Abstract
Aim: In active relapsing remitting multiple sclerosis (RRMS) patients requiring second-line treatment, the Dutch National Health Care Institute (ZiN) has not stated a preference for either alemtuzumab, fingolimod, or natalizumab. The aim was to give healthcare decision-makers insight into the differences in cost accumulation over time between alemtuzumab—with a unique, non-continuous treatment schedule—and fingolimod and natalizumab for second-line treatment of active RRMS patients in the Netherlands.
Methods: In line with ZiN’s assessment, a cost-minimization analysis was performed from a Dutch healthcare perspective over a 5-year time horizon. Resource use was derived from hospital protocols and summaries of product characteristics, and validated by two MS specialists. Unit costs were based on national tariffs and guidelines. Robustness of the base case results was verified with multiple sensitivity and scenario analyses.
Results: Alemtuzumab results in cost savings compared to fingolimod and natalizumab from, respectively, 3.3 and 2.8 years since treatment initiation onwards. At 5 years, total discounted costs per patient of alemtuzumab were €79,717, followed by fingolimod with €110,044 and natalizumab with €122,238, resulting in cost savings of €30,327 and €42,522 for alemtuzumab compared to fingolimod and natalizumab, respectively. Key drivers of the model are drug acquisition costs and the proportions of patients that do not require further alemtuzumab treatment after either two, three, or four courses.
Limitations: No treatment discontinuation and associated switching between treatments were incorporated. Consequences of JC virus seropositivity while continuing natalizumab treatment (e.g. additional monitoring) were omitted from the base case.
Conclusion: The current cost-minimization analysis demonstrates that, from the Dutch healthcare perspective, treating active RRMS patients with alemtuzumab results in cost savings compared to second-line alternatives fingolimod and natalizumab from ∼3 years since treatment initiation onwards. After 5 years, alemtuzumab’s cost savings are estimated at €30k compared to fingolimod and €43k compared to natalizumab.
Introduction
Multiple sclerosis (MS) is a chronic neurological disorder that is the leading cause of disability in young adults in developed countriesCitation1. In the Netherlands, MS occurs in one person per 1,000 inhabitants, and is 2.5-times more common in women than in menCitation1. Of the incident MS cases, 80–90% concern relapsing remitting multiple sclerosis (RRMS), which is characterized by episodes of clearly defined attacks of new or increasing neurologic symptoms (relapses), followed by partial or complete recovery (remission)Citation2. In the long-term, MS is associated with a progressive and irreversible disability (secondary progressive MS).
MS has a considerable impact on quality-of-life. Two cross-sectional studies reported that 42% of Dutch MS patients retired early due to MSCitation3,Citation4. In addition, the average loss of quality-adjusted life years (QALY) per year was estimated at 0.24 compared to an age- and gender-matched group of the general populationCitation4. Relapses and relapse severity are associated with worsening quality-of-lifeCitation3,Citation4.
The economic burden of MS is also high. The mean costs per patient per year in the Netherlands, including productivity losses, have been estimated at €30,938, €51,056, and €100,469 (cost year 2011) for MS patients with mild (Expanded Disability Status Scale [EDSS] 0–3), moderate (EDSS 4–6.5), and severe (EDSS 7–9) disability, respectively. A relapse was estimated to incur an additional cost of €8,195 over 12 monthsCitation3.
Disease-modifying therapies (DMTs) aim to reduce the frequency and severity of relapses in RRMS, and subsequently delay (or prevent or even reverse) disease worsening. In the Netherlands, interferon-β, glatiramer acetate, teriflunomide, and dimethyl fumarate are recommended as first-line treatments. Patients with rapidly evolving, severe RRMS, or with a high disease activity, who do not respond well to first-line treatments are eligible for second-line treatment with fingolimod (Gilenya) or natalizumab (Tysabri)Citation2. The novel DMT alemtuzumab (Lemtrada) has been registered and approved for reimbursement in both the first and second lineCitation2,Citation5. This article focuses on second-line treatment with alemtuzumab, fingolimod, or natalizumab for active RRMS in the Netherlands.
The efficacy and safety of alemtuzumab, fingolimod, and natalizumab have been investigated in a number of randomized controlled clinical trialsCitation6–10, vs interferon-β in the case of alemtuzumab and vs placebo in the case of fingolimod and natalizumab. Both the Institute for Clinical and Economic Review (ICER) and the Cochrane Collaboration performed a network meta-analysis comparing available DMTs for RRMS and arrived at similar results, based on the same trials included in these analyses for alemtuzumab, fingolimod, and natalizumab. ICER consequently judged (in 2017) that alemtuzumab and natalizumab have “an A rating—a high certainty of substantial net health benefit” compared to placebo, when weighing benefits and safety risksCitation11. Fingolimod was judged to produce “incremental or better net health benefits (B+)” compared to placeboCitation11. The Cochrane Collaboration concluded (in 2015) that “alemtuzumab, natalizumab and fingolimod are the best choices for preventing clinical relapses in people with RRMS”, based on evidence limited to the first 24 months of treatmentCitation12, and that insufficient evidence was available to evaluate treatments for the prevention of irreversible disability worsening and to obtain comparative safety profilesCitation12. In a network-meta analysis commissioned by the Dutch National Health Care Institute (Zorginstituut Nederland, ZiN), differences in efficacy and safety between these three treatments were considered either not statistically significant or based on low quality evidence (GRADECitation13)Citation5. While acknowledging the differences in mechanisms of action and safety profiles, ZiN did not differentiate between alemtuzumab, fingolimod, and natalizumab, based on therapeutic value in active RRMS patients requiring second-line treatment (2016)Citation5. From this viewpoint, a cost-minimization analysis (CMA) offers a relevant comparison.
In a CMA, pharmacoeconomic consequences of treatments are compared while considering treatment-related costs onlyCitation14. This type of analysis provides physicians, healthcare insurers, budget managers, and other healthcare decision-makers with insight into treatment-related expenditure over time. This insight is novel and of particular relevance regarding the most recently introduced DMT alemtuzumab, as its treatment schedule differs from those of fingolimod and natalizumab. Alemtuzumab is administered intravenously as a 5-day course in the first year and a 3-day course in the second year. In cases of disease activity beyond the second year, additional (3-day) courses may be provided at least 12 months apart. In the alemtuzumab clinical development program, the majority of patients (60Citation15 to 68%Citation16) did not require treatment after the second course. This unique treatment schedule thereby results in an initial investment, followed by a substantial drop in treatment costs after the second course. In contrast, fingolimod and natalizumab are intended as lifelong treatments with, respectively, daily oral and monthly intravenous administrations. Their associated costs are, therefore, rather constant and continuous over time. To provide a comprehensive understanding of the consequences of these differences in expenditure over time, the current CMA evaluates the costs of alemtuzumab compared to fingolimod and natalizumab for second-line treatment of active RRMS in the Netherlands.
Methods
A CMA was performed from the Dutch healthcare perspective, considering direct medical costs in the following categories: (1) drug acquisition costs, (2) administration costs, and (3) monitoring costs (). Costs associated with adverse events are generally considered beyond the scope of a CMA and were, thus, discarded from the base case, but investigated in a scenario analysis. Costs were reported in euros (cost year 2015) and, if necessary, inflated to 2015 price levels using the harmonized consumer price indexCitation17. Costs were discounted by 4% annuallyCitation18.
Table 1. Unit costs and units required for drug acquisition, drug administration, and monitoring.
In the base case analysis, a time horizon of 5 years was adopted to fully capture the alemtuzumab treatment practice that the majority of patients receive: two courses (one in year 1, one in year 2), followed by 48 months of obligatory monitoring after the last infusion (i.e. in years 2, 3, 4, and 5). It was assumed that no patients discontinued treatment, as patients who discontinue treatment most often switch to other treatments, and the assumption of no therapeutic differentiation underpinning this CMA does not apply to treatment sequences. Moreover, discontinuation is, in the strict sense, not possible for patients on alemtuzumab, due to alemtuzumab’s long-terms effects and non-continuous treatment schedule. Implications of this possible discrepancy with real-world practice will be addressed in the discussion section.
As mentioned, alemtuzumab-treated patients receive a minimum of two courses. Further courses can be administered at physician’s discretion, with an advised interval of 12 months between courses. The number of additional alemtuzumab courses that patients require were obtained from the CARE-MS II extension trial, that investigated alemtuzumab as second-line treatment: 28.8% received one additional course, 9.9% received two additional courses, and 1.5% received three additional courses within 5 years after treatment initiationCitation15. In the current analysis, it is assumed that additional alemtuzumab courses are administered without treatment interruption at intervals of 12 months; i.e. 28.8% received an additional course in year 3, 9.9% in years 3 and 4, and 1.5% in years 3, 4, and 5. This is a conservative assumption regarding discounting.
Assumptions regarding resource use were based on the summaries of product characteristics (SmPC)Citation19–21 and hospital protocolsCitation22–24, and were validated by two neurologists who specialize in MS (B.M.J.U. & S.T.F.M.F.). Unit costs were obtained from the drug cost database provided by ZiN (medicijnkosten.nl)Citation25, the Dutch Healthcare Authority DRG tariff databasesCitation26,Citation27, and the Dutch costing manualCitation28. From the latter, the averages of academic and peripheral hospital tariffs were used, assuming an equal distribution of the treated population over academic and peripheral hospitals.
Drug acquisition costs
Alemtuzumab acquisition costs comprise €35,000 (all drug costs excl. VAT) for the first course and €21,000 for the second course, based on five infusions of 12 mg on 5 consecutive days during the first course and three infusions during the secondCitation25. Pre-treatment medication adds another €161 and €101 to the first and second course. Additional 3-day courses beyond year 2 are associated with the same drug acquisition costs as the second course. Annual drug acquisition costs for fingolimod were calculated at €22,487, based on a once-daily oral dose of 0.5 mg, with 28 doses per package; and for natalizumab at €21,450, based on 4-weekly (i.e. 13 per year) intravenous administrations of 300 mgCitation25.
Administration costs
Based on Dutch expert opinion, all courses of alemtuzumab are assumed to be administered during hospital admission. This can be considered a conservative assumption, as with increasing experience courses may (partly) be administered on an outpatient basis. Total administration costs are €2,840 for the 5-day course in the first year and €1,704 for the 3-day course in the second year. Additional 3-day courses beyond year 2 are associated with the same administration costs as the second course ().
Fingolimod is an oral drug and, thus, not associated with administration costs, except for the first administration. Treatment initiation with fingolimod causes a transient (often asymptomatic) decrease in heart rate, which is monitored on the cardiology ward during 6 h after the first fingolimod dose. We applied the tariff of two electrocardiogram evaluations by a cardiologist (€44 eachCitation26) and an outpatient administration (€282Citation28), which includes medical and nursing staff, as well as infusion material costs. Natalizumab is also administered during an outpatient administration.
Monitoring costs
All patients, regardless of whether they are on alemtuzumab, fingolimod, or natalizumab, are monitored using contrast-enhanced magnetic resonance imaging (MRI) scans, laboratory tests, and physician and MS specialist nurse visits before treatment initiation, as well as during treatment. Women of fertile age (assumed at 80% × 71%Citation29) undergo a pregnancy test before treatment initiation. All procedures with their respective costs are listed in . Specific monitoring items for alemtuzumab, fingolimod, and natalizumab will be highlighted below.
Before treatment initiation with alemtuzumab, screening for hepatitis (B and C), human immunodeficiency virus (HIV), cervical human papilloma virus (HPV, only female patients [71%Citation29]), and tuberculosis (TBC) infections are required, as well as a complete blood count including leukocyte differentiation (CBC). Patients are also tested for varicella zoster virus (VZV) antibodies, and, if antibody negative (in ∼10%Citation30), vaccinated against VZV and re-tested for antibodies. While on treatment, renal function (urine screening and serum creatinine) and CBC are monitored monthly, thyroid function is monitored every 3 months, and female patients are screened for cervical HPV yearly (see ). Neurologist visits take place every 3 months. This monitoring protocol applies until 48 months after the last alemtuzumab course. Monitoring after this obligatory period is not recommended in the SmPCCitation21, nor in the EU risk management planCitation31, and has not yet been specified in Dutch MS guidelinesCitation1.
Patients on fingolimod treatment also require a pre-treatment VZV immune status test and, if necessary, a vaccination and re-test. Patients with a history of uveitis or diabetes (2.6% of treatment populationCitation32) need a pre-treatment ophthalmologic evaluation because of an increased risk of macular edema. Moreover, ophthalmologic evaluation after 3 months of treatment is mandatory for all patients treated with fingolimod. Neurologist visits take place every 3 months during the first year of treatment, and every 6 months afterwards. We assumed that skin checks for potential signs of basal cell carcinoma are performed by the neurologist during the regular visits (no additional costs associated)Citation33. Laboratory tests (CBC, liver function; see ) take place in parallel with the neurology visits.
Treatment preparation for natalizumab requires laboratory tests (CBC, liver function, anti-JCV antibody; see ), which are repeated every 6 months. Anti-JC virus (JCV) antibody tests are performed to monitor the risk of developing progressive multifocal leukoencephalopathy (PML). JCV seropositivity leads to either additional monitoring or treatment discontinuation and subsequent switching. As mentioned earlier, treatment discontinuation and subsequent switching are not modeled within the current CMA framework. Therefore, the alternative consequence of JCV seropositivity, namely additional monitoring, is also not taken into account in the base case. Neurologist visits take place every 6 months during the first and later years of treatment, and patients are monitored during the monthly administrations.
Base case, sensitivity, and scenario analysis
In the base case analysis, cumulative costs per patient for drug acquisition, administration, and monitoring were calculated over a 5-year period. Deterministic univariate analyses and probabilistic sensitivity analyses (PSA) were performed to assess the impact of parameter uncertainty on the results. In the univariate analyses, all model parameters carrying uncertainty were varied one by one to the lower and upper bounds of their 95% confidence intervals (CIs). Drug costs and resource use associated with drug administration were not varied, as these do not carry uncertainty. The proportions of patients requiring mandatory tests specified in the SmPCs were not varied (100%), only the frequency of testing. In the multivariate PSA, values were randomly drawn from the parameter distributions. This procedure was repeated 1,000 times to construct the 95% CI around the deterministic point estimates of the model results. Proportions were assumed to be beta-distributed, whereas costs and resource use were modeled using a gamma distribution. If no information about parameter uncertainty (standard error [SE] or confidence interval) was available, the SE was assumed at 20% of the mean.
Although based on therapeutic value, ZiN did not differentiate between alemtuzumab, fingolimod, and natalizumab for second-line treatment of active RRMS patients, it should be acknowledged that the nature of the adverse events associated with these treatments is diverse. To provide insight into the healthcare cost consequences of the respective safety profiles, adverse event costs were included in a scenario analysis. For each treatment, adverse events were included in case of a meaningful impact on adverse event costs, i.e. based on both severity and frequency. The included adverse events and their associated resource use were verified by the co-authoring clinical experts. A detailed overview is provided in the Supplementary materials.
Finally, the average numbers of alemtuzumab courses that would result in equal (discounted) costs vs fingolimod and vs natalizumab over 5 years were determined. In other words, using cost estimates for fingolimod and natalizumab over 5 years, we determined how many alemtuzumab courses per patient would be needed to reach equal costs.
Results
Base case results and sensitivity analyses
Base case results at 5 years are shown in . From a Dutch healthcare perspective, alemtuzumab is the least expensive treatment with total discounted costs per patient of €79,717 (with an average of 2.53 courses per patient), followed by fingolimod with €110,044 and natalizumab with €122,238, resulting in cost savings of €30,327 and €42,522 for alemtuzumab compared to fingolimod and natalizumab, respectively. In general, total costs are driven by drug acquisition costs. Drug costs of alemtuzumab are driven by the proportions of patients requiring additional courses beyond the second year. Drug costs of natalizumab are lower than those of fingolimod, but including administration costs for natalizumab results in natalizumab incurring the highest total costs. Monitoring costs are highest for alemtuzumab, but due to the lower 5-year drug costs compared with the other treatments, total discounted costs are lowest. The 95% CI around the incremental costs of alemtuzumab with respect to the comparators does not contain zero, meaning that the cost savings associated with alemtuzumab are statistically significant.
Table 2. Base case results at 5 years.
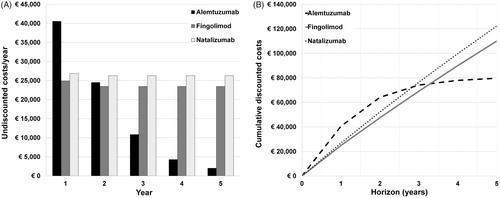
Alemtuzumab treatment costs develop differently over time than those of the other treatments. In the first year of treatment, alemtuzumab is associated with higher costs than fingolimod and natalizumab (€40,559 vs €24,886 and €26,859, see and ). In the second year of treatment, the costs of all treatments are similar (∼€25,000). In later years, the average annual alemtuzumab costs decrease sharply () because of a decreasing number of patients requiring courses, whereas average fingolimod and natalizumab costs remain equal over time. The cumulative discounted costs of alemtuzumab equal those of fingolimod after ∼3.3 years (95% CI = 3.17–3.45) and those of natalizumab after ∼2.8 years (95% CI = 2.55–3.12) (). Beyond 3 years, increases in cumulative alemtuzumab costs are relatively minimal, whereas cumulative fingolimod and natalizumab costs continue to increase by ∼€25,000 per year ().
Figure 1. A. Undiscounted costs per average patient per year for alemtuzumab, fingolimod and natalizumab. Average alemtuzumab costs decrease sharply after 2 years, because of a decreasing number of patients requiring courses. B. Cumulative discounted costs per average patient for alemtuzumab, fingolimod and natalizumab after 1 to 5 years. Alemtuzumab costs intersect with natalizumab costs at approximately 2.8 years, and with fingolimod costs at 3.3 years.
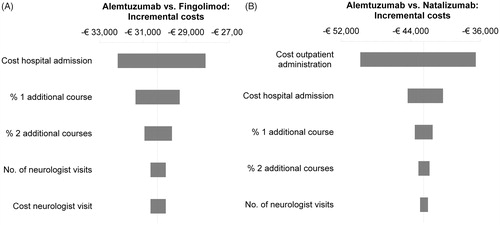
In all univariate sensitivity analyses, alemtuzumab remained cost-saving compared to fingolimod and natalizumab (). In the comparison with fingolimod, incremental savings associated with alemtuzumab are primarily influenced by hospital admission costs. When this cost is varied between the bounds of its 95% CI, the incremental savings associated with alemtuzumab range from €28,104–€32,159. The next most influential parameters are the proportion of patients requiring one additional course of alemtuzumab, the proportion requiring two additional courses of alemtuzumab, and the costs and number of neurologist visits per year. In the comparison with natalizumab, the incremental savings of alemtuzumab range from €36,530–€49,796 when the most-influential parameter “cost outpatient administration” is varied. Other influential parameters are again the cost of hospital admission, the proportion of patients requiring one additional course of alemtuzumab, the proportion requiring two additional courses of alemtuzumab, and the number of neurologist visits.
Figure 2. Overview of the 5-year results of the deterministic univariate sensitivity analyses of alemtuzumab versus fingolimod (A) and natalizumab (B). The bars represent the impact on incremental (discounted) costs when varying the parameters one by one to the lower and upper bounds of their 95% CIs.
Scenario analysis
At a horizon of 5 years, the incorporation of adverse event costs adds €783 to alemtuzumab costs, and €104 and €441 to fingolimod and natalizumab costs, resulting in total discounted costs per patient of €80,500, €110,148, and €122,679, respectively. Adverse events, thus, account for 0.97%, 0.13%, and 0.55% of the total discounted alemtuzumab, fingolimod, and natalizumab costs at 5 years. Additional monitoring of JCV-positive patients who continue natalizumab treatment beyond 2 years would add €742 annually, with a discounted total of €1,979 over years 3, 4, and 5—assuming 59.4% of patients being seropositiveCitation34 and 3-monthly MRIs with contrastCitation35,Citation36.
To further illustrate the magnitude of the cost savings with alemtuzumab, a scenario analysis (break-even analysis) was performed in which it was calculated that, for alemtuzumab to reach fingolimod costs at 5 years, alemtuzumab patients would need to receive 4.0 courses on average. Alemtuzumab and natalizumab would reach equal costs at 5 years if alemtuzumab patients received an average of 4.6 courses, which would mean that all patients receive four courses and 60% receive an additional fifth course.
Discussion
A CMA was performed from a Dutch healthcare perspective over a 5-year time horizon. The aim of this CMA was to compare the costs associated with alemtuzumab treatment to those associated with the second-line treatment alternatives fingolimod and natalizumab, for patients with active RRMS in the Netherlands. From the Dutch healthcare perspective, alemtuzumab was found to be cost-saving compared to fingolimod and natalizumab from, respectively, 3.3 and 2.8 years onwards. After 5 years, cost savings per average patient totaled €30,327 (95% CI = €27,536–€32,579) vs fingolimod and €42,522 (95% CI = €36,186–€50,283) vs natalizumab. The key driver of these cost savings is the cumulative difference in drug costs, which in turn is driven by the number of alemtuzumab courses patients require within 5 years. Additional alemtuzumab courses, i.e. courses administered after the initial two courses, accounted for 16% of total alemtuzumab drug costs. Sensitivity and scenario analyses did not alter the conclusion of alemtuzumab, resulting in cost savings within a period of 5 years compared to treatment with fingolimod and natalizumab. Hence, the results are considered robust.
The results must be interpreted within the framework of the current analysis. The premise underpinning this cost-minimization analysis was no therapeutic differentiation between alemtuzumab, fingolimod, and natalizumab in active RRMS patients requiring second-line treatment, according to the assessment of ZiNCitation5. Based on this specific assessment, and in view of inconclusive long-term comparative evidence, efficacy and safety were not modeled in the base case, nor were the costs associated with relapses and disability and associated quality-of-life. The results do, thus, not present a comprehensive overview of all costs associated with the management of active RRMS in the second-line, neither are the results applicable to a first-line comparison, since fingolimod and natalizumab are not recommended for the first-line treatment of active RRMS in the Netherlands.
To place the current research into an international context, we looked at assessments by the Swedish, British, and Canadian Health Technology Assessment (HTA) bodies that, like ZiN, place emphasis on health-economic analyses:
The Swedish Dental and Pharmaceutical Benefits Agency (TLV) assessed that alemtuzumab and natalizumab have an equivalent effect on disease worsening and relapse rate, but have different safety profiles and discontinuation rates. TLV assessed that alemtuzumab and fingolimod have a similar effect on disease worsening, but that fingolimod is somewhat less effective on the annual relapse rateCitation37.
NICE considered alemtuzumab at least as effective as fingolimod and natalizumab for people with highly active relapsing-remitting MS, despite beta interferon treatmentCitation38.
The Canadian Agency for Drugs and Technology in Health (CADTH) did not compare alemtuzumab with fingolimod or natalizumab in their final recommendationCitation39.
The standpoints taken by the HTA bodies indicate that there is no broad consensus regarding differentiation in therapeutic value between alemtuzumab, fingolimod, and natalizumab, which also showed from the differing conclusions drawn by ICER and the Cochrane Collaboration mentioned earlier. Nonetheless, our conclusion of alemtuzumab being the least expensive treatment, followed by fingolimod and natalizumab, is in alignment with the assessments by the other HTA bodies and ICER:
In the TLV analysis, alemtuzumab dominated natalizumab, as it resulted in cost savings of 520,000 SEK and 0.13 more QALYs over a lifetimeCitation37. In an additional analysis, fingolimod was less expensive than natalizumab, but more expensive than alemtuzumab; with comparative effectiveness not being assessed.
The evidence review group, commissioned by NICE, conducted a cost-effectiveness analysis of alemtuzumab compared with fingolimod for patients with highly active relapsing-remitting multiple sclerosis, despite beta interferon treatment. In the base case analysis, alemtuzumab dominated fingolimod, but NICE concluded that, over a lifetime, the most plausible ICER was £8,900 per QALY gained for alemtuzumab compared with fingolimod, with effect waning of 50% after 5 yearsCitation38.
In ICER’s analysis of first-line treatments, alemtuzumab dominated over natalizumab and fingolimod from a US healthcare perspectiveCitation11.
A further consideration regarding the current analysis framework is that our findings relate to hypothetical cohorts of patients remaining on their originally assigned treatment. Treatment discontinuation and switches between treatments were not taken into account as a consequence of the assumption of no therapeutic differentiation, as well as scarcity of long-term real-world evidence. For patients treated with natalizumab, it is well known that real-world discontinuation and subsequent treatment switching within 5 years is substantial due to patients becoming JCV seropositiveCitation40,Citation41; because JCV seropositivity increases the risk of acquiring PML. Incorporation of additional costs associated with treatment switching—as (re)initiation is more expensive than continuous use—would have increased the cost estimates mainly for patients starting on natalizumab treatment. Moreover, JCV seropositive patients who continue natalizumab treatment require more intense monitoring, with four MRI scans per year (€416 per MRI26). Omitting the consequences of JCV seropositivity, i.e. both treatment switching and increased monitoring, is a study limitation, but does not change the conclusion of natalizumab being the most expensive treatment at 5 years, as it under-estimates the cost associated with natalizumab. Lastly, we adopted a base case horizon of 5 years to capture the most common course of alemtuzumab treatment, i.e. two treatment courses followed by 48 months of monitoring. Although a longer horizon would have better reflected the chronic nature of MS, no extrapolation was done beyond the follow-up duration of the clinical trials.
As the most recent Dutch guidelines for the treatment of RRMS were published in 2012 and alemtuzumab treatment has only been available since 2014, uncertainty exists around the associated resource use in Dutch clinical practice. We adopted a realistic base case that reflects current alemtuzumab treatment practice, validated by two clinical experts who are authors on this paper (B.M.J.U. & S.T.F.M.F.). Therefore, it is unlikely that resource use associated with alemtuzumab was under-estimated in our model. In addition, the univariate and scenario analyses showed that uncertainty regarding resource use has limited impact on incremental costs. Uncertainty that does have an impact exists around the cost of hospital admission and the proportions of alemtuzumab patients requiring one, two, or three additional courses of alemtuzumab after the first two courses. The cost of a hospital admission (€556) may appear low compared to the cost of an outpatient administration (€282). However, both costs were sourced from the Dutch costing manualCitation28 and were, therefore, considered consistent. Moreover, the applied hospital admission cost corresponds well to the average price of a hospital day, based on the DRG of a 5-day admission (€2.875Citation42/5 = €575). The proportions of patients requiring additional alemtuzumab courses could differ in real-world clinical practice from those observed in a trial setting. It could, therefore, be considered a limitation that we obtained these proportions from the CARE-MS II extension trial. However, given the current limited experience with alemtuzumab (European market approval in 2014), real-world publications concern either small studiesCitation43, an insufficient follow-up duration to observe additional coursesCitation44–46, or a different population than the one relevant for the current analysisCitation47. Despite being observed in a different patient population (off-label use in patients with aggressive RRMS), a real-world study in the UK reported similar proportions to those recorded in the CARE-MS II extension trial: 28% of patients received one additional alemtuzumab course (28.8% in CARE-MS-II), 11% received two additional courses (9.9%), and 1% received three additional courses (1.5%)Citation47. Larger and, in terms of patient population, potentially more applicable real-world studies are currently ongoingCitation46,Citation48. With the break-even analysis we have shown that only if these studies would report a much higher need for additional courses (i.e. ≥4 courses per patient compared to an average of 2.53 courses in the CARE-MS II study), our conclusion of cost savings after 5 years would be invalidated.
This study informs healthcare decision-makers about healthcare-related cost accumulation over time associated with treatment of active RRMS patients with alemtuzumab, fingolimod, and natalizumab in the Netherlands. Decision-makers potentially also consider that, within the Dutch reimbursement system, fingolimod, as an oral drug, is reimbursed via the drug reimbursement system (geneesmiddelenvergoedingssysteem), leading to direct compensation of pharmacies by healthcare insurers. Alemtuzumab and natalizumab are administered in-hospital and paid via the hospital budget. As hospital budgets are increasingly under pressure, hospitals face a challenge when prescribing expensive intramural drugs. This leads to a situation where the optimal healthcare decision from a hospital perspective may not correspond to that from a healthcare perspective. Our study indicates that, from a healthcare perspective and under the premise of no therapeutic differentiation, an initial investment in alemtuzumab will lead to cost savings compared with fingolimod and natalizumab from ∼3 years onwards.
Conclusion
A CMA was performed to evaluate the costs of alemtuzumab compared to fingolimod and natalizumab for second-line treatment of active RRMS in the Netherlands. The analysis was conducted under the premise of no therapeutic differentiation as underpinned by the opinion of ZiN. From a Dutch healthcare cost perspective, alemtuzumab was shown to result in cost savings compared to fingolimod and natalizumab from, respectively, 3.3 and 2.8 years since treatment initiation onwards. After 5 years, alemtuzumab cost savings totaled €30,327 compared to fingolimod and €42,522 compared to natalizumab, and thereby proved to be a cost-saving alternative.
Transparency
Declaration of funding
This study was sponsored by Sanofi Genzyme Netherlands, Naarden, The Netherlands.
Declaration of financial/other relationships
LWW and JvW are employed by Sanofi Genzyme Netherlands, Naarden, The Netherlands. MAP and MH are employed by Pharmerit International, a consulting company which provides services to Sanofi Genzyme Netherlands, amongst others. STFMF has received consulting and lecture fees from Abbott, Bayer Schering, Biogen Idec, GlaxoSmithKline, Merck Serono, Novartis, Roche, Sanofi Genzyme, TEVA Aventis, and UCB Pharma. BMJU has received research support and personal compensation for consulting from Biogen Idec, Sanofi Genzyme, Merck Serono, Novartis, Roche, and TEVA. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MAP, MH, LWW, and JvW were involved in the conception and design of the study; MAP and MH conducted all analyses; STFMF and BMJU validated the data inputs and provided suggestions for refinement; MAP and MH drafted the paper; all authors reviewed the paper for intellectual content, gave their final approval of the version to be published, and agree to be accountable for all aspects of the work.
Supplemental Materials
Download MS Word (40 KB)Acknowledgements
None reported.
References
- Nederlandse Vereniging voor Neurologie. Richtlijn Multiple Sclerose 2012, Nederlandse Vereniging voor Neurologie, Utrecht, The Netherlands. Editor: Bohn Stafleu van Loghum, Houten, The Netherlands.
- Zorginstituut Nederland. Middelen bij multipele sclerose. Farmacotherapeutisch kompas. Available from: https://www.farmacotherapeutischkompas.nl/inleidendeteksten/i/inlmiddelenbijmultipelesclerose.asp Zorginstituut Nederlands, Diemen, The Netherlands. [Last accessed 25 November 2015]
- Karampampa K, Gustavsson A, van Munster ETL, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis study: the costs and utilities of MS patients in The Netherlands. J Med Econ 2013;16:939-50
- Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life in multiple sclerosis in The Netherlands. Eur J Health Econ 2006;7(Suppl 2):S55-S64
- Zorginstituut Nederland. Alemtuzumab (Lemtrada) bij actieve relapsing remitting multiple sclerose (RRMS). Zorginstituut Nederland, Diemen, The Netherlands; 2016. Accessible through: https://www.zorginstituutnederland.nl/publicaties/rapport/2016/03/24/alemtuzumab-lemtrada-bij-actieve-relapsing-remitting-multiple-sclerose-rrms
- Kappos L, Radue E, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387-401
- Polman C, O’Connor P, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899-910
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829-39
- Cohen J, Coles A, Arnold D, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819-28
- Institute for Clinical and Economic Review (ICER). Disease-modifying therapies for relapsing-remitting and primary-progressive multiple sclerosis: effectiveness and value. ICER, Boston, Massachusetts, United States. Available through: https://icer-review.org/wp-content/uploads/2016/08/CTAF_MS_Final_Report_030617.pdf [Last accessed March 28, 2018]
- Tramacere I, Del Giovane C, Salanti G, et al. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 2015; Issue 9
- GRADE Working Group. Grading of recommendations assessment, development and evaluation. Available from: http://www.gradeworkinggroup.org/ [Accessed: 28 March 2018]
- Drummond M, Sculpher M, Torrance G, et al. Methods for the economic evaluation of health care programmes. 3rd edn. New York: Oxford University Press; 2005
- Fox EJ, Arnold DL, Cohen JA, et al. Durable efficacy of alemtuzumab on clinical outcomes over 5 years in CARE-MS II with most patients free from treatment for 4 years. Eur Congr Treat Res Mult Scler 2015:P1102
- Havrdova E, Arnold D, Cohen J, et al. Durable efficacy of alemtuzumab on clinical outcomes over 5 years in treatment-naive patients with active relapsing-remitting multiple sclerosis with most patients not receiving treatment for 4 year: CARE-MS I extension study. Abstract published in: Multiple Sclerosis Journal, Volume 21 Issue 11 Supplement, September 2015.
- Organisation for Economic Co-operation and Development (OECD). Harmonized consumer price index. Available from: http://stats.oecd.org/. OACD, Paris, France. [Last accessed 29 September 2015]
- Zorginstituut Nederland. Richtlijn Voor Het Uitvoeren van Economische Evaluaties in de Gezondheidszorg. Zorginstituut Nederland, Diemen, The Netherlands; 2015
- Novartis Europharm Limited. Summary of product characteristics Gilenya. Novartis, Horsham, UK; 2011
- Elan Pharma International Ltd. Summary of product characteristics Tysabri. Elan Pharma International Ltd, Athlone, Ireland; 2006.
- Genzyme Therapeutics Ltd. Summary of product characteristics Lemtrada. Genzyme Therapeutics Ltd, Oxford. UK; 2014
- Hospital protocol 1. Hospital Protocol: Natalizumab (Tysabri); 2015
- Hospital protocol 2. Hospital Protocol: Fingolimod (Gilenya); 2015
- Hospital protocol 3. Hospital Protocol: Alemtuzumab (Lemtrada); 2015
- Zorginstituut Nederland. Medicijnkosten. Available from: https://www.medicijnkosten.nl/ Zorginstituut Nederland, Diemen, The Netherlands. [Last accessed 13 October 2015]
- Nederlandse Zorgautoriteit (NZa). DBC zorgproducten tariefapplicatie. Available from: http://dbc-zorgproducten-tarieven.nza.nl/nzaZpTarief/ZoekfunctieDot.aspx NZa, Utrecht, The Netherlands. [Last accessed 13 October 2015]
- Nederlandse Zorgautoriteit (NZa). Open DIS Data. Available from: http://www.opendisdata.nl/zorgproduct/ NZa, Utrecht, The Netherlands. [Last accessed 15 March 2016]
- Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, et al. Kostenhandleiding: Methodologie van Kostenonderzoek En Referentieprijzen Voor Economische Evaluaties in de Gezondheidszorg. Institute for Medical Technology Assessment, Erasmus University Rotterdam, Rotterdam, The Netherlands; 2015
- Nationaal Kompas Volksgezondheid RIVM. Cijfers multiple sclerose 2007 (prevalentie, incidentie en sterfte naar leeftijd en geslacht). RIVM, Bilthoven, The Netherlands, 2017. Available from: http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/achtergrondcijfers-bij-rangordetabellen/ [Accessed 28 September 2015]
- Donker G, van der Haar E. Waterpokken: vaccinatie invoeren of niet? Huisarts Wet 2009;52:165
- European Medicines Agency (EMA)/Committee for Medicinal Products for Human Use (CHMP). European Public Assessment Report (EPAR)- Alemtuzumab. EMA/CHMP; London, UK; 2013
- De Smet MD, Taylor SRJ, Bodaghi B, et al. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res 2011;30:452-70
- European Medicines Agency (EMA). New recommendations to minimise risks of the rare brain infection PML and a type of skin cancer with Gilenya. EMA, London, UK; 2015. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2015/12/news_detail_002447.jsp&mid=WC0b01ac058004d5c1 [Last accessed 14 November 2017]
- Borchardt J, Berger J. Re-evaluating the incidence of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler Relat Disord 2016;8:145-50
- VUmc MS Centrum Amsterdam. Natalizumab (Tysabri®). VUmc, Amsterdam, The Netherlands; 2015 Available from: https://www.vumc.nl/afdelingen/patientenfolders-brochures/zoeken-alfabet/T/tysabri_natalizumab.pdf [Last accessed 11 January 2018]
- Wattjes MP, Wijburg MT, Vennegoor A, et al. MRI characteristics of early PML-IRIS after natalizumab treatment in patients with MS. J Neurol Neurosurg Psychiatry 2016;87:879-84
- Tandvårds- och läkemedelsförmånsverket. Lemtrada (Alemtuzumab): Hälsoekonomiskt Kunskapsunderlag. Tandvårds- och läkemedelsförmånsverket (TLV) , Stockholm, Sweden; 2014
- National Institute of Health and Care Excellence. TA312: Alemtuzumab for treating relapsing remitting multiple sclerosis. NICE, London, UK; 2014. Available from: https://www.nice.org.uk/guidance/ta312/history [Last accessed 23 November 2017].
- Canadian Agency for Drugs and Technologies in Health. CDEC final recommendation: Alemtuzumab. CADTH, Ottawa, Canada; 2015. Available from: https://www.cadth.ca/sites/default/files/cdr/complete/cdr-complete-SR0405-Lemtrada-June-22-2015.pdf [Last accessed 23 November 2017]
- Van Rossum J, Vennegoor A, Killestein J. The effect of prolonged natalizumab treatment on anxiety and safety in JC virus-seropositive MS patients: a follow-up study. Mult Scler 2014;20:1668-9
- Alroughani R. High discontinuation rate of natalizumab in JC virus seropositive patients due to significant anxiety and safety feelings. Mult Scler 2014;20:1667-8
- Nederlandse Zorgautoriteit (NZa). Ziekenhuisopname met maximaal 5 verpleegdagen tijdens een eerste behandeltraject bij multipele sclerose in 2015. Zorgproduct 069599018. Open DIS Data. NZa; Utrecht, The Netherlands; 2016. Available from: www.opendisdata.nl [Last accessed 20 January 2016]
- Fortin J, Jacques F, Siktai Fokko V, et al. Switching from natalizumab to alemtuzumab in patients with RRMS: real-world experience. In: European Committee for Treatment and Research in Multiple Sclerosis; ECTRIMS Online Library. 14 September, 2016:EP1498
- LaGanke C, Adcock A. Clinical outcomes of 200 multiple sclerosis patients switching from natalizumab to alemtuzumab in a single United States MS center (P3.117). Neurology 2016;86(16 Suppl) http://n.neurology.org/content/86/16_Supplement/P3.117
- Ayrinac X. Efficacy and safety of alemtuzumab in 150 patients with active relapsing-remitting MS: two-year follow-up in France. In: European Committee for Treatment and Research in Multiple Sclerosis; 2017:P1149. ECTRIMS Online Library. 27 October, 2017; 200804 (https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/200804/xavier.ayrignac.efficacy.and.safety.of.alemtuzumab.in.150.patients.with.active.html?f=media=1)
- Thomas K. Observational study to evaluate real-world effectiveness in multiple sclerosis patients treated with alemtuzumab in Germany: TREAT-MS study preliminary results. In: European Committee for Treatment and Research in Multiple Sclerosis 2. 2017;EP1680. ECTRIMS Online Library, 25 October, 2017; 199700 (http://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/199700/katja.thomas.observational.study.to.evaluate.real-world.effectiveness.in.html)
- Willis M, Harding K, Pickersgill T, et al. Alemtuzumab for multiple sclerosis: long term follow-up in a multi-centre cohort. Mult Scler J 2016;22:1215-23
- Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017;16:271-81
