Abstract
Aims: Cost effectiveness analysis (CEA) is a useful tool for estimating the value of an intervention in relation to alternatives. In cardiovascular disease (CVD), CEA is especially important, given the high economic and clinical burden. One key driver of value is CVD mortality prevention. However, data used to inform CEA parameters can be limited, given the difficulty in demonstrating statistically significant mortality benefit in randomized clinical trials (RCTs), due in part to the frequency of fatal events and limited trial durations. This systematic review identifies and summarizes whether published CVD-related CEAs have incorporated mortality benefits, and the methodology among those that did.
Materials and methods: A systematic literature review was conducted of CEAs of lipid-lowering therapies published between 2000–2017. Health technology assessments (HTA) and full-length manuscripts were included, and sources of mortality data and methods of applying mortality benefits were extracted. Results were summarized as proportions of articles to articulate common practices in CEAs of CVD.
Results: This review identified 100 studies for inclusion, comprising 93 full-length manuscripts and seven HTA reviews. Among these, 99% assumed a mortality benefit in the model. However, 87 of these studies that incorporated mortality differences did so despite the trials used to inform model parameters not demonstrating statistically significant differences in mortality. None of the 12 studies that used statistically significant findings from an individual RCT were based on active control studies. In a sub-group analysis considering the 60 CEAs that incorporated a direct mortality benefit, 48 (80%) did not have RCT evidence for statistically significant benefit in CVD mortality.
Limitations and conclusions: The finding that few CEA models included mortality inputs from individual RCTs of lipid-lowering therapy may be surprising, as one might expect that treatment efficacy should be based on robust clinical evidence. However, regulatory requirements in CVD-related RCTs often lead to insufficient sample sizes and observation periods for detecting a difference in CVD mortality, which results in the use of intermediate outcomes, composite end-points, or meta-analysis to extrapolate long-term mortality benefit in a lifetime CEA.
Introduction
Healthcare expenditures in the US have been increasing, with spending in 2014 estimated at $3 trillion, or 17.5% of GDPCitation1. Efforts are underway to control spending, with the focus ranging from systemic changes in payment and insurance design to more careful assessment of the value of technologies being utilized. One such method of assessing the value of an innovation compared with existing alternatives is cost-effectiveness analysis (CEA).
CEA is a well-established framework for estimating the costs per unit of incremental benefit provided by a new technologyCitation2. In CEA, the incremental economic impact of therapeutic options is estimated and divided by the incremental clinical benefit, with the result referred to as the incremental cost-effectiveness ratio and used as a measure of value, often over the course of a lifetime. As real-world costs are often not sufficiently captured through clinical trials, and many long-term clinical outcomes are not observable due to the limited time horizons of trials, simulation models are commonly used to conduct CEAs assessing the lifelong impact of trial-tested interventions. Model-based CEAs are used to inform decision-making throughout the world, despite their more recent adoption in the USCitation3. The need for such analyses is driven by the increasing discussion around rising healthcare costs. Bibliographic analyses performed by researchers using the Tufts Cost Effectiveness Registry have shown that CEA publications both in the US and internationally have grown dramatically over the past 25 yearsCitation4, along with a corresponding increase in healthcare providers and administrators utilizing the information.
A clinical area where CEA is frequently performed is cardiovascular disease (CVD), due to its significant clinical, economic burden, and the emergence of high cost and high value therapies. In 2015, 41.5% of US citizens, and over 90% of those over the age of 80, had some form of CVDCitation5 (defined as hypertension, coronary heart disease, stroke, congestive heart failure, or atrial fibrillation). Given the aging population in the US, the impact of CVD is only expected to increase. Of similar concern is the trend of increasing costs. Direct healthcare spending in CVD prevention and treatment was recently estimated at $231 billion, and, when including indirect costs, it was estimated at more than $650 billion in 2015Citation6. The high burden and high costs associated with CVD have led to an increase in published CEAs that aim to inform decision-makers on how best to allocate valuable resources to manage this disease.
As CEAs are increasingly used by non-health economists such as clinicians, payers, and policy-makers to inform value assessments, it is important to ensure that modeling approaches are well-established and understood by this expanded audience. One aspect that may not be sufficiently transparent is how efficacy, a main driver of economic analyses, is incorporated into models, as often times the limited data from RCTs need to be extrapolated to estimate a lifetime treatment benefit. A specific area of potential confusion related to efficacy is the methodology for incorporating the impact of interventions on mortality, when the treatment benefit is not independently assessed in RCTs.
In this systematic review, we identified and summarized how published CVD-related CEAs have modeled mortality benefits. We specifically assessed how many published CEAs included a mortality difference between comparators, and, among those, the sources and methods for how treatment effect was incorporated. This was not an attempt to advocate for a particular methodology, given the extensive research that has been conducted around the appropriateness of using intermediate outputsCitation7–9. Rather, we aimed to provide quantitative measures of what was done in previously published analyses. It was hypothesized that, because of limitations in RCTs of lipid-lowering therapies, the majority of CEA would model a mortality impact that was not found to be statistically significant in individual RCTs. These findings can help illustrate a practical challenge in CEAs, highlight the need to develop better, more precise recommendations from CEA experts on modeling inputs, describe approaches when encountering a lack of hard end-points to provide insights into common practices to readers of CEA literature, and aid in designing future research.
Methods
Search strategy
We conducted a thorough search of the literature in compliance with the National Institute for Health and Care Excellence (NICE) and the Institute for Quality and Efficiency in HealthCare (IQWiG) standards to identify relevant cost-effectiveness studies of lipid-lowering therapies (statins or ezetimibe). The following databases were searched: MEDLINE (Ovid); Embase; Econlit; and NHS Economic Evaluation Database (EED). Medical Subject Headings (MeSH) and keyword searches were used and edited as necessary (Supplemental Tables 1a–d). Health technology assessment (HTA) documents assessing cost-effectiveness models of lipid-lowering therapies were identified through a search of the Center for Reviews and Dissemination (CRD) electronic bibliographic database and country-specific HTA websites. In total, 19 agencies across 16 countries were included and searched manually. Conference proceedings were excluded from the search, as the word limits for abstracts led to insufficient details for abstraction of relevant modeling methods. The search was conducted for articles published in the English language between January 2000 and February 2017.
Study selection
A multi-stage process was used for identifying relevant articles for abstraction. Initially, each title and abstract from the peer-reviewed literature was screened by two researchers. Among those chosen for full-text review, each publication was reviewed by multiple members of the research team to determine inclusion eligibility, and disagreements were resolved via consensus. HTA documents were similarly assessed for eligibility against inclusion/exclusion criteria. Eligibility is shown in , and was limited to studies of lipid-lowering therapies in adults >18 years of age for whom treatment is recommended.
Table 1. Study inclusion criteria applied when screening articles and abstracting data.
Data abstraction
For included CEA, bibliographic data and mortality-specific details were abstracted. In cases where the CEA publication did not provide sufficient detail, the original trials (sources) informing mortality parameters were also reviewed. After data was extracted from all sources, relevant information was then reviewed again by a third, more senior researcher to ensure that all data had been collected and correctly categorized. A quality-assurance process was undertaken by external clinical and methodological experts to verify all abstracted data and classifications. Discrepancies were resolved by consensus.
For each CEA, we categorized the source of mortality data as direct (from an RCT or meta-analysis) or indirect (based on a risk equation, such as the Framingham Risk Score, or using a difference in intermediate end-points, such as incidence of a CVD event that increased the risk of mortality post-CVD event). Within the source RCTs, we determined whether the trials compared the studied intervention to an active control or to a placebo, had demonstrated a differential risk of cardiovascular mortality between treatments, and whether that difference had been shown to be statistically significant in a trial. We also assessed whether the mortality effect had been measured in the source study as an individual end-point or as a composite end-point that included both fatal and non-fatal cases. Findings were reported for the entire collection of abstracted articles, and a sensitivity analysis was conducted assessing those studies that used a mortality benefit as directly reported from the source.
Results
Search and screening overview
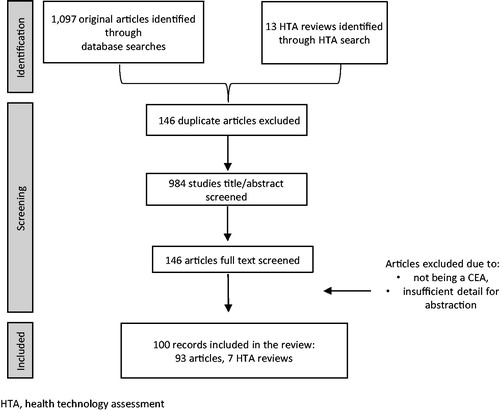
The search of all relevant databases yielded 1,110 studies including published literature and formal HTAs identified through individual agency websites (). After screening the literature, 133 full text articles and 13 HTA were accepted for abstraction. Among those abstracted, 46 studies were further excluded, with the primary reason being a lack of details regarding methodology to extract necessary components. A final set of 100 articles were included in the review, with details found in Supplemental Table 2Citation10–109.
Summary of included articles
Included studies ranged in publication date from 2000–2016, with 30 published in 2010 or more recently. Fifty-nine studies assessed lipid-lowering therapy against placebo, while 41 compared the benefits from intensification of lipid-lowering therapy or active controlled studies. There were a wide variety of model outcomes predicted, with some strictly estimating all-cause mortality, whereas others considered multiple separate CVD-related causes of mortality (e.g. coronary heart disease, stroke).
Studies applying mortality benefit, direct or indirect
Among the 100 included CEAs, 99 assumed a CVD mortality benefit in their analysis. Of these, only 12 CEAs were based on an individual RCT with mortality benefit that was shown to be statistically significant, while 87 of the studies incorporated mortality differences despite the trials used to inform model parameters not demonstrating statistically significant mortality differences. When stratifying by whether the source study had an active vs placebo control arm, none of the CEA that were based on trials using active controls had RCT evidence for statistically significant CVD mortality benefit. The majority of placebo controlled studies used to inform CEA inputs also did not find statistically significant mortality evidence ().
Table 2. Results: frequency of statistically significant mortality data utilized within cost effectiveness analyses of lipid-lowering therapies.
Studies applying a direct mortality benefit
Of the 99 CEAs that included a mortality benefit, 61% used direct information (RCT or meta-analysis) as the source for this benefit. Among the 60 studies using direct mortality information, 35 (58%) used a source trial that reported an individual end-point of death, whereas 25 (42%) used a composite end-point, such as the combined rate of fatal and non-fatal events. Of the CEA that used indirect information, 24 used trial findings (such as a decrease in LDL-C) entered into a risk calculator (e.g. Framingham Risk Score, SCORE Risk Equation) to model CVD mortality. The remaining 15 used trial findings of differences in intermediate outcomes, such as incidence of stroke or CHD, and applied a case-fatality rate from a separate source to indirectly model a mortality benefit (). Among the 60 CEA that used direct information, the majority used individual trials as opposed to meta-analyses. For those that used individual trial data for CVD mortality benefit, 20 of 43 studies (47%) applied a composite end-point ().
Figure 2. Results: source of mortality data and use of composite and individual end-points within CEA that incorporated a direct effect.
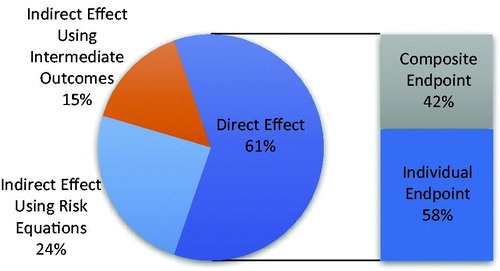
Table 3. Results: quantitative assessment of mortality data sources and format of data used among studies modeling a direct mortality effect.
Sensitivity analysis considering only studies that directly incorporated CVD mortality benefit
Sixty CEAs (60%) directly incorporated CVD mortality benefits in the model, out of which 48 studies (80%) included mortality benefit that did not have RCT evidence to support a statistically significant benefit between treatments. Of these studies, 19 relied on source trials that used active controls, while 41 were based on trials that were placebo controlled. Similar to the main analysis, none of the 19 studies with active controlled comparators had RCT evidence for CVD mortality benefit, and only 12 of 41 studies (29%) of the placebo controlled comparators had evidence of CVD mortality supported by clinical trial ().
Table 4. Sensitivity analysis of studies that incorporated a direct CVD mortality benefit.
Discussion
This systematic review of the literature identified 100 English-language CEAs that fulfilled the inclusion criteria of assessing lipid-lowering therapies in adults for prevention of CVD mortality. The reviewed articles, including manuscripts and HTA reports, primarily relied on data from meta-analyses or trials that did not find a statistically significant mortality difference between comparators. The articles were published in a wide range of peer-reviewed journals, and provided insights that could be used in driving important payer decisions.
Most likely, published CEAs did not rely strictly on statistically significant mortality findings, because it can be difficult, costly, and time consuming to design a trial that is powered to detect a statistically significant impact on mortality. A recent meta-analysis of clinical trials of lipid-lowering therapies reported that, of 27 studies assessed, only three showed statistically significant mortality outcomesCitation110. All three trials (LIPID, 4S, and HPS) were older (published between 1997–2002), had longer duration (>5 years), and were placebo-controlledCitation111–113. Among the six recent trials that compared more or less intensive LDL cholesterol lowering therapy, none found a statistically significant impact on mortality associated with treatmentCitation114–119.
With the improved management of hypertension and other risk factors, an increase in use of anti-platelet therapies, and substantial progress in the acute management of MI/stroke, survival in CVD patients has increased. This longer survival presents challenges in demonstrating CVD mortality benefit within a trial, where follow-up is limited and the sample size is selected to assess intermediate or composite end-points rather than CVD mortality alone. The regulatory landscape is also evolving, as current registration trials in CVD are increasingly powered to assess composite end-points. It would be beneficial if regulatory agencies and others assessing trials and subsequent CEAs provided formal guidance into appropriate study designs and how to incorporate trial data into lifetime economic models. In certain disease areas, such as metastatic cancer, collecting mortality data within the timeframe of a clinical trial is plausible, given that median overall survival time can be within 1 year of diagnosis or initiation of treatment. In a subsequent study, it could be interesting to investigate the types of data that are used in CEAs of other disease areas, to assess whether this is specific to chronic conditions.
CEAs are used to inform decision-making, and decisions must be made with the best available evidence. Therefore, model-based analyses are often forced to weigh trade-offs in using different sources of information not originally designed for economic analysis to inform parameters. Published modeling guidelines suggest that there is no single source that is preferable in all situations, but rather that researchers should weigh potential biases and utilize the best available evidenceCitation120. In rare cases, there may be a single head-to-head clinical trial designed to answer all relevant questions. In other cases, combining findings from RCTs with real world evidence can reduce biases. It is recommended that, in the absence of an ideal data source, it is preferable to combine data from multiple sources or infer the potential impact beyond the scope of an observation period, as preliminary cost-effectiveness results using best available data can be informative to decision-makers. The benefits of transparency in describing methods and results is that, as new data become available, the model can be updated. In the case of CVD, the long-term survival benefits of lipid-lowering therapy have been repeatedly shown over the past 20+ yearsCitation110, providing support for the link between a benefit in intermediate outcomes (e.g. stroke or MI prevented) and a decrease in mortality.
This analysis was conducted to provide a preliminary quantitative estimate of how mortality is incorporated within CVD CEAs, as opposed to making a formal recommendation or advocating for use of a specific methodology. One could envision an additional analysis that builds off this work in which predicted outcomes from historic models that used RCT data showing statistically significant results could be compared with those using non-statistically significant results or other types of data. This may help identify whether the practices identified can provide reasonable estimates.
While efforts were taken to adhere to guidelines regarding best practices in conducting systematic literature reviews, this study should be viewed in light of its limitations. Due to the detailed nature of the study question being examined, there were some articles that were excluded, despite their apparent relevance, because they did not provide sufficient transparency into the methods. There is no reason to believe that this limitation would introduce bias in any direction. Additionally, this analysis summarized the methods for incorporating mortality, and did not consider additional methodological decisions required when conducting CEA of lipid-lowering therapies, such as the choice to include quality-adjustments or gauging the strength of evidence used; this could be a potentially beneficial area for future research. Our investigation of methods for incorporating mortality also focused solely on the base case methods and findings, and did not evaluate how these assumptions were assessed in sensitivity analyses. This could provide further insights into the implications of modeling methodology, and would be an interesting area for future studies. Finally, our goal was to summarize the current practice, as opposed to assessing the quality of included studies or conducting a meta-analysis.
Conclusions
We assessed the use of intermediate outcomes, composite end-points, and non-statistically significant findings from RCT in CEA, using lipid-lowering therapy for CVD prevention as an example. In this review of 100 studies, we found that nearly all CEA incorporated mortality benefit, but most were not informed by statistical significance directly based on clinical trials. Limitations in clinical trials to assess mortality in chronic diseases, such as CVD, include insufficient sample sizes and limited observation periods. The clinical experience with lipid-lowering therapies in the past 20 years has led to exercises including the use of intermediate outcomes and composite end-points when conducting CEA, which has become a standard practice when assessing the value of lipid-lowering therapies. Subsequent analyses could help in determining whether this approach is also used in other disease areas, and additional investigation into the optimal data sources to use in CEA in the absence of RCT evidence could be warranted.
Transparency
Declaration of funding
This study has been sponsored by Amgen.
Declaration of financial/other relationships
JO, MB, TB, and AH are employees of the Partnership for Health Analytic Research, LLC, which received payment for conducting the analyses described in this manuscript. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplementary material
Download Zip (316.9 KB)Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats: Health Expenditures [Internet]. FastStats. 2017. https://www.cdc.gov/nchs/fastats/health-expenditures.htm. [Last accessed 11 April 2017].
- Gold MR, Seigel JE, Russell LB, et al., editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996
- Towse A, Pritchard C, Devlin N, editors. Cost-Effectiveness Thresholds: Economic and Ethical Issues. King's Fund and Office of Health Economics; 2002. p 56-68
- Neumann PJ, Thorat T, Shi J, et al. The changing face of the cost-utility literature, 1990–2012. Value Health 2015;18:271-7
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e146-e603
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:e38-e360
- Casali PG, Bruzzi P, Bogaerts J, et al. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: a European consensus position paper. Ann Oncol 2015;26:300-6
- Landais P, Daures J-P. Clinical trials, immunosuppression and renal transplantation: new trends in design and analysis. Pediatr Nephrol 2002;17:573-84
- Sawaya GF, Guirguis-Blake J, LeFevre M, et al. Update on the methods of the US preventive services task force: Estimating certainty and magnitude of net benefit. Ann Intern Med 2007;147:871-5
- All Wales Therapeutics and Toxicology Centre. AWMSG Secretariat Assessment Report. Rosuvastatin (Crestor®). Penarth, Vale of Glamorgan, Wales Reference number: 1311. 2012
- Annemans L, Marbaix S, Webb K, et al. Cost effectiveness of atorvastatin in patients with type 2 diabetes mellitus: a pharmacoeconomic analysis of the collaborative atorvastatin diabetes study in the Belgian population. Clin Drug Investig 2010;30:133-42
- Ara R, Pandor A, Stevens J, et al. Prescribing high-dose lipid-lowering therapy early to avoid subsequent cardiovascular events: is this a cost-effective strategy? Eur J Prev Cardiol 2012;19:474-83
- Ara R, Pandor A, Tumur I, et al. Cost effectiveness of ezetimibe in patients with cardiovascular disease and statin intolerance or contraindications: a Markov model. Am J Cardiovasc Drugs 2008;8:419-27
- Ara R, Pandor A, Tumur I, et al. Estimating the health benefits and costs associated with ezetimibe coadministered with statin therapy compared with higher dose statin monotherapy in patients with established cardiovascular disease: results of a Markov model for UK costs using data registries. Clin Ther 2008;30:1508-23
- Araujo DV, Bahia L, Souza CP, et al. Cost-effectiveness and budget impact analysis of rosuvastatin and atorvastatin for LDL-cholesterol and cardiovascular events lowering within the SUS scenario. Int J Atheroscler 2007;2:189-94
- Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus “usual” care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin 2002;18:220-8
- Barrios V, Lobos JM, Serrano A, et al. Cost-effectiveness analysis of rosuvastatin vs generic atorvastatin in Spain. J Med Econ 2012;15(Suppl1):45-54
- Bennett K, Kabir Z, Barry M, et al. Cost-effectiveness of treatments reducing coronary heart disease mortality in Ireland, 2000 to 2010. Value Health J Int Soc Pharmacoecon Outcomes Res 2009;12:10-15
- Berto P, Munro V, Gaddi A, et al. Cost-effectiveness analysis for statin therapies in the primary prevention of coronary heart disease in Italy. Clin Drug Investig 2000;20:109-21
- Brandle M, Davidson MB, Schriger DL, et al. Cost effectiveness of statin therapy for the primary prevention of major coronary events in individuals with type 2 diabetes. Diabetes Care 2003;26:1796-801
- Buller N, Gillen D, Casciano R, et al. A pharmacoeconomic evaluation of the Myocardial Ischaemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study in the United Kingdom. PharmacoEconomics 2003;21(Suppl 1):25-32
- Casciano R, Tarride J-E, Breton MC, et al. A pharmacoeconomic evaluation of the myocardial ischemia reduction with aggressive cholesterol lowering (MIRACL) study in Canada. Can J Clin Pharmacol J Can Pharmacol Clin 2004;11:e179-90
- CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542-51
- Chan PS, Nallamothu BK, Gurm HS, et al. Incremental benefit and cost-effectiveness of high-dose statin therapy in high-risk patients with coronary artery disease. Circulation 2007;115:2398-409
- Chau J, Cheung BM, McGhee SM, et al. Cost-effectiveness analysis of applying the Cholesterol and Recurrent Events (CARE) study protocol in Hong Kong. Hong Kong Med J Xianggang Yi Xue Za Zhi 2001;7:360-8
- Conly J, Clement F, Tonelli M, et al. Cost-effectiveness of the use of low- and high-potency statins in people at low cardiovascular risk. CMAJ Can Med Assoc J J Assoc Medicale Can 2011;183:E1180-8
- Cook JR, Yin D, Alemao E, et al. Cost-effectiveness of ezetimibe coadministration in statin-treated patients not at cholesterol goal: application to Germany, Spain and Norway. PharmacoEconomics 2004;22(Suppl 3):49-61
- Davies A, Hutton J, O’Donnell J, et al. Cost-effectiveness of rosuvastatin, atorvastatin, simvastatin, pravastatin and fluvastatin for the primary prevention of CHD in the UK. Br J Cardiol 2006;13:196-202
- Delea TE, Jacobson TA, Serruys PW, et al. Cost-effectiveness of fluvastatin following successful first percutaneous coronary intervention. Ann Pharmacother 2005;39:610-16
- de Vries FM, Denig P, Visser ST, et al. Cost-effectiveness of statins for primary prevention in patients newly siagnosed with type 2 diabetes in The Netherlands. Value Health 2014;17:223-30
- Erickson KF, Japa S, Owens DK, et al. Cost-effectiveness of statins for primary cardiovascular prevention in chronic kidney disease. J Am Coll Cardiol 2013;61:1250-8
- Feher MD, Langley-Hawthorne CE, Byrne CD. Cost-outcome benefits of fibrate therapy in type 2 diabetes. Br J Diabetes Vasc Dis 2003;3:124-30
- Fidan D, Unal B, Critchley J, et al. Economic analysis of treatments reducing coronary heart disease mortality in England and Wales, 2000–2010. QJM 2007;100:277-89
- Fragoulakis V, Kourlaba G, Maniadakis N. Economic evaluation of statins in high-risk patients treated for primary and secondary prevention of cardiovascular disease in Greece. Clin Outcomes Res CEOR 2012;4:135-43
- Franco OH, der Kinderen AJ, De Laet C, et al. Primary prevention of cardiovascular disease: cost-effectiveness comparison. Int J Technol Assess Health Care 2007;23:71-9
- Gandhi S, Gandhi S, Fox K, et al. Cost-effectiveness of rosuvastatin in comparison with generic atorvastatin and simvastatin in a Swedish population at high risk of cardiovascular events. Clin Outcomes Res 2012;4:1-11
- Ganz DA, Kuntz KM, Jacobson GA, et al. Cost-effectiveness of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor therapy in older patients with myocardial infarction. Ann Intern Med 2000;132:780-7
- Glasziou PP, Eckermann SD, Mulray SE, et al. Cholesterol-lowering therapy with pravastatin in patients with average cholesterol levels and established ischaemic heart disease: is it cost-effective? Med J Aust 2002;177:428-34
- Gómez-Gerique JA, Casciano R, Stern L, et al. A pharmacoeconomic evaluation of the effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes in Spain. Eur J Health Econ HEPAC Health Econ Prev Care 2004;5:278-84
- Greving J, Visseren F, de Wit G, et al. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ 2011;342:d1672
- Grover SA, Coupal L, Zowall H, et al. How cost-effective is the treatment of dyslipidemia in patients with diabetes but without cardiovascular disease? Diabetes Care 2001;24:45-50
- Grover SA, Coupal L, Zowall H, et al. Cost-effectiveness of treating hyperlipidemia in the presence of diabetes: who should be treated? Circulation 2000;102:722-7
- Grover S, Coupal L, Lowensteyn I. Preventing cardiovascular disease among Canadians: is the treatment of hypertension or dyslipidemia cost-effective? Can J Cardiol 2008;24:891-8
- Grover SA, Ho V, Lavoie F, et al. The importance of indirect costs in primary cardiovascular disease prevention: can we save lives and money with statins? Arch Intern Med 2003;163:333-9
- Heart Protection Study Collaborative. Lifetime cost effectiveness of simvastatin in a range of risk groups and age groups derived from a randomised trial of 20 536 people. BMJ 2006;333:1145
- Heart Protection Study Collaborative Group. Statin cost-effectiveness in the United States for people at different vascular risk levels. Circ Cardiovasc Qual Outcomes 2009;2:65-72
- Herregods M-C, Daubresse J-C, Michel G, et al. Discovery Belux: comparison of rosuvastatin with atorvastatin in hypercholesterolaemia. Acta Cardiol 2008;63:493-9
- Kang H-Y, Ko S-K, Liew D. Results of a Markov model analysis to assess the cost-effectiveness of statin therapy for the primary prevention of cardiovascular disease in Korea: The Korean Individual-Microsimulation Model for Cardiovascular Health Interventions. Clin Ther 2009;31:2919-30
- Khoury H, Wagner M, Merikle E, et al. Cost-effectiveness of atorvastatin in the primary prevention of major cardiovascular events in patients with type 2 diabetes in Canada. Can J Diabetes 2009;33:363-74
- Kohli M, Attard C, Lam A, et al. Cost effectiveness of adding ezetimibe to atorvastatin therapy in patients not at cholesterol treatment goal in Canada. PharmacoEconomics 2006;24:815-30
- Kongnakorn T, Ward A, Roberts CS, et al. Economic evaluation of atorvastatin for prevention of recurrent stroke based on the SPARCL trial. Value Health 2009;12:880-7
- Lafuma A, Colin X, Solesse A. Cost-effectiveness of atorvastatin in the prevention of cardiovascular events in diabetic patients: A French adaptation of CARDS. Arch Cardiovasc Dis 2008;101:327-32
- Laires PA, Ejzykowicz F, Hsu T-Y, et al. Cost-effectiveness of adding ezetimibe to atorvastatin vs switching to rosuvastatin therapy in Portugal. J Med Econ 2015;18:565-72
- Liew D, Park H-J, Ko S-K. Results of a Markov model analysis to assess the cost-effectiveness of a single tablet of fixed-dose amlodipine and atorvastatin for the primary prevention of cardiovascular disease in Korea. Clin Ther 2009;31:2189-203
- Lim SS, Vos T, Peeters A, et al. Cost-effectiveness of prescribing statins according to pharmaceutical benefits scheme criteria. Med J Aust 2001;175:459-64
- Lindgren P, Graff J, Olsson AG, et al. Cost-effectiveness of high-dose atorvastatin compared with regular dose simvastatin. Eur Heart J 2006;28:1448-53
- Lindgren P, Buxton M, Kahan T, et al. Cost-effectiveness of atorvastatin for the prevention of coronary and stroke events: an economic analysis of the Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA). Eur J Cardiovasc Prev Rehabil Off J Eur Soc Cardiol Work Groups Epidemiol Prev Card Rehabil Exerc Physiol 2005;12:29-36
- Lorgelly PK, Briggs AH, Wedel H, et al. An economic evaluation of rosuvastatin treatment in systolic heart failure: evidence from the CORONA trial. Eur J Heart Fail 2010;12:66-74
- MacDonald GP. Cost-effectiveness of rosuvastatin for primary prevention of cardiovascular events according to Framingham Risk Score in patients with elevated C-reactive protein. J Am Osteopath Assoc 2010;110:427-36
- Mark DB, Knight JD, Cowper PA, et al. Long-term economic outcomes associated with intensive versus moderate lipid-lowering therapy in coronary artery disease: Results from the Treating to New Targets (TNT) Trial. Am Heart J 2008;156:698-705
- Marshall T. Coronary heart disease prevention: insights from modelling incremental cost effectiveness. BMJ 2003;327:1264
- Marshall T. The cost-effectiveness of drug treatments for primary prevention of cardiovascular disease: a modelling study. Eur J Cardiovasc Prev Rehabil Off J Eur. Soc Cardiol Work Groups Epidemiol Prev Card Rehabil Exerc Physiol 2006;13:523-8
- McConnachie A, Walker A, Robertson M, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J 2014;35:290-8
- Heart Protection Study Collaborative Group. Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: economic analysis of a randomised trial in 20 536 individuals. The Lancet 21;365:1779–85
- Mihaylova B, Schlackow I, Herrington W, et al. Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in CKD: results of the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 2016;67:576-84
- Mould-Quevedo JF, Gutiérrez-Ardila MV, Ordóñez Molina JE, et al. Cost-effectiveness analysis of atorvastatin versus rosuvastatin in primary and secondary cardiovascular prevention populations in Brazil and Columbia. Value Health Reg Issues 2014;5:48-57
- Mullins CD, Rattinger GB, Kuznik A, et al. Cost-effectiveness of intensive atorvastatin treatment in high-risk patients compared with usual care in a postgeneric statin market: economic analysis of the Aggressive Lipid-lowering Initiation Abates New Cardiac Events (ALLIANCE) study. Clin Ther 2008;30(Pt2):2204-16
- Nagata-Kobayashi S, Shimbo T, Matsui K, et al. Cost-effectiveness of pravastatin for primary prevention of coronary artery disease in Japan. Int J Cardiol 2005;104:213-23
- Nash A, Barry M, Walshe V. Cost effectiveness of statin therapy for the primary prevention of coronary heart disease in Ireland. Ir Med J 2006;99:144-5
- National Institute for Health and Care Excellence. Ezetimibe for treating primary heterozygous-familial and non-familial hypercholesterolaemia [Internet]. Technol Apprais Guid TA385. 2016. https://www.nice.org.uk/guidance/ta385 11 April 2017
- Nherera L, Calvert NW, DeMott K, et al. Cost-effectiveness analysis of the use of a high-intensity statin compared to a low-intensity statin in the management of patients with familial hypercholesterolaemia. Curr Med Res Opin 2010;26:529-36
- Ohsfeldt RL, Gandhi SK, Smolen LJ, et al. Cost effectiveness of rosuvastatin in patients at risk of cardiovascular disease based on findings from the JUPITER trial. J Med Econ 2010;13:428-37
- Ohsfeldt RL, Olsson AG, Jensen MM, et al. Cost-effectiveness of rosuvastatin 20 mg for the prevention of cardiovascular morbidity and mortality: a Swedish economic evaluation of the JUPITER trial. J Med Econ 2012;15:125-33
- Olsson A, Casciano R, Stern L, et al. A pharmacoeconomic evaluation of aggressive cholesterol lowering in Sweden. Int J Cardiol 2004;96:51-7
- Onishi Y, Hinotsu S, Nakao YM, et al. Economic evaluation of pravastatin for primary prevention of coronary srtery disease based on risk prediction from JALS-ECC in Japan. Value Health Reg Issues 2013;2:5-12
- Peura P, Martikainen J, Soini E, et al. Cost-effectiveness of statins in the prevention of coronary heart disease events in middle-aged Finnish men. Curr Med Res Opin 2008;24:1823-32
- Pharmaceutical Benefits Advisory Committee. Public summary document: Ezetimibe with simvastatin tablet, 10 mg–20 mg, Vytorin®. Commonwealth of Australia PBAC; 2009
- Pharmaceutical Benefits Advisory Committee. Review of statin therapies. Commonwealth of Australia PBAC; 2012
- Pharmaceutical Benefits Advisory Committee. Public summary document: Ezetimibe with simvastatin, tablet, 10 mg–20 mg, Vytorin®. Commonwealth of Australia PBAC; 2012
- Pilote L, Ho V, Lavoie F, et al. Cost-effectiveness of lipid-lowering treatment according to lipid level. Can J Cardiol 2005;21:681-7
- Pinto CG, Carrageta MO, Miguel LS. Cost-effectiveness of rosuvastatin in the prevention of ischemic heart disease in Portugal. Value Health 2008;11:154-9
- Prosser LA, Stinnett AA, Goldman PA, et al. Cost-effectiveness of cholesterol-lowering therapies according to selected patient characteristics. Ann Intern Med 2000;132:769-79
- Raikou M, McGuire A, Colhoun HM, et al. Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia 2007;50:733-40
- Ramsey SD, Clarke LD, Roberts CS, et al. An economic evaluation of atorvastatin for primary prevention of cardiovascular events in type 2 diabetes. PharmacoEconomics 2008;26:329-39
- Reckless J, Davies G, Tunceli K, et al. Projected cost-effectiveness of ezetimibe/simvastatin compared with doubling the statin dose in the United Kingdom: findings from the INFORCE study. Value Health 2010;13:726-34
- Robberstad B, Hemed Y, Norheim OF. Cost-effectiveness of medical interventions to prevent cardiovascular disease in a sub-Saharan African country—the case of Tanzania. Cost Eff Resour Alloc CE 2007;5:3
- Rosen VM, Taylor DCA, Parekh H, et al. Cost effectiveness of intensive lipid-lowering treatment for patients with congestive heart failure and coronary heart disease in the US. PharmacoEconomics 2010;28:47-60
- Rubinstein A, Colantonio L, Bardach A, et al. Estimation of the burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of preventative interventions to reduce this burden in Argentina. BMC Public Health 2010;10:627
- Rubinstein A, García Martí S, Souto A, et al. Generalized cost-effectiveness analysis of a package of interventions to reduce cardiovascular disease in Buenos Aires, Argentina. Cost Eff Resour Alloc 2009;7:10
- Russell MW, Huse DM, Miller JD, et al. Cost effectiveness of HMG-CoA reductase inhibition in Canada. Can J Clin Pharmacol J Can Pharmacol Clin 2001;8:9-16
- Schwartz GG, Ganz P, Waters D, et al. Pharmacoeconomic evaluation of the effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. Am J Cardiol 2003;92:1109-12
- Scottish Medicines Consortium. ADVICE: rosuvastatin, 5 mg, 10 mg, 20 mg, film-coated tablets (Crestor®) SMC No. (725/11). Glasgow, Scotland: SMC; 2011
- Scuffham PA, Chaplin S. An economic evaluation of fluvastatin used for the prevention of cardiac events following successful first percutaneous coronary intervention in the UK. PharmacoEconomics 2004;22:525-35
- Scuffham PA, Chaplin S. A cost-effectiveness analysis of fluvastatin in patients with diabetes after successful percutaneous coronary intervention. Clin Ther 2005;27:1467-77
- Scuffham PA, Kósa J. The cost-effectiveness of fluvastatin in Hungary following successful percutaneous coronary intervention. Cardiovasc Drugs Ther 2006;20:309-17
- Sigvant B, Henriksson M, Lundin F, et al. Asymptomatic peripheral arterial disease: is pharmacological prevention of cardiovascular risk cost-effective? Eur J Cardiovasc Prev Rehabil 2011;18:254-61
- Slejko JF, Page RL, Sullivan PW. Cost-effectiveness of statin therapy for vascular event prevention in adults with elevated C-reactive protein: implications of JUPITER. Curr Med Res Opin 2010;26:2485-97
- Soini EJO, Davies G, Martikainen JA, et al. Population-based health-economic evaluation of the secondary prevention of coronary heart disease in Finland. Curr Med Res Opin 2010;26:25-36
- Straka RJ, Mamdani M, Damen J, et al. Economic impacts attributable to the early clinical benefit of atorvastatin therapy – a US managed care perspective. Curr Med Res Opin 2007;23:1517-29
- Taylor DCA, Pandya A, Thompson D, et al. Cost-effectiveness of intensive atorvastatin therapy in secondary cardiovascular prevention in the United Kingdom, Spain, and Germany, based on the Treating to New Targets study. Eur J Health Econ 2009;10:255-65
- Tonkin AM, Eckermann S, White H, et al. Cost-effectiveness of cholesterol-lowering therapy with pravastatin in patients with previous acute coronary syndromes aged 65 to 74 years compared with younger patients: Results from the LIPID study. Am Heart J 2006;151:1305-12
- Tsevat J, Kuntz KM, Orav EJ, et al. Cost-effectiveness of pravastatin therapy for survivors of myocardial infarction with average cholesterol levels. Am Heart J 2001;141:727-34
- van Hout B. Cost-effectiveness of HMG coenzyme reductase inhibitors. Whom to treat? Eur Heart J 2001;22:751-61
- van Nooten F, Davies GM, Jukema JW, et al. Economic evaluation of ezetimibe combined with simvastatin for the treatment of primary hypercholesterolaemia. Neth Heart J 2011;19:61-7
- Wagner M, Lindgren P, Merikle E, et al. Economic evaluation of high-dose (80 mg/day) atorvastatin treatment compared with standard-dose (20 mg/day to 40 mg/day) simvastatin treatment in Canada based on the Incremental Decrease in End-Points Through Aggressive Lipid-Lowering (IDEAL) trial. Can J Cardiol 2009;25:e362-9
- Wagner M, Goetghebeur M, Merikle E, et al. Cost-effectiveness of intensive lipid lowering therapy with 80 mg of atorvastatin, versus 10 mg of atorvastatin, for secondary prevention of cardiovascular disease in Canada. Can J Clin Pharmacol J Can Pharmacol Clin 2009;16:e331-45
- Walshe V, Nash A, Barry M. Cost effectiveness of statin therapy for primary prevention of coronary heart disease. Ir Med J 2006;100:144-5
- Ward S, Lloyd Jones M, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess Winch Engl 2007;11:1-160, iii-iv
- Zechmeister I, Stollenwerk B, Ara R, et al. Statins for the secondary prevention of cardiovascular diseases: An analysis of expected health gains and cost-utility in Austria. HTA Project Report; Vienna, Austria: Ludwig Boltzmann Gesellschaft GmbH 2008
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. The Lancet 2015;385:1397-405
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-57
- Miettinen TA, Pyörälä K, Olsson AG, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S). Circulation 1997;96:4211-18
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet Lond Engl 2002;360:7-22
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504
- de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004;292:1307-16
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35
- Pedersen TR, Faergeman O, Kastelein JJP, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005;294:2437-45
- Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Armitage J, Bowman L, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet Lond Engl 2010;376:1658-69
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387-97
- Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices—Modeling Studies. Value Health 2003;6:9-17