Abstract
Aims: The EINSTEIN-Extension trial (EINSTEIN-EXT) found that continued treatment with rivaroxaban for an additional 6 or 12 months (vs placebo) after 6–12 months of initial anticoagulation significantly reduced the risk of recurrent venous thromboembolism (VTE) with a small non-significant increased risk of major bleeding (none fatal or in critical site). This study aimed to compare total healthcare cost between rivaroxaban and placebo, based on the EINSTEIN-EXT event rates.
Methods: Total healthcare cost was calculated as the sum of treatment and clinical event costs from a US managed care perspective. Treatment duration and event rates were obtained from the EINSTEIN-EXT study. Adjustment on treatment duration was made by assuming a 10% non-adherence rate. Drug costs were based on wholesale acquisition costs. Cost estimates for clinical events (i.e. recurrent deep vein thrombosis [DVT], recurrent pulmonary embolism, major bleeding, clinically relevant non-major bleeding) were determined from the literature. Results were examined over a ±20% range of each cost component and over 95% confidence intervals (CIs) of event rate differences in deterministic (one-way) and probabilistic sensitivity analyses (PSA).
Results: Total healthcare cost was $1,454 lower for rivaroxaban-treated (vs placebo-treated) patients in the base-case, with a lower clinical event cost fully offsetting drug cost. The cost savings of recurrent DVT alone (–$3,102) was greater than drug cost ($2,723). Total healthcare cost remained lower for rivaroxaban in the majority (73%) of PSA (cost difference [95% CI] = –$1,454 [–$2,396, $1,231]).
Limitations: This study was conducted over the 1-year observation period of the EINSTEIN-EXT trial, which limited “real-world” applicability and examination of long-term economic impact. Assumptions on drug and clinical event costs were US-based and, thus, not applicable to other healthcare systems.
Conclusions: Total healthcare costs were estimated to be lower for patients continuing rivaroxaban therapy compared to those receiving placebo in VTE patients who had completed 6–12 months of VTE treatment.
Introduction
Venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is a common disease that is associated with increased risk of recurrence and mortality, and could be potentially preventableCitation1,Citation2. According to the Centers for Disease Control and PreventionCitation3, ∼1–2 per 1,000 individuals could have VTE each year in the US, resulting in ∼60,000–100,000 annual deaths. After a first VTE episode, many patients remain at high risk for recurrence, which is associated with deterioration in quality-of-life and increased mortalityCitation4,Citation5. Studies have shown that recurrent VTE can occur in up to 25% of patients within the next 5 years following a first VTE episode and 30% within 10 yearsCitation3,Citation6,Citation7. The chronic nature of VTE carries a substantial economic burden in the US. It has been shown that over 500,000 hospitalizations are attributed to VTE each year in the USCitation8. Moreover, studies on recurrent VTE episodes have demonstrated substantial increases in healthcare costs related to incident VTE eventsCitation8,Citation9.
Current standard of care consists of initial treatment with anticoagulants, often with a low molecular weight heparin or a vitamin K antagonist (VKA), mainly warfarinCitation10,Citation11. Rivaroxaban, a newly developed oral direct factor Xa inhibitor anticoagulant licensed for VTECitation12, has been demonstrated as a viable alternative therapy to traditional VKA therapy with advantages of minimal drug and food interactions and no requirement for routine coagulation monitoringCitation13. The American College of Chest Physicians (ACCP) guidelines now suggest its use, as other non-vitamin K antagonist oral anticoagulants, over VKA therapyCitation14.
Although there is no clear consensus on the optimal duration VTE patients should remain on anticoagulants, the ACCP guidelines recommend anticoagulant treatment for at least 3 months following an acute episode of VTE to prevent recurrent VTECitation14. In addition, in patients with a first or recurrent unprovoked VTE who have a low or moderate bleeding risk, the guidelines recommend extending anticoagulant therapy over 3 monthsCitation14.
The EINSTEIN-Extension (EINSTEIN-EXT) trial results showed that extending treatment with rivaroxaban beyond 6 months in those with unprovoked VTE reduced the risk of recurrent VTE without substantially increasing the risk for major bleeding events when compared to placeboCitation15. While these findings on extended anticoagulant treatment in the EINSTEIN-EXT study are compelling, there are currently no studies evaluating the potential cost implications associated with extended rivaroxaban treatment compared to no treatment.
Healthcare costs of VTE events can be multifactorial, primarily driven by inpatient care, treatments, and complicationsCitation1,Citation2. Evaluating costs associated with treatment, cost associated with potential increased bleeding events, and reduced cost associated with fewer recurrent VTE events would be of great value for payers to better evaluate the cost-effectiveness of extended treatment with rivaroxaban relative to placebo in VTE patients.
Therefore, the objective of this study was to compare cost associated with extended treatment with rivaroxaban vs placebo, based on the observed recurrent VTE and bleeding rates in the EINSTEIN-EXT study.
Methods
Study population
The study population was based on the population of the EINSTEIN-EXT study (ClinicalTrials.gov NCT00439725), which was a multi-center (in over 30 countries), randomized, double-blind, placebo-controlled study consisting of 1,197 adult patients with confirmed symptomatic DVT and/or PE enrolled after the initial 6–12 months of treatment (rivaroxaban = 602 and placebo = 594, one excluded due to invalid informed consent). Of note, in the clinical trial, patients in whom anticoagulant treatment for their index PE or DVT should be continued were excluded. Patient demographic and clinical characteristics were described in the EINSTEIN-EXT studyCitation15,Citation16. For both cohorts, mean age was 58 years and 58% of the patients were men. Among all VTE patients, ∼ 60% had DVT as the initial diagnosis (rivaroxaban = 64.1% and placebo = 60.0%), 15% had a previous VTE (rivaroxaban = 17.9% and placebo = 14.1%), and 8% had a known thrombophilic condition (rivaroxaban = 8.1% and placebo = 8.1%). Moreover, most VTEs (DVT or PE) at index were unprovoked VTEs (rivaroxaban = 73.1% and placebo = 74.2%).
Cost comparison framework
A cost comparison analysis was conducted to compare the total healthcare direct costs, comprising drug cost and clinical event cost, in VTE patients who received extended treatment with rivaroxaban vs placebo from a US managed care payer’s perspective. Drug cost was defined as the cost associated with rivaroxaban use in the rivaroxaban cohort and calculated as the product of unit cost of drug and duration of treatment, while there was no drug cost associated with the placebo cohort. Clinical event costs were defined as annual incremental costs of care associated with clinical events (vs without clinical events) and calculated as the product of the 1-year Kaplan-Meier (KM) survival rate from the EINSTEIN-EXT study and the annual incremental cost of care for that event. Specifically, the annual incremental costs of care associated with a clinical event included the cost associated with managing the occurrence of the event, as well as the incremental medical cost during the period after the event occurrence. Four clinical event rates that were assessed in the EINSTEIN-EXT study were considered in the cost comparison model, including recurrent DVT, recurrent PE, major bleeding, and clinically relevant non-major bleeding. Although mortality was assessed in the EINSTEIN-EXT study, it was not included in the current study, due to a very small and likely to be spurious mortality difference between cohorts (rivaroxaban vs placebo; difference in mortality = 2/10,000 patients). Costs were assessed over a 1-year time horizon, as the EINSTEIN-EXT study on which our study was based had a maximum of 1-year follow-up. Moreover, patients in both cohorts were assumed to have the same medical spending before the occurrence of a clinical event. All the analyses in this study were conducted using Microsoft Excel 2016 software.
Cost comparison inputs
Treatment
Patients in the EINSTEIN-EXT study were randomly assigned to receive either continued treatment with rivaroxaban (20 mg once daily) or placebo after having been treated previously with a VKA or rivaroxaban. To calculate the drug cost associated with rivaroxaban, the duration of treatment (DOT) was estimated based on the intended DOTs from the trial, with an assumption of a non-adherence rate of 10%, to account for the fact that patients in clinical trials may not be fully adherent to the assigned treatments. Specifically, 59.8% of patients in the rivaroxaban cohort had 6 months intended DOT, and the remaining patients had a 12 month intended DOT. The weighted average of the intended DOT was 8.4 months, which was then adjusted based on the 10% non-adherence rate and was used to calculate the drug cost in the base-case analysis (i.e. 7.57 months). The observed DOT was shorter than the intended DOT in the EINSTEIN-EXT study, due to the study design (event driven, superiority study). The median observed DOTs were 6.0 and 8.8 months in rivaroxaban-treated patients with 6-month and 12-month intended DOT, respectively. Thus, the weighted average of the observed DOT of 7.1 months was used to calculate drug cost for the rivaroxaban cohort in a sensitivity analysis.
Clinical events
In the EINSTEIN-EXT study, recurrent DVT and recurrent PE were defined as symptomatic recurrent DVT and symptomatic recurrent non-fatal or fatal PE, respectively, based on the diagnostic criteria specified in that study; major bleeding was defined as any bleed contributing to death, or occurring in a critical site, or associated with a fall in hemoglobin of >2 g per deciliter or transfusion of >2 units or both; and clinically relevant non-major bleeding was defined as overt bleeding not meeting the criteria for major bleeding, but associated with medical intervention, unscheduled contact with a physician, interruption or discontinuation of study treatment, or associated with any other discomfort, such as pain or impairment of activities of daily life.
Compared to placebo recipients, rivaroxaban-treated patients in the EINSTEIN-EXT study were found to have lower 1-year KM rates of recurrent VTE (event rate per 10,000 patients in rivaroxaban vs placebo cohorts was 145 vs 662 for recurrent DVT, and 154 vs 355 for recurrent PE, respectively), but higher rates of major bleeding and clinically relevant non-major bleeding (event rate per 10,000 patients in rivaroxaban vs placebo cohorts was 68 vs 0 for major bleeding, and 766 vs 183 for clinically relevant non-major bleeding, respectively).Citation17 All major bleeds (three gastrointestinal bleeds and one menorrhagia) were non-fatal bleeds, and associated with transfusions or transient hemoglobin reductions.
Unit costs
Unit cost of rivaroxaban was estimated based on the wholesale acquisition cost (WAC) package price from the Red BookCitation18. Unit costs for clinical events were identified from a targeted literature search. All costs were inflated to 2016 dollars based on Consumer Price Index (CPI) medical services data from the US Bureau of Labor Statistics. The unit cost for each cost model component (i.e. treatment and four clinical events) is listed in .
Table 1. Base-case model inputs and ranges used in one-way sensitivity analysis.
In the base-case analysis, the unit cost for rivaroxaban (i.e. for 1 month of treatment) was estimated at $359.61, which is the 2016 drug price for a 30-pill package of 20 mg daily dose rivaroxaban obtained from Red BookCitation18.
Our literature search showed that, although previous studies have assessed costs associated with the clinical events of interest, most of the reported cost estimates only included costs associated with managing the event itself, but not costs associated with managing the consequences of the eventCitation19–23. Due to the lack of information on annual incremental cost associated with recurrent DVT and PE in the literature, the unit costs for both recurrent DVT and PE events were estimated at $60,000 per event, based on the adjusted incremental annual total all-cause healthcare cost of care associated with recurrent VTE (vs non-recurrent VTE) estimated in two studies: $62,181 (in 2016 US dollars) by Lefebvre et al.Citation4 and $59,784 (in 2016 US dollars) by Lin et al.Citation8. Both studies used healthcare insurance claims databases and controlled for patient baseline characteristic differences. Therefore, the cost estimates from the two studies reflected the incremental annual all-cause cost of care due to occurrence of recurrent VTE rather than patient differences. Of note, in the EINSTEIN-EXT trial, 67.9% of recurrent VTE events were DVT, a proportion in a similar range to that reported in both recurrent VTE groups of the cited studies (Lefebvre et al.Citation4 and Lin et al.Citation8: ∼75%). In addition, the unit cost of $16,161 per major bleeding event was also set at an adjusted incremental annual total all-cause healthcare cost associated with major gastrointestinal (GI) bleeding (vs no bleeding), which was estimated in another claims analysis study among warfarin-treated patients with atrial fibrillationCitation10. Finally, the unit cost for the clinically relevant non-major bleeding event was set at $364 per event, based on the estimated annual incremental cost associated with minor GI bleeding (vs no bleeding) from the same studyCitation10. Since the unit cost for a clinical event may vary across populations, type of event (such as different type of bleeding event), or other clinical factors, the effect of unit cost assumptions on the results was examined through sensitivity analyses.
Sensitivity analyses
Two sensitivity analyses related to drug cost estimation were conducted: (1) a 15% drug discount was applied to the unit cost for rivaroxaban to acknowledge that insurance payers in the US market may likely receive rebates on drug purchases from pharmaceutical companies; and (2) the weighted average of the observed DOT instead of the intended DOT was used.
Moreover, to account for the uncertainties surrounding the model inputs, and to examine the impact of model inputs on the cost comparison model, deterministic (i.e. one-way) and probabilistic sensitivity analyses were conducted. A one-way sensitivity analysis was conducted by changing one model input (i.e. unit costs or clinical event rates) at a time, while keeping all the other model inputs constant at their base-case values, while a probabilistic sensitivity analysis was conducted by changing all model inputs simultaneously.
In the one-way sensitivity analysis, unit costs were varied over the ±20% range of their base values, and clinical event rate differences were varied over their corresponding 95% confidence intervals (CIs) that were reported in the EINSTEIN-EXT study ()Citation17. In the probabilistic sensitivity analysis, a Monte Carlo simulation with 1,000 iterations was conducted. In each iteration, model inputs were sampled at random from their respective assumed probability distributions, and total healthcare cost differences were calculated. Specifically, the unit cost for rivaroxaban was assumed to follow a triangular distribution with the WAC price discounted by 15% as the mode, the WAC price as the maximum, and –20% of the mode value as the minimum. Unit costs for clinical events were assumed to follow gamma distributions, with means equal to their base values and standard deviations estimated from the 95% CIs of the base values obtained from the corresponding referencesCitation10,Citation24. Clinical event rate differences were assumed to follow normal distributions, with means equal to their base values and standard deviations estimated from the 95% CIs of rate differences obtained from the EINSTEIN-EXT study. The 95% CI of the total healthcare cost difference corresponding to the 2.5th and 97.5th percentiles of 1,000 cost estimates was obtained, and the proportion of iterations with total healthcare cost difference in favor of rivaroxaban was also reported.
Results
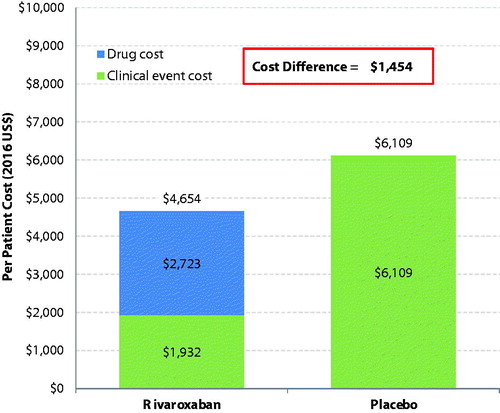
In the base-case analysis, the total drug cost per patient in the rivaroxaban cohort was $2,723 for duration of treatment of 7.57 months, compared to $0 in the placebo cohort (). As for the clinical events (), compared to patients in the placebo cohort, patients in the rivaroxaban cohort had a lower cost due to recurrent VTE with a cost difference of –$4,308 per patient ($1,794 vs $6,102), but a higher cost due to bleeding (cost difference per patient was $110 and $21 for major bleeding event and clinically relevant non-major bleeding event, respectively). The overall clinical event cost per patient was $1,932 in the rivaroxaban cohort and $6,109 in the placebo cohort, leading to a clinical event cost difference per patient of –$4,177 between rivaroxaban and placebo ().
Table 2. Drug costs per patient per year in rivaroxaban and placebo cohorts.
Table 3. Clinical event costs per patient per year in rivaroxaban and placebo cohorts.
The total healthcare cost difference between cohorts accounted for both treatment and clinical event costs (). Compared to placebo, rivaroxaban had a much lower overall clinical event cost, which completely offset the higher drug cost, and resulted in a total healthcare cost lower by $1,454 per patient (rivaroxaban vs placebo: $4,654 vs $6,109). The cost savings of recurrent DVT alone (−$3,102) was greater than drug cost ($2,723). Compared to the base-case analysis using the intended DOT of 7.57 months, the sensitivity analysis using the observed DOT of 7.1 months for the rivaroxaban cohort showed that patients in the rivaroxaban cohort had a smaller incremental drug cost (rivaroxaban vs placebo: $2,553 vs $0), and, thus, a greater total healthcare cost saving (rivaroxaban vs placebo: –$1,623). Moreover, when a 15% drug discount was applied to the rivaroxaban unit cost, a greater total healthcare cost difference (rivaroxaban vs placebo: –$1,863) was also observed, due to the reduced total drug costs associated with rivaroxaban.
Figure 1. Total healthcare cost difference per patient per year between rivaroxaban- and placebo-treated patients.
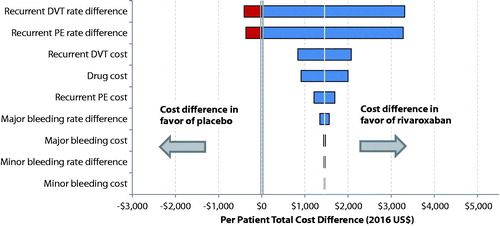
The impact of model inputs on the total healthcare cost, which was assessed in the one-way sensitivity analysis by varying each model input across the specified ranges (), is illustrated using a tornado diagram (). The tornado diagram was arranged in order of decreasing width, indicating that variations in inputs at the top have the greatest impact on the total healthcare cost difference, while variations in inputs at the bottom have the smallest impact. The 1-year KM rate difference of recurrent DVT was the leading cost driver, followed by the 1-year KM rate difference of recurrent PE, unit cost of recurrent DVT, unit cost for drug, and unit cost for recurrent PE (). Moreover, rivaroxaban was always associated with a lower total healthcare cost compared to placebo when unit costs were varied by ±20% or when the rate difference for major or clinically relevant non-major bleeding events varied within their 95% CIs (). However, the total healthcare cost differences turned in favor of placebo when the rate differences of recurrent DVT or PE events between cohorts took the value of the lower bound of their 95% CIs, although the magnitudes of the higher costs associated with rivaroxaban were small (∼$400).
Figure 2. Total healthcare cost difference per patient per year between rivaroxaban- and placebo-treated patients estimated in one-way sensitivity analysis. (1) Unit costs associated with drug, recurrent DVT, recurrent PE, major bleeding, or clinically relevant non-major bleeding varied by having its base unit cost decreased or increased by 20%. (2) The variation of a clinical event rate (i.e. recurrent DVT, recurrent PE, major bleeding, or clinically relevant non-major bleeding) was assessed based on the corresponding 95% confidence intervals (CIs) of rate differences between cohorts that were reported in the EINSTEIN-EXT study (Wells et al.Citation17).
When accounting for the variabilities of model inputs simultaneously, the PSA results showed that the total healthcare cost was lower in rivaroxaban-treated patients in the majority (73%) of the 1,000 cost estimates obtained (cost difference [95% CI], rivaroxaban vs placebo = –$1,454 [–$2,396, $1,231]).
Discussion
Using the EINSTEIN-EXT study event rates and the incremental healthcare cost of care for clinical events from literature, this study estimated and compared the total healthcare cost associated with extended rivaroxaban vs placebo among patients with VTE who had completed 6–12 months of anticoagulation. Study results indicated that continued treatment with rivaroxaban for an additional 6 or 12 months was associated with a lower total healthcare cost than placebo in the base-case analysis and in the majority of sensitivity analyses. The base-case results showed that, although, compared to placebo-treated patients, rivaroxaban-treated patients had a higher drug cost, the lower clinical event cost resulting from fewer recurrent VTE events in the rivaroxaban cohort fully offset the drug cost and led to a lower total healthcare cost. The total healthcare cost difference per patient was –$1,454 lower in the rivaroxaban cohort than in the placebo cohort in the base-case analysis, and a greater cost savings associated with rivaroxaban (–$1,863) was observed when a 15% discount was applied to rivaroxaban drug price. When accounting for the large variability around model inputs simultaneously in the PSA, 73% of the 1,000 simulated data showed that rivaroxaban had a lower total healthcare cost.
The one-way sensitivity analysis showed that the base-case finding of lower total healthcare cost associated with rivaroxaban over placebo was consistent across the ranges of most model inputs, except for the rate difference of recurrent DVT and recurrent PE. A higher total healthcare cost in rivaroxaban (vs placebo) was observed when the rate differences of recurrent DVT or PE took the lower bounds of their 95% CIs. However, since the probability of observing the boundary value of the 95% CIs for a rate difference in real-world settings may be low, the higher cost associated with rivaroxaban based on the lower bound of the 95% CI may be unlikely to be observed. Moreover, with the ranges used for each model input (i.e. ±20% for unit costs and 95% CIs for event rates), the one-way sensitivity analysis showed that the 1-year KM rate difference of recurrent DVT had the greatest impact on the total healthcare cost difference between rivaroxaban and placebo, followed by the 1-year KM rate difference of recurrent PE, recurrent DVT cost, and drug cost. Relative to other model inputs, rate differences and unit cost due to bleeding events had less impact on the total healthcare cost difference.
To the best of our knowledge, this is the first study to estimate costs associated with extended treatment with rivaroxaban vs placebo using a cost comparison analysis in the US healthcare system. Our findings of lower total healthcare cost of extended treatment with rivaroxaban (vs placebo) are comparable to previous studies showing rivaroxaban to be cost effective when compared with standard initial VTE therapyCitation11,Citation25–28. A recent cost effectiveness study based on the EINSTEIN DVT/PE trial dataCitation29,Citation30 showed that rivaroxaban therapy yielded a per patient cost savings of $2,471 over the 1-year time horizon and had longer quality-adjusted life-years compared to enoxaparin + VKACitation27. In a similar study in the UK setting, which also used event rates from the EINSTEIN-DVT/PE trials, the authors constructed a Markov model and demonstrated that, compared to standard VTE treatment, rivaroxaban was associated with lower costs for treatment duration modeled at 3, 6, and 12 months in the overall VTE population and in the sub-population stratified by type of VTECitation11. Another study also showed that patients receiving rivaroxaban had lower median hospital charges in the 6 months following a diagnosis of low-risk VTE compared to patients receiving low-molecular-weight heparin and then warfarinCitation26. One study which assessed medical costs associated with extended treatment with new oral anti-coagulants (NOACs) vs placebo based on data from clinical trials (RE-SONATE, EINSTEIN-EXT, and AMPLIFY-EXT) found that extended treatment with dabigatran, rivaroxaban, and apixaban was associated with lower medical costs, due to the reduction in VTE recurrenceCitation31. Another study using data from the EINSTEIN-EXT trial and a Markov model over a 40-year time horizon also showed that extended treatment with rivaroxaban was cost-effective relative to placeboCitation32.
This study is subject to a number of limitations. First, since patient level healthcare utilization data were not collected in the EINSTEIN-EXT study, this study estimated the cost differences between treatment groups based on the clinical event rates observed from the trial and the annual incremental healthcare costs of care associated with events identified from the literature. Moreover, unit costs for clinical events were estimated based on a few studies identified during the targeted literature search, while real-world costs for managing clinical events may vary greatly across hospitals/centers. Particularly, due to limited published data, the unit costs for major and clinically relevant non-major bleeding events used in the base-case analysis were based on the incremental annual cost associated with major and minor GI bleeding events only. However, the one-way sensitivity analysis varying unit cost assumptions yielded results consistent with the base-case analysis, suggesting that the study finding of a lower total healthcare cost in rivaroxaban- vs placebo-treated patients is robust across a range of relevant costs. The ±20% range of costs examined was arbitrary, and results may differ over a wider set of costs. Third, it was assumed that healthcare cost prior to the clinical events was the same between the two cohorts. This assumption may not be valid for patients who developed a clinical event earlier vs later in the course of follow-up. Fourth, this study evaluated the impact of extended treatment with rivaroxaban only on direct medical costs, but did not assess the potential impact on indirect costs. It is possible that recurrent VTE prevention reduces productivity loss and improves quality-of-life, which in turn would increase the societal cost savings associated with rivaroxaban. Fifth, although the assumptions on patient non-adherence rate and drug discount on rivaroxaban were somewhat arbitrary, the use of low values for non-adherence rate (10%) and drug discount (15%) provided conservative drug cost estimates for rivaroxaban. Sixth, as the current study relied on the data from the EINSTEIN-EXT trial that followed patients for the intended treatment duration, it is unknown whether rivaroxaban-treated patients would experience an increased risk of recurrent VTE following the discontinuation of rivaroxaban and, thus, would be associated with additional clinical event costs. Future studies should assess the cost impact of continued anticoagulation with rivaroxaban over a longer duration of follow-up. Seventh, as this study focused on rivaroxaban, whether the findings could be applicable to other NOACs remains unclear. Moreover, the “real-world” applicability of this study may be limited, since data used in this study were obtained from the EINSTEIN-EXT trial, in which patients were selected based on stringent inclusion and exclusion criteria, given close continuous care and monitoring, and measured for specific clinical events. Future cost analyses using claims-based data, which contain real-world detailed information on healthcare resource utilization and cost, may be considered. Finally, although unit cost assumptions in this study were based on the US healthcare system, the EINSTEIN-EXT study was conducted in an international setting. It is unknown whether differences in healthcare systems may have affected the results of this study.
Conclusions
Based on data from the EINSTEIN-EXT study, this study conducted a cost comparison analysis and found that extended treatment of rivaroxaban in VTE patients had a lower total healthcare cost when compared to patients on placebo. The reduction in recurrent VTE events in the rivaroxaban cohort led to greater cost savings when compared to the increased costs associated with bleeding complications and treatment. Moreover, recurrent DVT and PE rates had the greatest impact on the total healthcare cost difference between rivaroxaban and placebo, followed by recurrent DVT cost and drug cost. In summary, the EINSTEIN-EXT trial showed the clinical benefit of extended rivaroxaban treatment, and our study using the EINSTEIN-EXT trial event rates suggests an economic advantage of extended rivaroxaban treatment. Future research, such as cost-effectiveness analyses, are warranted to corroborate these findings.
Transparency
Declaration of funding
This research was funded by Janssen Scientific Affairs, LLC, Raritan, NJ, USA.
Declaration of financial/other relationships
PSW has received research funding from BMS/Pfizer; has participated on advisory boards for Bayer Healthcare; has acted as a consultant to Janssen Scientific Affairs, LLC; and has served on a writing committee for Itreas. AWAL is an employee of Bayer Pharma AG. FL, PL, DL, and YX are employees of Groupe d’analyse, Ltée., a consulting company that has received research grants from Janssen Scientific Affairs, LLC. VA, CC, MD, and JS are employees of Janssen Scientific Affairs, LLC. BL and LH are employees of Janssen Research & Development, LLC. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Parts of this study were presented at the QCOR 2017 Scientific Sessions; April 2–3, 2017; Arlington, VA.
References
- Anderson FA, Zayaruzny M, Heit JA, et al. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol 2007;82:777-82
- Grosse SD, Nelson RE, Nyarko KA, et al. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res 2016;137:3-10
- Centers for Disease Control and Prevention. Venous thromboembolism: a public health concern. 2015. Atlanta, GA: CDCP
- Lefebvre P, Laliberté F, Nutescu EA, et al. All-cause and disease-related health care costs associated with recurrent venous thromboembolism. Thromb Haemost 2013;110:1288-97
- Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol 2009;29:298-310
- Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis 2006;21:23-9
- Prandoni P. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996;125:1
- Lin J, Lingohr-Smith M, Kwong WJ. Incremental health care resource utilization and economic burden of venous thromboembolism recurrence from a U.S. Payer perspective. J Manag Care Pharm 2014;20:174-86
- MacDougall DA, Feliu AL, Boccuzzi SJ, et al. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Heal Pharm 2006;63(20Suppl6):S5-15
- Ghate SR, Biskupiak J, Ye X, et al. All-cause and bleeding-related health care costs in warfarin-treated patients with atrial fibrillation. J Manag Care Pharm 2011;17:672-84
- Bamber L, Muston D, McLeod E, et al. Cost-effectiveness analysis of treatment of venous thromboembolism with rivaroxaban compared with combined low molecular weight heparin/vitamin K antagonist. Thromb J 2015;13:20
- XARELTO (rivaroxaban) tablets [prescribing information]. 2015. Titusville, NJ: Janssen Pharmaceuticals, Inc
- Laliberté F, Cloutier M, Nelson WW, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin 2014;30:1317-25
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315-52
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510
- Once-daily oral direct factor Xa inhibitor rivaroxaban in the long-term prevention of recurrent symptomatic venous thromboembolism in patients with symptomatic deep-vein thrombosis or pulmonary embolism. The Einstein-Extension study. ClinicalTrials.gov. 2014. Available at: https://clinicaltrials.gov/show/NCT00439725
- Wells PS, Prins MH, Levitan B, et al. Long-term anticoagulation with rivaroxaban for preventing recurrent VTE: a benefit-risk analysis of EINSTEIN-extension. Chest 2016;150:1059-68
- Truven Health Analytics. Micromedex solution: Red Book online search results. Greenwood Village, CO: Truven Health Analytics; 2016. Available at: http://www.micromedexsolutions.com/
- Bookhart BK, Haskell L, Bamber L, et al. Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. J Med Econ 2014;17:691-5
- Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm 2007;13:475-86
- LaMori JC, Shoheiber O, Mody SH, et al. Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther 2015;37:62-70
- Bullano MF, Willey V, Hauch O, et al. Longitudinal evaluation of health plan cost per venous thromboembolism or bleed event in patients with a prior venous thromboembolism event during hospitalization. J Manag Care Pharm 2005;11:663-73
- Miller JD, Ye X, Lenhart GM, et al. Cost-effectiveness of edoxaban versus rivaroxaban for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) in the US. Clin Outcomes Res 2016;8:215-26
- Casciano JP, Dotiwala Z, Kemp R, et al. Economic burden of recurrent venous thromboembolism: analysis from a U.S. hospital perspective. Am J Heal Pharm 2015;72:291-300
- Piedade A, Paladini L, Tobaruella F, et al. Budget impact model (BIM) of rivaroxaban in comparison with enoxaparin plus warfarin in the treatment of venous thromboembolism (VTE) under the perspective of the private health system. Value Health 2015;18:A383-4
- Kahler ZP, Beam DM, Kline JA. Cost of treating venous thromboembolism with heparin and warfarin versus home treatment with rivaroxaban. Acad Emerg Med 2015;22:796-802
- Lefebvre P, Coleman CI, Bookhart BK, et al. Cost-effectiveness of rivaroxaban compared with enoxaparin plus a vitamin K antagonist for the treatment of venous thromboembolism. J Med Econ 2014;17:52-64
- Heisen M, Treur MJ, Heemstra HE, et al. Cost-effectiveness analysis of rivaroxaban for treatment and secondary prevention of venous thromboembolism in the Netherlands. J Med Econ 2017;20:813-24
- Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510
- Investigators TE. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97
- Amin A, Jing Y, Trocio J, et al. Evaluation of medical costs avoided when new oral anticoagulants are used for extended treatment of venous thromboembolism based on clinical trial results. J Thromb Thrombolysis 2014;40:131-8
- Coleman CI, Limone BL, Bookhart BK, et al. Cost-effectiveness analysis of extended duration anticoagulation with rivaroxaban to prevent recurrent venous thromboembolism. Thromb Res 2014;133:743-9

