomatic SREs on the cost-effectiveness of denosumab vs zoledronic acid.
Sensitivity analyses
Deterministic sensitivity analyses
Tornado diagrams were used to identify the model parameters that were key drivers of the results, applying a ±30% input parameter variation. For all tumor types, the relative SRE rate for denosumab vs zoledronic acid had the highest impact on the ICER (). The second most influential parameter across all tumor types was the relative real-world-adjusted SRE rate. The QALY decrement due to IV infusion of zoledronic acid also impacted highly on ICERs (). There were some differences in the variables that impacted on the ICER between the different tumor types: pathologic fracture and the resultant QALY decrement were more important in breast cancer than in the other tumor types (). QALY decrement due to radiation to bone and cost of this treatment had a greater influence on the ICER in prostate cancer and OST than in breast cancer ().
Probabilistic sensitivity analyses
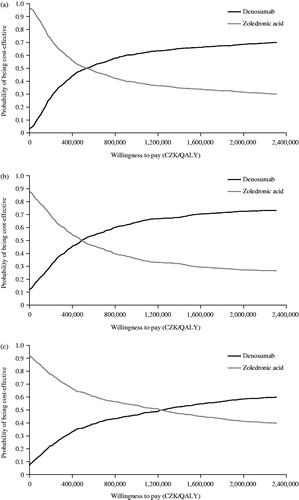
PSAs were also performed to assess the uncertainty associated with the different parameters used in the model. The model was run for 1000 Monte Carlo simulations. Cost-effectiveness acceptability curves for prostate cancer and breast cancer showed that denosumab was the preferred treatment over zoledronic acid (). Although not official, the typical threshold used in cost-effectiveness analyses in the Czech Republic is 1.2 million CZK. Using this threshold, treatment of patients with prostate cancer with denosumab had a 0.64 chance of being cost-effective (). Similarly, for breast cancer and OST, the probability of denosumab being cost-effective was 0.67 () and 0.49 (), respectively.
Discussion
Cost-effectiveness analyses are important for payers to make informed decisions on the allocation of healthcare resources. Payers need to identify the treatment options that offer the greatest benefit for a given costCitation37. In the analyses described here, the use of denosumab resulted in fewer SREs, more QALYs, and higher total treatment costs compared with the use of zoledronic acid.
Denosumab provided a cost-effective treatment option vs zoledronic acid. The main drivers of the cost-effectiveness results in our study were the treatment effect (clinical efficacy) of denosumab, in comparison with zoledronic acid, and the baseline frequency of SREs for patients receiving zoledronic acid (and consequent rate ratio to adjust the clinical trial SRE rates to reflect observations from clinical practice). The different distributions of SREs across the tumor types were also key determinants of the variables which were most impactful in the three populations. The proportion, for instance, of patients who experience pathologic fractures is higher in breast cancer relative to the other tumor types (58% vs 27% in prostate cancer and 31% in OST). As pathologic fracture is the second most expensive SRE type in the Czech Republic, the average SRE cost and the overall SRE cost savings from the use of denosumab were higher in the breast cancer analysis compared to prostate cancer and OST, and the base case results were more sensitive to changes of the cost of pathologic fracture. Similarly, the majority of prostate cancer patients (66%) experience radiation to the bone, which justifies the higher sensitivity of the results in changes of the cost and QALY decrement of this SRE type.
SREs are considered an objective and clinically relevant end-point for the evaluation of complications in patients with advanced cancerCitation38. They are often severe and debilitatingCitation39, and result in substantial HRU and costsCitation40. Therefore, the impact of SREs on patients and healthcare systems are considerable and, by reducing these events, the burden can be lessened. In our model, denosumab use resulted in lower costs associated with patient and SRE management, and fewer AEs, together with gains in QALYs, compared with zoledronic acid. Similar results were reported in a cost-effectiveness analysis of denosumab vs zoledronic acid in patients with bone metastases secondary to solid tumors in the US. The value of denosumab is derived from its clinical efficacy, favorable safety, and efficient administrationCitation30.
Zoledronic acid is an appropriate comparator in these analyses, because many patients with cancer and bone metastases will likely receive a bone-targeted agent during the course of their disease management, of which zoledronic acid is one of the most commonly used in Europe so farCitation41. As reported by Lipton et al.Citation16, denosumab showed a clinical benefit across patients with advanced solid tumors and bone metastases in comparison with zoledronic acid, including in patients with a prior history of SREs. A budget impact analysis of denosumab vs zoledronic acid in Austria, Sweden, and Switzerland predicted SRE rate reductions and corresponding budget offsets, even in the scenario in which the price of zoledronic acid was reduced, reflecting the release of the generic formulationCitation42.
The availability of Czech-specific inputs on quality-of-life is, in general, extremely rare. Local data on SRE- and AE-specific QALY decrements are lacking. Therefore, extrapolation was used for this cost-effectiveness analysis conducted in the Czech setting by adopting disutilities available in the literature, which is widely accepted by experts and local authorities. Based on the available evidence, the rate of SREs observed in clinical practice is higher than the rate observed in the clinical trialsCitation9,Citation29. Some potential reasons for this relate to the restrictions on patient populations included in clinical trial settings. Inclusion and exclusion criteria may have meant that populations with less severe disease were enrolled (e.g. patients with an Eastern Cooperative Oncology Group performance score of 0–2 or patients with a life expectancy of more than 6 months). Additional evidence regarding the SRE rates observed in real-world clinical practice in the Czech Republic would improve the accuracy of the modeling results reported here. To the best of our knowledge, such country-specific evidence is not available. The uncertainty around these SRE rates, as well as the other main parameters included in the model, was tested in the probabilistic sensitivity analysis, in which in the majority of the simulations performed, the ICERs derived were below the typical cost-effectiveness threshold of 1.2 million CZK used in the Czech Republic.
In local clinical practice both zoledronic acid and denosumab are being used entirely in line with their SmPC and considered RCTs. According to label, zoledronic acid can be administered either once in 3 or 4 weeks. Local clinicians confirm that the frequency once in 4 weeks is the one used in Czech clinical practice. This frequency was, therefore, applied for the purposes of the cost-effectiveness analysis.
The cost of drug administration was substantially lower with denosumab than with zoledronic acid, reflecting different routes of administration between the two treatments. Non-interventional time and motion studies reported substantial reductions in preparation and administration times with denosumab compared with zoledronic acidCitation43,Citation44. Administration route may also contribute to treatment adherence, which, in turn, will improve patient outcomes. In the scenario analysis presented here, in which there was no treatment discontinuation, more SREs were avoided and more QALYs gained than in the base case. There were cost increases for ICERS per QALY gained and per SRE avoided in this scenario compared with baseline, but these effects were minimal.
In addition to the inputs considered in this model, other factors may impact on cost-effectiveness, such as patient preference. A systematic review found that two-thirds of studies reported a clear patient preference for SC over IV drug deliveryCitation45, attributed to the time saving and potential for treatment at homeCitation45. A retrospective analysis in the US of patients with solid tumors and bone metastases who received a bone-targeted agent found that patients treated with denosumab were more likely to remain on treatment and showed better adherence than those receiving IV bisphosphonatesCitation46. In a study of patient preference in France, Germany, and the UK, patients considered delaying SREs, avoiding renal impairment, and delaying pain worsening as the most important factors when deciding upon a treatment optionCitation43. Engaging patients in treatment decision-making is important, and can lead to improved outcomesCitation47; therefore, patient preference may be an additional valuable parameter that could be included in cost-effective analysis models such as those presented here.
In this analysis, as for the study by Stopeck et al.Citation30, SRE and drug administration QALY decrement inputs were based on published TTO studies in the general population. The findings of the cost-effectiveness analyses presented here, coupled with the clinical efficacy data, support the finding that denosumab is cost-effective compared with zoledronic acid.
Conclusions
Constraints on healthcare resources are increasing; therefore, understanding the incremental cost-effectiveness of treatment options is becoming more important. Across tumor types, denosumab was associated with fewer SREs, improved QALYs, and higher total costs over a lifetime. The estimated ICERs per QALY gained and SREs avoided indicate that SC denosumab is cost-effective compared with IV zoledronic acid for the prevention of SREs in patients with bone metastases secondary to prostate cancer, breast cancer, and OST in the Czech Republic, over a range of scenarios.
Transparency
Declaration of funding
This research was funded by Amgen (Europe) GmbH.
Declaration of financial/other relationships
JC was employed by Amgen and held stock. JF has been a consultant for Amgen, Bayer, Pierre-Fabre, Sanofi, Roche, BMS, MSD, Novartis, and Celgene. PJ, YQ, TB, and ML are employed by Amgen and hold stock. MK and BP are involved in some Amgen projects as external vendors. CG is employed by Amgen. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing support, funded by Amgen (Europe) GmbH, was provided by Liz Hartfield, PhD, from Oxford PharmaGenesis, UK, and Emma Booth from Amgen (Europe) GmbH. Editorial support was provided by Sarah Petrig of Amgen (Europe) GmbH.
References
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23
- Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res 2013;13:483-96
- Qian Y, Song X, Zhang K, et al. Short-term disability in solid tumor patients with bone metastases and skeletal-related events. J Med Econ 2015;18:210-8
- Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005;16:579-84
- Body JJ, Pereira J, Sleeboom H, et al. Health resource utilization associated with skeletal-related events: results from a retrospective European study. Eur J Health Econ 2015;17:711-21
- Hoefeler H, Duran I, Hechmati G, et al. Health resource utilization associated with skeletal-related events in patients with bone metastases: results from a multinational retrospective–prospective observational study – a cohort from 4 European countries. J Bone Oncol 2014;3:40-8
- Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. Springerplus 2014;3:328
- Hechmati G, Cure S, Gouepo A, et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ 2013;16:691-700
- Pereira J, Body JJ, Gunther O, et al. Cost of skeletal complications from bone metastases in six European countries. J Med Econ 2016;19:611-8
- European Medicines Agency, XGEVA (denosumab) summary of product characteristics. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002173/WC500110381.pdf. Accessed April 5, 2016
- European Medicines Agency, Zometa (zoledronic acid) summary of product characteristics. 2006. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000336/WC500051730.pdf. Accessed April 5, 2016
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-22
- Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer 2014;22:679-87
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132-9
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012;48:3082-92
- Czech Republic General Health Insurance Company. List of reimbursed health procedures. 2015. Czech Republic General Health Insurance https://www.vzp.cz/poskytovatele/ciselniky/zdravotni-vykony. Accessed July 28, 2016
- Expert Panel., Amgen data on file.
- Czech State Institute for Drug Control (SÚKL). List of reimbursed medicines. 2015. http://www.sukl.cz/sukl/seznam-cen-a-uhrad-lp-pzlu-k-1-12-2015. Accessed July 28, 2016
- Amgen. XGEVA (denosumab) summary of product characteristics. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002173/WC500110381.pdf. Accessed April 5, 2016
- Novartis Europharm Limited. Zometa (zoledronic acid) summary of product characteristics. 2006. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000336/WC500051730.pdf. Accessed April 5, 2016
- Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 3. Value Health 2012;15:812-20
- Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev 2005;3:CD003474
- Saad F, Gleason D, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94:1458-68
- Botteman M, Barghout V, Stephens J, et al. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol 2006;17:1072-82
- Carter JA, Joshi A, Kaura S, et al. Cost effectiveness of zoledronic acid in the management of skeletal metastases in hormone-refractory prostate cancer patients in France, Germany, Portugal, and the Netherlands. J Med Econ 2011;14:288-98
- De Cock E, Hutton J, Canney P, et al. Cost-effectiveness of oral ibandronate compared with intravenous (i.v.) zoledronic acid or i.v. generic pamidronate in breast cancer patients with metastatic bone disease undergoing i.v. chemotherapy. Support Care Cancer 2005;13:975-86
- De Cock E, Hutton J, Canney P, et al. Cost-effectiveness of oral ibandronate versus IV zoledronic acid or IV pamidronate for bone metastases in patients receiving oral hormonal therapy for breast cancer in the United Kingdom. Clin Ther 2005;27:1295-310
- Hatoum HT, Lin SJ, Smith MR, et al. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer 2008;113:1438-45
- Stopeck A, Rader M, Henry D, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ 2012;15:712-23
- Matza LS, Chung K, Van Brunt K, et al. Health state utilities for skeletal-related events secondary to bone metastases. Eur J Health Econ 2014;15:7-18
- Skoupá J, Annemans L, Hájek P. Health economic data requirements and availability in the European Union: results of a survey among 10 European countries. Value Health 2014;4:53-7
- National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) program. SEER*Stat Database: incidence. http://seer.cancer.gov/seerstat. Accessed April 5, 2016
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res Treat 2012;131:231-8
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 2011;14:177-83
- National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) program. SEER*Stat Database: incidence. http://seer.cancer.gov/seerstat. Accessed April 5, 2016
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97
- Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 2015;26:368-74
- Ibrahim T, Farolfi A, Mercatali L, et al. Metastatic bone disease in the era of bone-targeted therapy: clinical impact. Tumori 2013;99:1-9
- Duran I, Fink MG, Bahl A, et al. Health resource utilisation associated with skeletal-related events in patients with bone metastases secondary to solid tumours: regional comparisons in an observational study. Eur J Cancer Care 2016; doi: 10.1111/ecc.12452
- Lebret T, Casas A, Cavo M, et al. The use of bisphosphonates in the management of bone involvement from solid tumours and haematological malignancies – a European survey. Eur J Cancer Care 2016; doi: 10.1111/ecc.12490
- Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eur J Hosp Pharm Sci Pract 2013;20:227-31
- Body J, Mebis J, Peeters M, et al. A time and motion study of denosumab subcutaneous injection and zoledronic acid intravenous infusion in patients with metastatic bone disease in Belgium. Value Health 2015;18:A485
- Pedrazzoli P, Caraceni A, Beano A, et al. Results from a time and motion study of denosumab subcutaneous injection and zoledronic acid intravenous infusion in patients with metastatic bone disease from Italian sites. Value Health 2015;18:A485-6
- Stoner KL, Harder H, Fallowfield LJ, et al. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient 2015;8:145?153
- Hernandez RK, Quigley J, Pirolli M, et al. Patients with bone metastases from solid tumors initiating treatment with a bone-targeted agent in 2011: a descriptive analysis using oncology clinic data in the US. Support Care Cancer 2014;22:2697-705
- Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ 2007;335:24-7
- Amgen. Data on file (Clinical study reports 20050103, 20050136 and 20050244)
- Amgen. Data on file
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006
- International Agency for Research on Cancer, WHO. Chapter 2: Rates and rate standardization. In: N.E. Breslow and N.E. Day Eds Statistical methods in cancer research volume II: the design and analysis of cohort studies. Lyon, France: Oxford University Press; 1987, pp 48-81
- Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm 2010;16:693-702
Appendix
Probabilistic sensitivity analyses
Background
The purpose of this appendix is to describe the inputs used for probabilistic sensitivity analyses (PSAs) in the Czech cost-effectiveness model for the prevention of skeletal-related events (SREs) in patients with solid tumors (categorized as: breast cancer, prostate cancer, and other solid tumors). PSAs permit the assessment of the total uncertainty across all model inputs. This is accomplished by sampling the inputs according to distributions that reflect the range of likely values for each input, and repeating this process to create a distribution of possible outcomes. The resulting distribution of incremental cost per quality-adjusted life-year (QALY) results can then be compared against various willingness-to-pay thresholds to estimate the likelihood of a treatment being cost-effective according to those thresholds.
Model input values
Most of the model inputs included in the PSAs were sampled as described in the PSA Appendix of the Stopeck et al.Citation30 study. For completeness, this document details how the simulated values were estimated for all model parameters.
Where possible, inputs were derived from confidence intervals (CIs), or other measures of uncertainty, where available for each parameter. In the event that there were no appropriate measures provided for a particular input, reasonable estimates were constructed based on other relevant data as described below. The parameter estimates discussed below do not incorporate covariance terms, which were not available in most cases ().
Skeletal-related event rates
The gamma distribution was used to describe SRE rates (which were converted to probabilities in the model), because this distribution has the same range as rates (from 0–1). The two parameters for the gamma distribution, alpha and beta, can be estimated as functions of the mean and variance of a given sample distribution using a method of moments approach (see Briggs et al.Citation50, Chapter 4). The crude SRE rate with zoledronic acid was sampled using the gamma distribution, with a mean equal to the rate. In order to have a consistent, computationally efficient approach, the variances were based on the variance estimate for a rate, which can be approximated by the count of events divided by the squared person-time of observationCitation51. Distributions for relative rates (i.e. relative treatment effect and off-therapy SRE relative rate) were incorporated using log-normal distributions to reflect the log-linear nature of the estimation processCitation50.
Skeletal-related event distribution by type
The sampling of the distribution of the four primary individual SRE types (pathologic fracture, surgery to bone, radiation to bone, and spinal cord compression) was performed using a Dirichlet distribution for multinomial data. Each SRE type was sampled according to an independent gamma distribution with a common beta parameter, according to the method suggested by Briggs et al.Citation50 (technical appendix to Chapter 4) and the International Agency for Research on CancerCitation51. More specifically, we used a gamma distribution with the mean (alpha parameter) equal to the count of events, and variance equal to the mean (as in a Poisson distribution, forcing the beta parameter to be 1.0). The alpha parameters were divided by 10 before sampling, because values over 300 cause errors in Microsoft Excel. The output of these four gamma distributions was normalized to sum to 100%, and the resulting proportions by SRE type were used in the model to generate average costs and average QALY decrements.
Real-world skeletal-related event rate adjustment factor
The real-world SRE rate adjustment factor was sampled using a log-normal distribution. In the absence of a standard error, the 95% CI was assumed to fall within ±30% of the mean. The standard error was calculated on the log scale using the natural log (ln) of the lower bound of the 95% CI minus the natural log of the upper bound of the 95% CI to give the width of the 95% CI (i.e. ln[mean ×1.3] − ln[mean ×0.7]). This value was then divided by 2 × 1.96, to estimate the standard error.
Mortality risk
Because the model assumes the same mortality risk for denosumab and zoledronic acid, changes to the underlying mortality risk estimate will not affect the incremental cost per QALY results. However, to incorporate additional uncertainty into the model, the three underlying parameters required to specify the generalized gamma mortality risk distribution were sampled from a normal distribution.
Adverse event and discontinuation rates
As for SRE rates, adverse event and discontinuation rates (which were converted to probabilities in the model) were sampled using the gamma distribution (the gamma distribution ranges from 0–∞, similar to the rate of SREs).
Quality-adjusted life-year decrements
The model does not incorporate health state utilities directly; instead, the utility decrements caused by SREs have been assessed using a time trade-off study and incorporated into the model. The base case SRE QALY decrements and QALY decrements for subcutaneous injections and intravenous infusions came from time trade-off analysesCitation31, and were sampled from normal distributions. The standard error for each health state was calculated as the standard deviation divided by the square root of the size of the population in the study. Baseline utilities were not sampled, because the model is based on QALY decrements, and the baseline utility does not affect the incremental results.
Costs
In general, uncertainty around costs reflects a variety of factors, including the duration of the condition, the amount of resources required, and location (i.e. the site of service and/or the region within a country). Standard errors for all costs were taken from a US study of the weighted average inpatient and outpatient costs of SREs associated with the care of patients with breast cancer and prostate cancer, and expressed as a proportion of the meanCitation52. All costs were multiplied by the largest ratio in the study (0.16) to estimate standard errors as a conservative assumption (i.e. to maximize the uncertainty in costs). All costs were sampled using a gamma distribution, with the alpha and beta parameters defined by the mean and variances as described previouslyCitation51.
Table A1. Prostate cancer probabilistic inputs.
Table A2. Breast cancer probabilistic inputs.
Table A3. Other solid tumors probabilistic inputs.