Abstract
Background: Atrial fibrillation (AF) causes a significant health and economic burden to the Dutch society. Dabigatran was proven to have at least similar efficacy and a similar or better safety profile when compared to vitamin K antagonists (VKAs) in preventing arterial thromboembolism in patients with AF.
Objective: To evaluate the cost-effectiveness and monetary benefit of dabigatran vs VKAs in Dutch patients with non-valvular AF. Value-based pricing considerations and corresponding negotiations on dabigatran will be explicitly considered.
Methods: The base case economic analysis was conducted from the societal perspective. Health effects and costs were analysed using a Markov model. The main model inputs were derived from the RE-LY trial and Dutch observational data. Univariate, probabilistic sensitivity, and various scenario analyses were performed.
Results: Dabigatran was cost saving compared to VKAs. A total of 4,552 QALYs were gained, and €13,892,288 was saved in a cohort of 10,000 AF patients. The economic value of dabigatran was strongly related to the costs of VKA control that are averted. Notably, dabigatran was cost saving compared to VKAs if annual costs of VKA control exceeded €159 per person, or dabigatran costs were below €2.81 per day.
Conclusion: Dabigatran was cost saving compared to VKAs for the prevention of atrial thromboembolism in patients with non-valvular AF in the Netherlands. This result appeared robust in the sensitivity analysis. Furthermore, volume based reduction of the price in the Netherlands will further increase the monetary benefits of dabigatran.
Introduction
Atrial fibrillation (AF) is defined as a disorder in the atrium, resulting in irregular arterial contractionCitation1,Citation2. Most common complications associated with AF are heart failure, systemic embolism (SE), ischemic stroke (IS), and cerebrovascular accidents (CVAs). In particular, risk of stroke has previously been estimated to be at least 5-times higher in patients with AF compared with patients with normal sinus rhythm. Also, strokes associated with AF often have higher rates of disability and mortality, with an estimated more than half of all patients (64%) dying within 1 year after an AF-related stroke compared to 34% for patients with a non-AF related stroke.
A high prevalence of AF and its related complications can cause a significant health and economic burden to the Dutch healthcare system. In general, AF is the most common cause of sinus dysrhythmia, prevalent among ∼1% of the total populationCitation3,Citation4. The incidence and prevalence of AF in the Netherlands increase with age, up to, for example, a prevalence of 17.8% in those aged >85 yearsCitation5. The specific age distribution of AF implies that AF can be considered as virtually non-prevalent in those aged <25 years and that more than half of all AF patients is >75 years of ageCitation6. Although the incidence and prevalence for AF is higher for men than for women, the total number of AF patients in both genders is comparable due to the higher life expectancy of womenCitation7. It is estimated that there are ∼296,000 patients with AF in 2015Citation5,Citation8.
In the Netherlands, 78,174 years of life are lost because of stroke, resulting in 191,320 disability adjusted life-years lost annually. Stroke is the second largest contributor to the total disease burden in the NetherlandsCitation9. Approximately 15–20% of all ischemic strokes (IS) is caused by AFCitation10. In 2011, 2.5% of healthcare cost in the Netherlands were caused by stroke, amounting to €2.3 billionCitation11.
Several stroke risk assessment tools are available to predict the risk of stroke in AF patients. The CHADS2 score—reflecting the presence of Cardiac failure, Hypertension, Age, Diabetes, and Stroke (doubled if present)—is a risk assessment tool for stroke risk that is still reported in most AF trials with years of follow-upCitation1,Citation2. The CHADS2 is further extended with vascular disease, age groups, and sex into the CHA2DS2-VASc score. Based on the 2012 ESC guidelines on AF, non-vitamin K antagonist anti-coagulants (NOACs) are preferred for patients with one or more stroke risk factors; i.e. a CHA2DS2-VASc score of 1 and aboveCitation1,Citation2. While vitamin K antagonists (VKAs) reduce the risk of thromboembolism and CVAs (notably stroke), these agents are not preferred because they offer less safety and convenience compared to NOACsCitation1,Citation2. Furthermore, high variability in effects of VKA treatment exists, posing possible risks to patients undergoing this treatment. Therefore, patients receiving VKA therapy should undergo regular coagulation monitoring to remain in an acceptable range of the International Normalized Ratio (INR)Citation1,Citation2. INR values outside the acceptable range of 2.0–3.0 result in a higher risk of thromboembolism (INR < 2.0) or bleeding (INR > 3.0)Citation12–14. Other safety issues with VKAs are related to several possible drug and dietary interactionsCitation15.
Dabigatran is a synthetic reversible thrombin inhibitor, which binds to thrombin with a high affinity and specificity, resulting in anti-coagulation. Dabigatran belongs to the class of NOACs, with rivaroxaban, apixaban, and edoxaban being the others. One major advantage of NOACs is that there is no need for monitoring at specialized thrombosis centers, with potential cost savings.
The recommended daily dose of dabigatran is 150 mg twice daily. A dose of 110 mg capsule twice daily can be individually considered for patients between 75–80 years, at the discretion of the physician, when the thromboembolic risk is low and the bleeding risk is high. Patients aged 80 years or above should be treated with 110 mg twice daily due to the general increased risk of bleeding in this populationCitation16,Citation17. It is estimated that ∼60% of treated AF-patients are younger than 80 years of ageCitation18.
The efficacy and safety of dabigatran 150 mg and 110 mg compared to the VKA warfarin in patients with non-valvular AF was examined in the RE-LY trial (ClinicalTrials.gov number, NCT00262600)Citation16. The results of this trial indicated the superiority of dabigatran 150 mg compared to VKAs in preventing stroke and SE events, but a similar level of protection against major hemorrhages. Furthermore, a similar efficacy but better safety profile of dabigatran 110 mg compared to VKAs was also observed.
Looking at the changing environment within healthcare, with increasing demand and costs for healthcare while budgets are under pressure, it is seen and might be further expected that patients will have more difficult and more restricted access to innovative interventions. Notably, governments and healthcare payers are reluctant to pay for increased ease of use for the patients in drug treatment, inclusive of potential absence of monitoring for NOACs. Furthermore, due to separated budgets for drugs, specialized hospital care, thrombosis services, and informal care, potential savings for dabigatran due to less INR monitoring costs or even decreased hospital and informal care costs cannot be collected within the budget where the drug expenditures take place and might, therefore, be considered separately rather than integrated. Yet, ideally various healthcare budget holders, government, and pharmaceutical companies share the same goal of improving and optimizing care and cure of patients within the limited budgets within a strong cooperation of all relevant healthcare stakeholders, and taking an integrative and societal overall perspective. Notably, it is the latter perspective that drives our approach in this paper. From this perspective, it also follows that, based on recommendations of the Dutch National Health Care Institute and Health Council, price and volume of dabigatran have been negotiated between the government and the manufacturer of dabigatran, within perception of value-based pricing and patient access scheming.
In this paper, we aim to estimate the cost-effectiveness of dabigatran vs VKAs for the prevention of arterial thromboembolism in Dutch patients with non-valvular AF. The economic analysis will be based on RE-LY trial resultsCitation16,Citation17 and Dutch observational data. The evaluation will be extended to include value-based pricing considerations and corresponding negotiations.
Methods
Model
Cost-effectiveness analyses were conducted including both differences in survival as well as differences in life-years adjusted for utility of health states (quality-adjusted life years; QALYs). The RE-LY trial was used as the key data sourceCitation16,Citation17. The CHADS2-score distribution as used in the base case was derived from Korenstra et al.Citation19 ().
Table 1. Dutch AF patients (base case)Citation19 and RE-LY trialCitation16 CHADS2 score distribution applied in the model.
A Markov model—embedded in a decision tree—was developed that followed AF patients through the natural course of the disease until the end of their life. The clinical events or complications included in the model were primary and recurrent IS, hemorrhagic stroke (HS), transient ischemic attack (TIA), SE, acute myocardial infarction (AMI), intracranial hemorrhage (ICH), extracranial hemorrhage (ECH), and death. Extracranial hemorrhage mainly consisted of gastro-intestinal bleeding. The key consequences of the clinical events are changing treatment status, reductions in quality-of-life, disability levels, or death. A simplified schematic presentation can be found in . In particular, the model distinguishes health states resulting from the combinations previous stroke/no previous stroke, no treatment/1st treatment/subsequent treatment, and independent/moderate disability dependent. Tunnel states for the maximum duration of one cycle were added to reflect temporary treatment discontinuations after ECHs. Initially, a cohort of 10,000 AF-patients populated the model, followed with 3-month cycles and including half-cycle corrections. The model allowed for analytic time horizons of 2 years (median follow-up in RE-LY), 3 years, 5 years, 10 years, 15 years, and lifetime. In the base case analysis, the time horizon was set at lifetime, to fully grasp the lifelong consequences of stroke and hemorrhage. The model was extensively described by Sorensen et al.Citation20 and Zheng et al.Citation21.
Figure 1. Schematic representation of the Markov model embedded in a decision tree. A Markov model was developed that followed atrial fibrillation (AF) patients through the natural course of the disease, with patients at risk of relevant clinical events until the end of their life. The clinical events included primary and recurrent ischemic stroke (IS), hemorrhagic stroke (HS), transient ischemic attack (TIA), systemic embolism (SE), acute myocardial infarction (AMI), intracranial hemorrhage (ICH), extracranial hemorrhage (ECH), and death. The key consequences of the clinical events are changing treatment status, resulting disability levels (DL), and/or reduction in quality-of-life, and death.
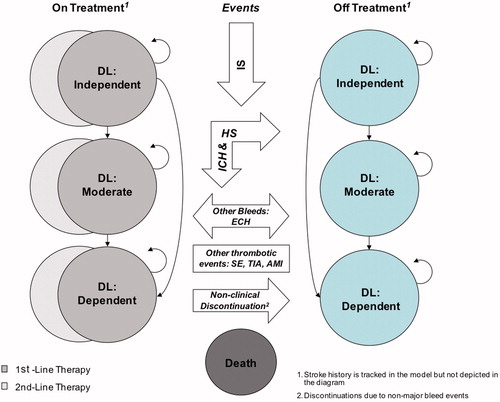
Transition probabilities
Transition probabilities were derived from the rates of stroke and other events as found in the RE-LY trialCitation16,Citation17. Specifically, CHADS2-dependent transition probabilities for IS in patients receiving dabigatran 150 mg bid, dabigatran 110 mg bid, or VKAs were estimated from the event rates per CHADS2 categoryCitation20. ICH risk is reported to increase after 80 years of age, and a relative risk as compared to those of 80 years and below of 1.8 was integrated in the model for patients over 80 years of ageCitation16,Citation17. Also, ECH risk is reported to be higher in older patients; in particular, those aged 70 years or higher. Therefore, a reduced ECH risk for patients less than 70 years was used (relative risk at 0.5)Citation22. In the base case, we did not correct for the center’s mean Time in Therapeutic Range (cTTR) and, as such, relative risks and transition probabilities were derived directly from the RE-LY trial. This is a conservative approach as the mean TTR in the RE-LY trial of 64% was better than the TTR of 55% in the Dutch situationCitation16,Citation19. Mortality due to clinical events such as IS, ICH, and ECH was estimated using the transition probability of the event and the probability of dying from the specific event. Age-related death was obtained from Statistics Netherlands’Citation23.
The model included over 500 transition probabilities, of which over 80 were CHADS2 score dependent.
QALYs
To estimate QALYs, utilities () were multiplied by the time spent in a health state. We took a conservative approach by using the utilities unadjusted for age, gender, race, ethnicity, income, education, and comorbidities. Utilities were obtained from a catalog of EQ-5D scores for the UKCitation24,Citation25. Additionally, marginal disutilities, reflecting the effective decreases in utility level, were applied for each clinical event for the duration of 3 months (i.e. one cycle). Resulting QALYs were discounted at 1.5% according to the Dutch guidelinesCitation26.
Table 2. Utility values used in the base case.
Costs
As the cost-effectiveness analysis was performed from the societal perspective, both direct and indirect costs were considered, medical as well as non-medical (). First, direct medical costs inside the healthcare sector were included in the model, in particular, drug costs, general practitioner (GP) visits, annual resource use costs for VKA monitoring, event costs (e.g. costs for hospitalization and those related to first episodes), follow-up costs (e.g. costs related to sequela and long-term morbidity), and costs related to discontinuation of therapy (e.g. costs for additional GP visits and prescription fees for alternate drug treatments). In the base case the drug costs for dabigatran were €2.30 per day, irrespective of the dose. Second, non-medical costs were used, including traveling expenses, informal care costs, and opportunity costs, notably regarding production lossesCitation27. Costs were determined for the year 2014 and discounted at 4%Citation26.
Table 3. Dutch unit costs for resources used applied in the model (in 2014€).
Base case and scenarios
In the base case evaluation, the cost-effectiveness of dabigatran was compared to VKAs from the societal perspective. To explore the impact of using only direct medical costs, a scenario analysis from the healthcare perspective was performed. In a scenario analysis, the CHADS2 distribution, as found in the RE-LY trial, was used instead of the CHADS2 distribution of Dutch patients. In the RE-LY trial, fewer non-hemorrhagic strokes and major bleedings were found in VKA-treated patients in centers with better INR control. However, the benefit of dabigatran over VKA in reducing intracranial hemorrhage was consistent, irrespective of the quality of INR controlCitation28. Therefore, a scenario was explored with a cTTR of >70%, to evaluate the influence of the quality of INR control. In this scenario, only the relative risks of intracranial bleeding and myocardial infarction in dabigatran vs VKAs patients in the RE-LY trial were used in the model. All other relative risks and derived transition probabilities, such as non-hemorrhagic stroke and bleedings, were set at the level of VKAs.
The monetary benefit of dabigatran was assessed at a threshold of €0 per QALY gained. The monetary benefit was extrapolated to the Dutch society using a projection of 296,000 AF patientsCitation8.
Sensitivity analysis
Both univariate and multivariate probabilistic sensitivity analyses (PSA) were performed. All relevant parameters, such as discounting; quality of INR control; age; time horizon; clinical effectiveness values; costs; utilities; and disutilities, were included in the univariate sensitivity analysis. For the PSA, appropriate distributions were chosen. In particular, baseline risks were assumed to have beta distributions, while relative risks were assumed to be log-normally distributed. Event costs and utilities were assumed to follow gamma and beta distributions, respectively.
Results
In the base case, for a cohort of 10,000 AF-patients, 4,454 life years and 4,552 QALYs were gained for dabigatran over VKAs (). Correspondingly, discounted incremental total cost savings were estimated at €13,892,288, in favor of dabigatran. Notably, the build-up of costs of dabigatran vs VKAs cost consisted of extra treatment costs at €34,008,204, savings on costs of events at €4,469,910, savings on follow-up costs at €16,069,395, and savings on indirect costs at €27,361,188. Drug treatment costs included INR monitoring and dose adjustment for VKAs. Ergo, dabigatran 150 mg bid for patients below the age of 80 years and 110 mg bid for patients 80 years and older was a dominant strategy compared to VKAs in the base case.
Table 4. Life-time results from the model-based cost-effectiveness analysis in the base case (number of events; costs in 2014 €).
The expected monetary benefits were €1,389 per patient at a cost-effectiveness threshold of €0 per QALY gained. For the Dutch population of 296,000 AF patients, expected monetary benefits amounted to €411 million.
In the base case, dabigatran prevented 412 cases of ischemic stroke compared to VKAs. Compared to VKAs, dabigatran prevented 152 cases of fatal ischemic stroke. The number of cases of intracranial hemorrhage and hemorrhagic stroke prevented by dabigatran was 688; of these prevented cases, 357 were fatal. Excluding gastro-intestinal hemorrhage, the total number of hemorrhage cases prevented by dabigatran compared to VKAs was 98. Non-fatal gastro-intestinal bleeding occurred in 526 cases extra for dabigatran compared to VKAs. Five cases of fatal hemorrhage occurred extra in the dabigatran population. An extra 258 cases of acute myocardial infarction, of which three were fatal, occurred in dabigatran compared to VKA treated patients.
In a scenario considering only direct medical costs, net costs were €13.5 million, compared to cost savings in the base case (). The corresponding ICER was €2,959 per QALY gained. The impact of employing the CHADS2 distribution of the RE-LY trial instead of the distribution of Dutch patients was limited to lower cost-savings and slightly higher health benefits compared to the base case. Simulating a scenario to reflect the cTTR >70% population of the RE-LY trial yielded lower health gains and showed net costs for dabigatran compared to VKA.
Table 5. Scenario and deterministic univariate sensitivity analysis for dabigatran vs VKAs for stroke prevention in atrial fibrillation.
In univariate sensitivity analyses, cost savings and favorable incremental health effects generally remained, except when the time horizon was strictly limited to 2 years and if costs of dabigatran were drastically raised or costs of INR-control drastically reduced. Notably, dabigatran was cost saving compared to VKAs if annual costs of VKA control exceeded €159 per person per year or dabigatran costs were below €2.81 per day.
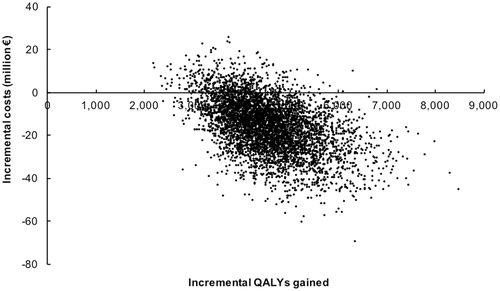
The scatterplot resulting from the probabilistic sensitivity analysis is shown in . The Monte Carlo simulation (5,000 replicates) for dabigatran vs VKA revealed that dabigatran was cost saving compared to VKA in 90.2% of the replicates. At a probability of 95% that dabigatran is cost-effective, the corresponding willingness-to-pay was €1,070 per QALY gained. At a willingness-to-pay of €7,134 per QALY gained, the probability that dabigatran was cost-saving reaches 100%.
Figure 2. Monte Carlo simulation (5,000 replicates) of the cost-effectiveness of dabigatran vs VKAs for stroke prevention in atrial fibrillation. The probability that dabigatran is cost saving compared to VKA is 90.2%. At a probability of 95% that dabigatran is cost-effective, the corresponding willingness-to-pay is €1,070 per QALY gained. At a willingness-to-pay of €7,134 per QALY gained, the probability that dabigatran is cost-saving reaches 100%.
Discussion
The cost-effectiveness of dabigatran vs VKAs for prevention of atrial thromboembolism was evaluated for Dutch patients with non-valvular AF. Dabigatran 150 mg bid for patients below the age of 80 years and 110 mg bid for patients aged 80 years and older vs VKAs was found to be cost saving. In the base case analysis, a total of 4,552 QALYs were gained, and €13,892,288 was saved in a cohort of 10,000 AF patients. From the healthcare perspective, including only direct medical cost, the ICER amounted to €2,959 per QALY gained. Using the CHADS2 distribution of the RE-LY trial decreased the cost-savings and increased the health gains. In a scenario evaluating a high quality of INR control, i.e. a cTTR >70%, an ICER of €3,518 per QALY gained was found.
Several studies examined the cost-effectiveness and cost-utility of applying dabigatran compared to VKAs in different country-settings (e.g. UK, Belgium, Germany, Canada, Sweden, USA)Citation20,Citation21,Citation29–32. Yet, our study is the first one to provide the health and economic effects of applying dabigatran compared to VKAs in the Dutch setting. The results of the aforementioned studies indicate a wide range of ICERs from €2,807/QALY in the Belgian setting to €294,349/QALY in the German setting. These major differences in economic consequences associated with the use of anti-coagulants could be attributed to the choice of the study perspective (i.e. societal vs healthcare provider), the choice of first line/second line treatment (e.g. only dabigatran 150 mg or 110 mg or a switch between the two dosages), modeling approach (e.g. Markov model or discrete event simulation model), the health states included in the model (e.g. the German study did not include SE and hemorrhagic stroke state), country-specific costs, and discount rates. Such variability in the modeling approaches hampers a direct comparison across the study results. The presented favorable health economic profile is substantiated by the study of Freeman et al.Citation33. At €2.30 per day (our base case price, $3.16), dabigatran would be cost-saving in the evaluation by Freeman et al.Citation33. Compared to the NOACs apixaban and rivaroxaban, dabigatran was found to be dominant in NVAF patients in the UKCitation21.
The key drivers of the cost-effectiveness of dabigatran 150 mg bid relative to trial-based VKAs were dabigatran’s ability to reduce the IS rate by 0.34 events per 100 patient-years, and to reduce ICH rate by 0.45 events per 100 patient-years. Particularly important was the reduction of fatal ICH by 0.25 fatal events per 100 patient-years. In addition, dabigatran was increasingly cost-effective as the utility for the independent, disability free health state increases relative to health states with disabilities, which magnifies the health improvements conferred by dabigatran.
Rigorous univariate sensitivity analyses revealed that the estimated net costs and health benefits for the base case are relatively insensitive to variation in the parameters, and our results may, therefore, be considered robust. A decrease of €0.20 in drug costs for dabigatran increased the cost-savings from €13.9 to €19.2 million. Dabigatran will still be cost-saving compared to VKA if dabigatran drug costs are lower than €2.82 per day. In a break-even analysis, it was shown that dabigatran is cost saving at assumed pricing compared to VKA over an annual cost of €159 per person for INR control. As the benefit of reduced stroke risk is received with increasing treatment duration, dabigatran is more cost-effective when administered over a life-time treatment horizon than in shorter durations of 2 and 10 years. The PSA showed that the probability of health losses is very low (). The probability of cost-saving is 90.2% and, for a willingness-to-pay of €1,070 per QALY, the probability of being cost-effective for dabigatran is 95%.
The sensitivity analysis with respect to the cost of dabigatran is especially interesting with regards to the price-volume agreement. This agreement has been negotiated between the Ministry of Health, Welfare, and Sports, and Boehringer Ingelheim, the manufacturer of dabigatran. Due to the potential high impact on the drug budget of above €100 million per year and the pressure on healthcare budgets in general it has been agreed between both parties to give patients access to dabigatran at a lower initial price of €2.30, followed by further decreasing prices when the volume increases. This means that the prevention of arterial thromboembolism in patients with non-valvular AF with dabigatran instead of VKA is even more cost saving than calculated in the base case. Therefore, the expected monetary benefits to the Dutch society are also higher than projected in this study. Unfortunately, as the actual average price of dabigatran per day is not disclosed to the Dutch society, the real monetary value of dabigatran is also unknown to the Dutch society.
A key strength of the evaluation is that estimates of clinical outcomes for dabigatran and its primary comparator, VKAs, are taken from a head-to-head randomized clinical trial. This allows the most important determinant of cost-effectiveness, the relative risk of stroke when treated with dabigatran vs VKAs, to be assessed precisely. Also, the model incorporated country-specific costs, nation-specific mortality due to other causes, and CHADS2 score related allocation of Dutch patients. Moreover, wide parameter ranges have been explored using both one-way and probabilistic sensitivity analyses, providing a clear understanding of the drivers of cost-effectiveness and the magnitude of uncertainty in the estimate.
Our study has several potential limitations. One of the limitations is the uncertainty involved in extrapolating data derived from clinical trials with a 2 year follow-up to lifetime events in patients. Furthermore, the inclusion of patients in the RE-LY trial was based on the CHADS2 score instead on the CHA2DS2-VASc score. The advantages of the CHA2DS2-VASc score compared to the CHADS2 score is the tool reliability in identifying patients at a true risk for stroke, which otherwise might be neglected in the assessment with the CHADS2 score. As the more sensitive CHA2DS2-VASc score now commonly is used to identify AF patients eligible for anti-coagulation, we used the on average lower CHADS2 score of Dutch AF patients in our evaluation instead of the higher CHADS2 score in the RE-LY trial. Unfortunately, due to the still limited data on clinical outcomes, we were not able to include health economic effects of idarucizumab in our evaluation. Idarucizumab is a monoclonal antibody fragment to reverse the anti-coagulant effect of dabigatranCitation34.
Conclusion
In conclusion, dabigatran may serve as a cost-effective alternative to VKAs in combination with INR monitoring in Dutch patients with non-valvular AF. Compared to VKAs, dabigatran prevented more strokes, caused less intra-cranial hamorrhages, and saved costs to the Dutch society. Due to an undisclosed lower price of dabigatran, monetary benefits are expected to be even higher than projected in this study.
Transparency
Declaration of funding
Boehringer Ingelheim paid personal fees to MVH to conduct this study.
Declaration of financial/other relationships
MVH reports grants from Bayer and personal fees from Boehringer Ingelheim during the conduct of the study. RGT reports grants and personal fees from Boehringer Ingelheim and personal fees from Bayer and Pfizer/Bristol Meyer Squibb, all outside the submitted work. BK is an employee of Boehringer Ingelheim. MJP received grants and honoraria from various pharmaceutical companies, inclusive of those developing, producing, and marketing new oral anti-coagulants (NOACs). JS and MSJ have no relevant or other relationships to disclose. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
Presented as a poster at the Dutch Federation for Innovative Drug Research (FIGON), The Netherlands, October 2016.
References
- Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47
- European Heart Rhythm A, European Association for Cardio-Thoracic S, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 2001;285:2370-5
- Iqbal MB, Taneja AK, Lip GY, et al. Recent developments in atrial fibrillation. BMJ 2005;330:238-43
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949-53
- van der Linden MWW, de Bakker GP, Schellevis DH, et al. Nationale studie naar ziekten en verrichtingen in de huisartspraktijk. Klachten en aandoeningen in de bevolking en in de huisartspraktijk. [Second national study on illnesses in the general practice] Utrecht/Bilthoven: NIVEL; 2004
- Opstelten W, Boode BS, Heeringa J, et al. [Summary of the practice guideline 'Atrial fibrillation' (first revision) from the Dutch College of General Practitioners]. Nederlands tijdschrift voor geneeskunde 2010;154:A1570
- Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746-51
- National Institute of Public Health and Enviroment. Volksgezondheid.info: Ranglijst ziekten op basis van ziektelast (in DALY’s) [Public health and health care: ranking diseases on disease burden (in DALY’s)] [Internet]. 2017. https://www.volksgezondheidenzorg.info/ranglijst/ranglijst-ziekten-op-basis-van-ziektelast-dalys
- You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2Suppl):e531S-75S
- Anonymous. Cost of stroke in The Netherlands (2011). National Institute for Public Health and the Environment; 2016. Available at: https://kostenvanziektentool.volksgezondheidenzorg.info/tool/nederlands/?ref=kvz_v2l1b1p4r4c2i0t1j0o3y6a-1g0d50s54z0f0w2
- Hylek EM, Skates SJ, Sheehan MA, et al. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996;335:540-6
- Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003;349:1019-26
- Cannegieter SC, Rosendaal FR, Wintzen AR, et al. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med 1995;333:11-17
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095-106
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE-LY trial. N Engl J Med 2010;363:1875-6
- Virjo I, Makela K, Aho J, et al. Who receives anticoagulant treatment with warfarin and why? A population-based study in Finland. Scand J Primary Health Care 2010;28:237-41
- Korenstra J, Wijtvliet EP, Veeger NJ, et al. Effectiveness and safety of dabigatran versus acenocoumarol in 'real-world' patients with atrial fibrillation. Europace 2016;18:1319-27
- Sorensen SV, Kansal AR, Connolly S, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105:908-19
- Zheng I, Sorensen SV, Gonschior AK, et al. Comparison of the cost-effectiveness of new oral anticoagulants for the prevention of stroke and systemic embolism in atrial fibrillation in a UK setting. Clin Ther 2014;36:2015-28.e2
- Fang MC, Go AS, Hylek EM, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriat Soc 2006;54:1231-6
- Statistics Netherlands 2016. Available at: http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=37360ned&D1=0,3&D2=a&D3=a&D4=94&HDR=G1,T&STB=G2,G3&VW=T
- Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making Int J Soc Med Decis Making 2011;31:800-4
- Post PN, Stiggelbout AM, Wakker PP. The utility of health states after stroke: a systematic review of the literature. Stroke 2001;32:1425-9
- The National Health Care Institute. Guidelines for the conduction of economic evaluations in health care. Diemen: The National Health Care Institute; 2016
- van den Berg B, Brouwer W, van Exel J, et al. Economic valuation of informal care: lessons from the application of the opportunity costs and proxy good methods. Soc Sci Med 2006;62:835-45
- Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975-83
- Pink J, Lane S, Pirmohamed M, et al. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ 2011;343:d6333
- Wouters H, Thijs V, Annemans L. Cost-effectiveness of dabigatran etexilate in the prevention of stroke and systemic embolism in patients with atrial fibrillation in Belgium. J Med Econ 2013;16:407-14
- Krejczy M, Harenberg J, Marx S, et al. Comparison of cost-effectiveness of anticoagulation with dabigatran, rivaroxaban and apixaban in patients with non-valvular atrial fibrillation across countries. J Thromb Thrombolysis 2014;37:507-23
- Davidson T, Husberg M, Janzon M, et al. Cost-effectiveness of dabigatran compared with warfarin for patients with atrial fibrillation in Sweden. Eur Heart J 2013;34:177-83
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1-11
- Pollack CV, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med 2017;377:431-441
- Robinson A, Thomson R, Parkin D, et al. How patients with atrial fibrillation value different health outcomes: a standard gamble study. J Health Serv Res Policy 2001;6:92-8
- The National Health Care Institute. Consumer reimbursement price. 2017. Available at: http://medicijnkosten.nl/
- Netherlands HCot. New anticoagulants: a well-dosed introduction. Vol. publication no. 2012/07. The Hague: Health Council of the Netherlands; 2012
- Hakkaart-van Roijen L, Tan SS, Bouwmans CAM. Handleiding voor kostenonderzoek, methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg [Manual for costing studies: methodes and standard prices]. Diemen: Health Care insurance board; 2010
- van Hout BA, Simoons ML. Cost-effectiveness of HMG coenzyme reductase inhibitors; whom to treat? Eur Heart J 2001;22:751-61
- Baeten SA, van Exel NJ, Dirks M, et al. Lifetime health effects and medical costs of integrated stroke services - a non-randomized controlled cluster-trial based life table approach. Cost Effect Resour Alloc C/E 2010;8:21
- Groot M. Cost-effectiveness of melagatran/ximelagatran for the prevention of venous thromboembolism following major elective orthopaedic surgery. Economic Evaluation Serving different actors in a changing environment; some examples [Thesis]. Rotterdam; 2006
- The Dutch Health Care Authority. The Hague: DRG tariffs; 2014. http://dbc-zorgproducten-tarieven.nza.nl/nzaZpTarief/ZoekfunctieDbc.aspx
- Vonkeman HE, Klok RM, Postma MJ, et al. Direct medical costs of serious gastrointestinal ulcers among users of NSAIDs. Drugs Aging 2007;24:681-90
- Soekhlal RR, Burgers LT, Redekop WK, et al. Treatment costs of acute myocardial infarction in the Netherlands. Neth Heart J. 2013;21:230-5

