?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims: Budesonide with multi-matrix technology (MMX) is an oral corticosteroid, shown to have high topical activity against ulcerative colitis (UC) while maintaining low systemic bioavailability with few adverse events. The aim of this study was to evaluate the cost-effectiveness of budesonide MMX versus commonly used corticosteroids, in the second-line treatment of active mild-to-moderate UC in the Netherlands.
Materials and methods: An eight-state Markov model with an 8 week cycle length captured remission, four distinct therapy stages, hospitalization, possible colectomy and mortality. Remission probability for budesonide MMX was based on the CORE-II study. Population characteristics were derived from the Dutch Inflammatory Bowel Disease South Limburg cohort (n = 598) and included patients with proctitis (39%), left-sided (42%) and extensive disease (19%). Comparators (topical budesonide foam and enema, oral budesonide and prednisolone) were selected based on current Dutch clinical practice. Treatment effects were evaluated by network meta-analysis using a Bayesian framework. Cost-effectiveness analysis was performed over a 5 year time horizon from a societal perspective, with costs, health-state and adverse event utilities derived from published sources. Outcomes were weighted by disease extent distribution and corresponding comparators.
Results: Budesonide MMX was associated with comparable quality-adjusted life year (QALY) gain versus foam and oral formulations (+0.01 QALYs) in the total UC population, whilst being cost-saving (EUR 366 per patient). Probabilistic sensitivity analysis evaluated an 86.6% probability of budesonide MMX being dominant (cost-saving with QALY gain) versus these comparators. Exploratory analysis showed similar findings versus prednisolone.
Limitations: Differing definitions of trial end-points and remission across trials meant indirect comparison was not ideal. However, in the absence of head-to-head clinical data, these comparisons are reasonable alternatives and currently offer the only comparison of second-line UC treatments.
Conclusions: In the present analysis, budesonide MMX was shown to be cost-effective versus comparators in the total UC population, for the second-line treatment of active mild-to-moderate UC in the Netherlands.
Introduction
Ulcerative colitis (UC) is a chronic, ulcer-causing inflammatory bowel disease (IBD) that affects millions of people worldwideCitation1. UC is the most common IBD and affects more than half of all IBD sufferers in the USCitation2. The global incidence and prevalence of the disease is rising, with the highest incidences reported in northern Europe (24.3 cases per 100,000), Canada (19.2 cases per 100,000) and Australia (17.4 cases per 100,000)Citation3–5. Prevalence rates are also highest in Europe, with 505 cases per 100,000 peopleCitation6. Prevalence of the disease in the Netherlands was estimated to be 7.7 per 10,000 people in populations from 1991–2003, while prevalence of IBD in Europe has been estimated at 432 per 100,000 people in 2010Citation7,Citation8. IBD and UC represent a significant healthcare burden, with an annual direct healthcare cost for IBD in Europe estimated to be EUR 4.6–5.6 billionCitation7. Indirect costs for IBD manifest as lack of productivity through unemployment (8% of European patients), sick leave (average of 3–6 weeks annually), and permanent work disability (two-fold increased)Citation7,Citation9. New treatments are therefore crucial to ease the economic burden of UC.
UC is characterized by symptoms confined to the large intestine (colon) and rectum, such as diarrhea with blood or pus and abdominal discomfortCitation10. Symptoms can last for several years, but the duration of outbreaks varies between patients and are often interspersed by periods of remissionCitation10. The disease can present at any age but is typically diagnosed (via colonoscopy) in patients under 30 years of ageCitation10. The goal of therapy is to improve patient quality of life by inducing and maintaining remission from active UCCitation10. Treatment guidelines advise oral or rectal administration of 5-aminosalicylic acids (5-ASAs) for first-line therapy of mild-to-moderate cases, which is associated with remission rates of up to 50%Citation11–13. However, more severe forms of UC require more potent therapy, and second-line treatment with corticosteroids is generally recommendedCitation13,Citation14. Prevalent second-line treatments include prednisolone and budesonide. Although prednisolone is more commonly used and often effective at inducing remission, its adverse event profile and lack of long-term clinical data mean it should not be used for maintenance therapyCitation14,Citation15. Additionally, adverse events associated with prednisolone have been linked with reduced patient adherence and increased healthcare and economic burdenCitation16. Oral budesonide, which utilizes a pH-dependent delivery mechanism, is well tolerated but fails to target the left colon, with release primarily at the distal ileum and proximal colon, making it less suitable for the treatment of UCCitation17,Citation18. Topical corticosteroids, such as budesonide foam and enema formulations, offer improved tolerability but also lack the ability to target the entire colon, and are generally associated with lower remission ratesCitation19,Citation20. Moreover, these therapies are associated with lower patient adherence, owing to their multiple times daily, rectal-only administrationCitation21.
An oral, once daily formulation of budesonide with multi-matrix technology (MMX) was launched in 2013. It is designed to induce remission in patients with active mild-to-moderate UC, with release of budesonide at a controlled rate throughout the colonCitation22,Citation23. The safety and efficacy of budesonide MMX versus placebo had been demonstrated in two randomized controlled trials (RCTs), CORE-I and CORE-II. Budesonide MMX once daily 9 mg formulations were associated with higher remission rates than placebo over 8 weeks of therapy in patients with active mild-to-moderate UC. In addition, budesonide MMX was shown to be well tolerated with few adverse events, in line with reports on other budesonide formulationsCitation24,Citation25. This data is consistent with a role for budesonide MMX as a first-choice second-line treatment for active mild-to-moderate UC ahead of oral prednisoloneCitation18,Citation26.
As budesonide MMX was recently approved for use in the Netherlands, the aim of the present analysis was to evaluate the cost-effectiveness of budesonide MMX versus commonly used corticosteroids for the second-line treatment of active mild-to-moderate UC in the Netherlands.
Methods
Modeling analysis
An eight-state Markov model was developed to reflect Dutch clinical practice, following Dutch guidelines for economic evaluations in healthcare ()Citation27. The model has a cycle length of 8 weeks which is consistent with most steroids’ maximum treatment duration. Remission was assessed at 8 weeks in most of the clinical trials used in the model. There is no current supportive evidence that budesonide MMX is used for a longer or shorter time than 8 weeks in clinical practice, therefore the cycle length seems appropriate to characterize steroid treatment course. The model starts with second-line initiation of corticosteroid treatment for a cohort of patients with active disease (steroid treatment in ). From this health state, patients can go into remission, improve and transfer to 5-ASA maintenance therapy or worsen, meaning further treatments (immunomodulators and infliximab) are required. Disease progression eventually leads to hospitalization and colectomy. Three types of health states were used in the structure: tunnel states meaning that patients cannot stay in the health state more than one cycle (steroid treatment, third- and fourth-line treatment, hospital), semi-absorbing states allowing patients to stay more than one cycle in the same state or transition to another state (remission, 5-ASA maintenance therapy, post-surgery) and absorbing states from which no transition is allowed (death). Consequently, staying in the corticosteroid treatment state for more than one cycle is not allowed as the cycle duration represents a full steroid treatment course. As budesonide MMX is used only in induction and not in maintenance, re-treatment with steroids is allowed after relapse as indicated in (transition between remission and steroid treatment). Treatment duration of immunomodulators in the third line was simulated by using three sub-tunnel states. Model outputs were reported in quality of life benefits (measured in quality-adjusted life years [QALYs]) and cost breakdowns (expressed in 2016 Euros [EUR]).
Figure 1. Markov model of ulcerative colitis treatment progression. Abbreviation. 5-ASA, 5-aminosalicylic acid.
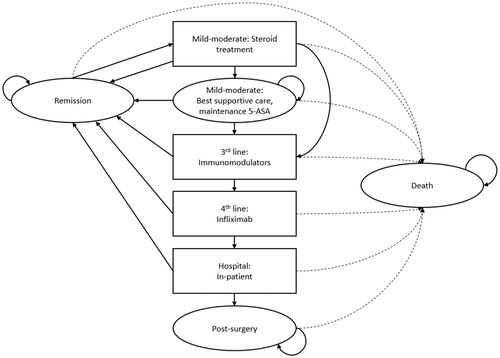
Comparators to budesonide MMX were chosen based on Dutch clinical practice in accordance with standard treatment guidelines for UC in the Netherlands and Europe, as well as guidance laid out by several Dutch physiciansCitation28,Citation29. Budesonide foam was used as the comparator for proctitis and left-sided UC, with oral budesonide as the comparator for extensive UC. Outcomes of QALYs and costs in the comparator arm were weighted according to the distribution of disease extent in the patient population. All treatment effects were defined in terms of remission, shown as the transition probability from any treatment stage to “remission” in the Markov model (), and were derived by network meta-analysis (NMA) (Supplemental Appendix 1).
The NMA was performed using a Bayesian framework, in accordance with the standard Bayesian Markov Chain Monte Carlo methods outlined by NICE technical support documentsCitation30. Eligible studies were defined by inclusion of RCTs and references to remission, with ineligible studies excluded for lack of these features. The proportion achieving remission was defined as a binary outcome and analyses were performed using binomial fixed (FE) and random effects (RE) models. Placebo was used as the reference treatment, as it was ubiquitous in all but one trial. Monte Carlo simulations were fitted using JAGS software, with the Markov chain Monte Carlo estimator being run for 50,000 burn-in simulations and monitored for a further 250,000. Inconsistencies between indirect and direct evidence were checked analytically by node splitting. Probabilities of remission were ranked by combining odds ratios of each regimen (relative to placebo) with the average estimate of the odds of response (with placebo) across studies. The probability of remission at 8 weeks was therefore defined as:
Prednisolone could not be included in the NMA because literature trials were not randomized. Instead, its remission rate was estimated by dividing the reported remission rate from Lennard-Jones et al. (68.42%) by the remission rate of its placebo (16.70%), then multiplying this by the remission rate of the placebo from the CORE-II study (4.5%)Citation25,Citation31. The resulting probabilities of remission at 8 weeks are displayed in .
Table 1. Network meta-analysis results.
Remission probabilities for 5-ASA maintenance therapy (37.2%), third-line azathioprine (53.0%), and fourth-line infliximab (33.9%) were estimated using data from published sourcesCitation32–34. Probabilities for relapse (10.68%), severe disease (1.64%), surgery (7.82%) and death (0.13%) were also based on published dataCitation35–39 (). This data was converted to an 8 week cycle length according to the equations by Fleurence and HollenbeakCitation40. Treatment specific probabilities for adverse events were taken from published sourcesCitation25,Citation41–46.
Table 2. Transition matrix with probabilities shared between interventions.
Costs and quality of life utilities
All costs were expressed in 2016 Euros (EUR). Pharmacy costs were taken from published 2016 prices inclusive of value added tax in the NetherlandsCitation47 (). Drug delivery costs were applied as recommended in the Dutch guidelines for economic evaluation in healthcareCitation48. Treatment regimens were based on those used in published studies for all therapies: budesonide MMX and oral budesonide (CORE-IICitation25), budesonide foam (Sandborn et al.Citation46), budesonide enema (Hanauer et al.Citation43), prednisolone (European ConsensusCitation9), 5-ASA maintenance therapy (Sandborn et al.Citation49), azathioprine/further prednisolone (Ardizzone et al.Citation33) and infliximab (Rutgeerts et al.Citation34).
Table 3. Pharmaceutical unit costs.
Additional healthcare costs were categorized into inpatient care (for cases where surgery was required, covering colectomy and post-surgery recovery) and outpatient care (covering physician visits and diagnostic procedures). Unit costs were estimated based on an economic evaluation of IBD in the Netherlands, and from the costing manual of the Institute for Medical Technology AssessmentCitation48,Citation50 (). The adverse event profiles of budesonide MMX and comparators were conservatively assumed to be similar, despite clinical trial evidence indicating a reduction in associated adverse events with budesonide MMX treatment. Furthermore, no additional costs were included for adverse events associated with budesonide MMX or comparator treatment as it is assumed that no healthcare resource is required for the management of adverse events related to budesonide MMX and comparators according to three Dutch physicians. Costs for adverse effects associated with third- and fourth-line treatments were based on those reported by Blommestein et al., inflated with consumer price indexCitation51,Citation52. Indirect costs were estimated using a human capital approach (workplace absenteeism) and based on a Dutch study on UC patientsCitation36. Based on this source, model inputs for employed patients with UC were absent for 0.21 days of paid work and 0.14 days of unpaid work per 8 week cycleCitation36. Loss of productivity per day was estimated at EUR 207.52 for paid work and EUR 83.58 for unpaid workCitation48.
Table 4. Unit costs of additional healthcare.
Health state utility associated with corticosteroid treatment was estimated to be 0.59 QALYs by Prenzler et al., with health state utility associated with 5-ASA maintenance therapy assumed to be the same ()Citation53. Utilities for subsequent health states (third- and fourth-line treatment, hospitalization, remission, post-surgery and death) were sourced from a publication by Arseneau et al.Citation54. Disutilities for hirsutism, insomnia, acne, fever, nausea, rash and fatigue experienced during third- and fourth-line treatment states were obtained from published studies (Supplemental Appendix 2)Citation55–58. These disutilities were multiplied by 0.75 to give a more conservative estimate when combined. Disutilities for all other adverse events during third- and fourth-line treatment states were assumed to be 0.02 with no adjustment for combinations.
Table 5. Utility inputs.
Patient population
The population comprised patients with mild-to-moderate ulcerative colitis with characteristics based on the Dutch Inflammatory Bowel Disease South Limburg cohort (n = 598), diagnosed between 2006 and 2010Citation37. The population was 49.7% male, with a mean age of 48.2 years and a mean weight of 77 kg. Disease extents at diagnosis were 38.8% proctitis, 41.6% left-sided UC and 19.6% extensive UC.
Time horizon and discounting
The time horizon was set at 5 years, with a cycle length of 8 weeks, matching the duration of treatment for budesonide MMX in induction used in the CORE-I and CORE-II trialsCitation24,Citation25. Discounting was applied at annual rates of 4% and 1.5% for future costs and clinical benefits, respectively, in line with the guideline for economic evaluation in healthcare in the NetherlandsCitation27.
Sensitivity and scenario analyses
One-way, deterministic sensitivity analysis was performed to evaluate the effect of variation of each model input parameter on budesonide MMX cost-effectiveness, including the relative effectiveness of budesonide MMX versus other corticosteroids, transition probabilities, unit costs, resource use and disutilities. Additionally, probabilistic sensitivity analysis with 1000 replications was performed by simultaneously varying these parameters. Comparators for the sensitivity analysis were budesonide foam for extensive and left-sided disease, and oral budesonide for proctitis. For both one-way and probabilistic sensitivity analyses, each model input was varied according to upper and lower estimates (using 95% confidence interval [CI] bounds, or when these are unavailable, ±20% and ±10% of the average value). Distributions associated with each parameter were based on the recommendations by Briggs et al.Citation59.
All clinically plausible combinations of comparators and disease extents were analyzed in scenario analyses to evaluate the impact of using different comparators for varying disease extents relative to the base case. Eight different combinations were evaluated, with budesonide foam and enema used as comparators for proctitis and left-sided UC, oral budesonide for left-sided and extensive UC, and prednisolone for extensive UC only. All possible combinations of these treatments for the specified disease extent were explored.
Results
Base case analysis
Budesonide MMX treatment was associated with a comparable improvement in quality of life versus comparators in a Dutch patient mix, gaining approximately 0.01 QALYs versus commonly used treatments (). Clinical benefits were mainly driven by higher remission rates with budesonide MMX than with other second-line therapies. As treatment effects of corticosteroids were applied for only one cycle, the slight improvement in QALYs result mostly from the difference in 8 week remission probabilities between comparators. Budesonide MMX was associated with mean cost savings of EUR 366 per patient versus comparators. This was primarily due to the lower cost of the drug itself, saving EUR 288 per patient versus comparators. Additional costs originating from 5-ASA maintenance therapy and progression health states (EUR -50), supplementary healthcare (EUR -14) and indirect costs (EUR -14) were all lower with budesonide MMX, but comparable to current treatments ().
Table 6. Budesonide MMX and comparator cost-effectiveness.
Deterministic sensitivity analysis
Budesonide MMX showed comparable improvements to quality of life and remained less costly across all one-way sensitivity analyses (). The largest variations in costs and QALYs were seen with the application of the upper and lower limits (95% CI) for remission probability with budesonide foam, ranging from EUR −424 to EUR −261, respectively. Quality of life benefit with budesonide MMX dropped slightly when the upper limit for remission probability of budesonide foam was applied but remained comparable. Conversely, budesonide MMX was projected to be notably more effective than budesonide foam when applying the lower limit for remission probability for the latter treatment. Applying adverse event costs for corticosteroids only slightly reduced the cost savings associated with budesonide MMX, and it remained dominant versus comparators.
Table 7. Deterministic sensitivity analysis inputs and outcomes.
Probabilistic sensitivity analysis
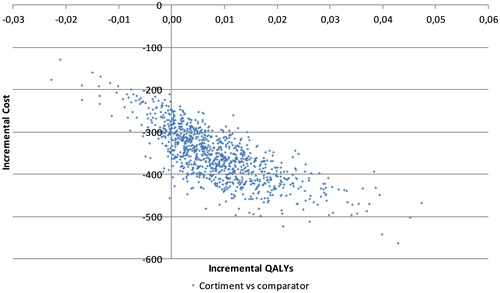
Probabilistic sensitivity analysis showed that budesonide MMX treatment was associated with a comparable mean improvement (+0.01 QALYs, approximately) and mean cost savings of EUR 354 per patient versus comparators. Budesonide MMX was associated with a mean of 3.477 QALYs (2.870–3.939 QALYs with 95% CI) and a mean cost of EUR 6248 (EUR 5075–7680, 95% CI), while comparators were associated with a mean of 3.468 QALYs (2.868–3.935 QALYs, 95% CI) and a mean cost of EUR 6601 (EUR 5364–8012, 95% CI) over the 5 year time horizon. A majority of iterations (86.6%) showed budesonide MMX to be dominant to comparators, with comparable improvements in quality of life but at a lower cost ().
Scenario analyses
Budesonide MMX continued to show comparable, or slightly improved, QALYs at a lower cost versus comparators across various scenario analyses, with only two comparator combinations causing a change in outcomes (). Budesonide MMX was associated with an improvement of 0.015 QALYs but a higher cost of EUR 87 per patient against a combination of budesonide enema for proctitis, oral budesonide for left-sided UC and prednisolone for extensive UC. This translated to an incremental cost-effectiveness ratio (ICER) of EUR 5692 per QALY gained for budesonide MMX versus these comparators. Against a combination of budesonide foam for proctitis, oral budesonide for left-sided UC and prednisolone for extensive UC, budesonide MMX was associated with an improvement of 0.016 QALYs but a higher cost of EUR 47 per patient. The corresponding ICER of budesonide MMX versus these comparators was EUR 2848 per QALY gained.
Table 8. Scenario analysis with alternative comparator combinations.
Discussion
The present modeling analysis evaluated the cost-effectiveness of budesonide MMX for the treatment of UC versus commonly used comparators in the Netherlands. Budesonide MMX was projected to reduce costs from healthcare payer and societal perspectives over a 5 year time horizon, whilst maintaining patients’ quality of life in the majority of analyses. Base case analysis estimated a mean cost saving of EUR 366 per patient with budesonide MMX over comparators, with a comparable improvement in quality-adjusted life years (+0.009 QALYs). Sensitivity analysis of the model design and input parameter values showed robust results in a majority of analyses. Assumptions regarding parameter values for comparator remission rates resulted in a less costly, less effective outcome.
Previous studies have evaluated similar extended-release oral medications for UC. Mesalazine MMX, a 5-ASA utilizing the same multi-matrix release technology, was associated with a higher rate of remission versus placebo with no increase in adverse events, similar to budesonide MMXCitation49,Citation60–62. Taken together with the CORE trials, the data suggests that MMX is very well toleratedCitation24,Citation25. In a UK analysis, mesalazine MMX was shown to be cost-effective versus oral mesalazine from a healthcare payer perspective, with an ICER of EUR 899 per QALY gainedCitation63. In the present analysis, budesonide MMX was comparable in terms of quality of life benefits but less costly versus all comparators (including oral budesonide) in the base case and all but one sensitivity analysis. Economic evaluation has provided evidence of value for MMX technology in both cases. However, differences in setting, comparators and perspective make any comparison between the two studies challenging. In a Polish analysis, budesonide MMX was shown to be dominant versus systemic oral corticosteroids, with cost savings of US$1505.86 and 0.47 QALY gain over a life-time horizonCitation64. The authors considered similar efficacy in terms of remission, but a better safety profile and bioavailability of budesonide MMX compared to systemic corticosteroids. Hence, the favorable safety profile of budesonide MMX helps to avoid severe steroid related adverse events while reducing costs and slightly improving QALYs which is consistent with the present analysis. However, differences in comparators and time horizon make comparison of the QALY gains in the two studies challenging. In a recent cost-minimization analysis submitted to the Scottish Medicines Consortium (SMC), budesonide MMX led to cost saving of £57 versus a weighted average comparator of other budesonide treatments over a time horizon of 1 year, which seems consistent with the present analysisCitation65.
A potential limitation of the present analysis was the paucity of clinical data on prednisolone for the NMA, which forms the basis of the present analysis. Budesonide MMX was shown to have a higher probability of remission (17.4%) than budesonide foam (13.1%), enema (16.1%) and oral formulations (13.1%), but the probability for prednisolone (18.4%) was estimated via a different method, as clinical trials did not fit the NMA criteria (). UC studies also often contain differing definitions of trial end-points, particularly regarding remission classification, which can make comparison of direct and indirect data problematicCitation66. However, in absence of suitable RCTs for prednisolone and head-to-head trials for all comparators, NMA data is a reasonable alternative (being endorsed by NICE in the UK) and currently offers the only comparison of remission probabilities across multiple second-line treatments for UCCitation30. While the NMA did not show a statistically significant difference in remission rates between budesonide MMX and comparators, conducting a cost-effectiveness analysis is still relevant to explore the QALY gains related to the better safety profile of budesonide MMX compared to other corticosteroids.
The shortcomings of current treatments have been widely reported and include multiple daily dosing regimens, rectal administrations and associated adverse events. These can all reduce patient adherence in routine clinical practice, particularly when the disease itself is inactiveCitation16. This increases the risk of relapse and associated economic burden. Budesonide MMX may therefore offer an attractive alternative for patients, with a once daily oral regimen and fewer adverse events, particularly in comparison with prednisolone, the current first-choice second-line medicationCitation14. The present health economic analysis can be considered conservative in that it did not capture the potential benefits of improved tolerability and patient adherence with budesonide MMX relative to other second-line treatmentsCitation16. In routine clinical practice, patients may prefer a therapy like budesonide MMX that offers a reduction in associated adverse events, potentially increasing adherenceCitation14. Incorporating these factors into the model inputs could potentially yield further clinical benefits with budesonide MMX versus comparators. Fewer adverse events associated with corticosteroid treatment for UC would also likely reduce clinical dependence and healthcare burden. However, economic assessment of such hypotheses requires further real-world evidence of budesonide MMX patient adherence versus comparators.
Conclusions
Budesonide MMX was estimated to be cost saving versus other commonly used second-line treatments for active mild-to-moderate UC in the Netherlands, reducing costs from a societal perspective and displaying comparable patient quality of life improvements, while offering reduced adverse events compared with current first-choice second-line treatment prednisolone. Further long-term and head-to-head trials are required to assess its place in the treatment paradigm and potential role as a maintenance therapy, but its current 9 mg formulation appears well suited as an alternative second-line induction therapy for UC in the Dutch setting.
Transparency
Declaration of funding
HEVA-HEOR received funding from Ferring Pharmaceuticals for analyzing the cost-effectiveness of budesonide MMX in the Netherlands, including the production of a technical report. Ossian Health Economics and Communications received funding from HEVA-HEOR for the writing of the present manuscript. Employees from HEVA-HEOR, Ossian Health Economics and Communications, and Ferring Pharmaceuticals were involved in reviewing and revising aspects of this manuscript.
Author contributions: A.G. and S.R. were involved in conception and design of the cost-effectiveness analysis, and drafting a technical report that was used as the basis of the final manuscript. O.G., Y.L.Y.S. and J.K. provided feedback on several drafts of the manuscript.
Declaration of financial/other relationships
A.G. and S.R. have disclosed that they are employees of HEVA-HEOR. O.G. and Y.L.Y.S. have disclosed that they are employees of Ferring Pharmaceuticals. J.K. has disclosed that he has no significant relationships with or financial interests in any commercial companies related to this study or article.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Appendix 2
Download MS Word (172.6 KB)Supplemental Appendix 1
Download MS Word (756.8 KB)Acknowledgements
Audrey Petitjean (HEVA-HEOR) was involved in the review of the technical report of the analysis, on which the manuscript is based. Michel Cucherat (University Lyon I) was involved in conducting the NMA used in the analysis, on which the manuscript is based. Sam Malkin and William Valentine (Ossian Health Economics and Communications) were involved in writing of the present manuscript.
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424-29
- Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol 2006;101:1559-68
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42
- Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut 1996;39:690-7
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785-94
- Burisch J, Jess T, Martinato M, Lakatos PL; on behalf of the European Crohn’s and Colitis Organisation, ECCO. The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7:322-37
- de Groof EJ, Rossen NGM, van Rhijn BD, et al. Burden of disease and increasing prevalence of inflammatory bowel disease in a population-based cohort in the Netherlands. Eur J Gastroenterol Hepatol 2016;28:1065-72
- Wilson B, Lonnfors S, Hommes DW, et al. P406 A European Crohn’s and ulcerative colitis patient life IMPACT survey. J Crohns Colitis 2012;6:S171
- NIDDK: Ulcerative colitis. US: National Institute of Diabetes and Digestive and Kidney Diseases, 2014. Available at: https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis [Last accessed 8 January 2018]
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749-53
- Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pract Gastroenterol Hepatol 2007;4:160-70
- Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2017;11:769-84
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501-23
- Truelove SC, Watkinson G, Draper G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. BMJ 1962;2:1708-11
- Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med 2003;114:39-43
- Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol 2008;14:354-77
- Danese S, Siegel CA, Peyrin-Biroulet L. Review article: integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther 2014;39:1095-103
- Lichtenstein GR, Bengtsson B, Hapten-White L, Rutgeerts P. Oral budesonide for maintenance of remission of Crohn’s disease: a pooled safety analysis. Aliment Pharmacol Ther 2009;29:643-53
- Rutgeerts P, Lofberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn’s disease. N Engl J Med 1994;331:842-5
- Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Aliment Pharmacol Ther 2006;23:577-85
- Uceris (budesonide) extended release tablets, for oral use [package insert]. Raleigh, NC: Salix Pharmaceuticals Inc., 2014
- Brunner M, Ziegler S, Di Stefano AFD, et al. Gastrointestinal transit, release and plasma pharmacokinetics of a new oral budesonide formulation. Br J Clin Pharmacol 2006;61:31-8
- Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 2012;143:1218-26
- Travis SPL, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014;63:433-41
- Dubinsky MC. Reviewing treatments and outcomes in the evolving landscape of ulcerative colitis. Postgrad Med 2017;129:538-53
- Guideline for economic evaluations in healthcare. The Netherlands: Zorginstituut Nederland [Dutch Care Institute], 2017. Available at: https://www.zorginstituutnederland.nl/binaries/content/documents/zinl-www/documenten/publicaties/publications-in-english/2016/1606-guideline-for-economic-evaluations-in-healthcare/Guideline+for+economic+evaluations+in+healthcare.pdf [Last accessed 8 January 2018]
- Travis SPL, Stange EF, Lémann M, et al. European evidence-based consensus on the management of ulcerative colitis: current management. J Crohns Colitis 2008;2:24-62
- Handleiding Behandeling IBD – 2014–2015 [Treatment Manual for IBD]. The Netherlands: Initiatief in Crohn en Colitis en Nederlandse Vereniging van Maag-. Darm- en Leverartsen [Initiative in Crohn’s and Colitis and Dutch Association of Stomach, Gut and Liver Doctors], 2015. Available at: https://www.crohn-colitis.nl/wp-content/uploads/2016/09/Modernisering-IBD-richtlijn-volwassenen-2009.pdf [Last accessed 17 May 2017]
- National Institute for Health and Care Excellence (NICE). NICE Decision Support Unit Technical Support Documents. London: National Institute for Health and Care Excellence (NICE), 2010 – 19 May 2017. Available at: https://www.ncbi.nlm.nih.gov/books/?term=collection-id_nicedsutsd%5BAttribute%5D [Last accessed 19 May 2017]
- Lennard-Jones JE, Longmore AJ, Newell AC, et al. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut 1960;1:217-22
- Lakatos PL, Lakatos L. Once daily 5-aminosalicylic acid for the treatment of ulcerative colitis; are we there yet? Pharmacol Res 2008;58:190-5
- Ardizzone S, Maconi G, Russo A, et al. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut 2006;55:47-53
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462-76
- Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009;44:431-40
- van der Valk ME, Mangen M-JJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut 2014;63:72-9
- Jeuring SFG, Bours PHA, Zeegers MP, et al. Disease outcome of ulcerative colitis in an era of changing treatment strategies: results from the Dutch population-based IBDSL cohort. J Crohns Colitis 2015;9:837-45
- Romberg-Camps M, Kuiper E, Schouten L, et al. Mortality in inflammatory bowel disease in the Netherlands 1991–2002: results of a population-based study: the IBD South-Limburg cohort. Inflamm Bowel Dis 2010;16:1397-410
- Mortality; key figures. The Netherlands: Centraal Bureau voor de Statistiek [Central Bureau of Statistics], 2018. Available at: http://statline.cbs.nl/Statweb/publication/?DM=SLEN&PA=37979ENG&D1=1&D2=0&D3=l&LA=EN&VW=T [Last accessed 8 January 2018]
- Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. PharmacoEconomics 2007;25:3-6
- Rubin DT, Sandborn WJ, Bosworth B, et al. Budesonide foam has a favorable safety profile for inducing remission in mild-to-moderate ulcerative proctitis or proctosigmoiditis. Dig Dis Sci 2015;60:3408-17
- Rhodes JM, Robinson R, Beales I, et al. Clinical trial: oral prednisolone metasulfobenzoate (Predocol) vs. oral prednisolone for active ulcerative colitis. Aliment Pharmacol Ther 2008;27:228-40
- Hanauer SB, Robinson M, Pruitt R, et al. Budesonide enema for the treatment of active, distal ulcerative colitis and proctitis: a dose-ranging study. U.S. Budesonide enema study group. Gastroenterology 1998;115:525-32
- Gross V, Bar-Meir S, Lavy A, et al. Budesonide foam versus budesonide enema in active ulcerative proctitis and proctosigmoiditis. Aliment Pharmacol Ther 2006;23:303-12
- Hartmann F, Stein J, BudMesa-Study Group. Clinical trial: controlled, open, randomized multicenter study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left-sided ulcerative colitis. Aliment Pharmacol Ther 2010;32:368-76
- Sandborn WJ, Bosworth B, Zakko S, et al. Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis. Gastroenterology 2015;148:740-50
- Medicijn kosten [Medication costs]. The Netherlands: Zorginstituut Nederland [Dutch Care Institute], 2017. Available at: https://www.medicijnkosten.nl/ [Last accessed 8 January 2018]
- Bijlage 1 – Kostenhandleiding – Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg [Appendix 1 – Cost Manual – Methodology of cost research and reference prices for economic evaluations in health care]. Erasmus Universiteit Rotterdam: Zorginstituut Nederland [Dutch Care Institute], 2016. Available at: https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg [Last accessed 8 January 2018]
- Sandborn WJ, Kamm MA, Lichtenstein GR, et al. MMX Multi matrix system mesalazine for the induction of remission in patients with mild-to-moderate ulcerative colitis: a combined analysis of two randomized, double-blind, placebo-controlled trials. Aliment Pharmacol Ther 2007;26:205-15
- van der Valk ME, Mangen M-JJ, Severs M, et al. Evolution of costs of inflammatory bowel disease over two years of follow-up. PLoS One 2016;11:e0142481
- Blommestein HM, de Groot S, Aarts MJ, et al. Cost-effectiveness of obinutuzumab for chronic lymphocytic leukaemia in The Netherlands. Leuk Res 2016;50:37-45
- Consumer prices; price index 2015. The Netherlands: Centraal Bureau voor de Statistiek [Central Bureau of Statistics], 2015. Available at: http://statline.cbs.nl/Statweb/publication/?DM=SLEN&PA=83131ENG&D1=0&D2=0&D3=195-206,208-219,221-232,234-245,247-258,260-271,273-276&LA=EN&VW=T [Last accessed 8 January 2018]
- Prenzler A, Yen L, Mittendorf T, von der Schulenburg J-M. Cost effectiveness of ulcerative colitis treatment in Germany: a comparison of two oral formulations of mesalazine. BMC Health Serv Res 2011;5:157
- Arseneau KO, Sultan S, Provenzale DT, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2006;4:1135-42
- Klassen AF, Newton JN, Mallon E. Measuring quality of life in people referred for specialist care of acne: comparing generic and disease-specific measures. J Am Acad Dermatol 2000;43(2):229-33
- Léger D, Morin CM, Uchiyama M, et al. Chronic insomnia, quality-of-life, and utility scores: comparison with good sleepers in a cross-sectional international survey. Sleep Med 2012;13:43-51
- Ekbäck MP, Lindberg M, Benzein E, Årestedt K. Health-related quality of life, depression and anxiety correlate with the degree of hirsutism. Dermatol Basel Switz 2013;227(3):278-84
- Shabaruddin FH, Chen L-C, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. PharmacoEconomics 2013;31:277-88
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Handbooks in Health Economic Evaluation, 2006
- Kamm MA, Lichtenstein GR, Sandborn WJ, et al. Randomised trial of once- or twice-daily MMX mesalazine for maintenance of remission in ulcerative colitis. Gut 2008;57:893-902
- Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 2007;132:66-75
- Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2007;5:95-102
- Brereton N, Bodger K, Kamm MA, et al. A cost-effectiveness analysis of MMX mesalazine compared with mesalazine in the treatment of mild-to-moderate ulcerative colitis from a UK perspective. J Med Econ 2010;13:148-61
- Pruszko C, Jachimowicz M, Kowalczyk M, et al. Economic implications of budesonide MMX advantage in ulcerative colitis treatment over systemic steroids: budesonide MMX decreases ulcerative colitis treatment costs. Poster PGI15. ISPOR 21st Annual International Meeting, Washington, USA, 21–25 May 2016
- Scottish Medicines Consortium. Resubmission. Budesonide 9m prolonged release tablet (Cortiment). SMC No. (1093/15). 9 September 2016. Available at: https://www.scottishmedicines.org.uk/media/1371/budesonide_cortiment_resub_final_sept_2016_for_website.pdf [Last accessed 16 May 2018]
- D’Haens G, Sandborn WJ, Feagam BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132:763-86
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011 (updated April 2012). Available at: http://www.nicedsu.org.uk [Last accessed 16 May 2018]
- Naganuma M, Aoyama N, Suzuki Y, et al. Twice-daily budesonide 2-mg foam induces complete mucosal healing in patients with distal ulcerative colitis. J Crohns Colitis 2016;10:828-36