Abstract
Aims: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). This analysis investigated the cost-effectiveness of the second-line treatment with tofacitinib, compared with adalimumab, both plus methotrexate (MTX), in patients with moderate-to-severe RA and an inadequate response to the first-line MTX, from a Taiwan National Health Insurance Administration perspective.
Materials and methods: A patient-level simulation model was used to project lifetime costs and quality-adjusted life-years (QALYs). Base-case analysis compared second-line treatment with tofacitinib 5 mg twice daily plus MTX vs adalimumab 40 mg every 2 weeks plus MTX. Patients switched or discontinued treatment due to a lack or loss of effectiveness or a serious adverse event. Efficacy was measured by change in Health Assessment Questionnaire-Disability Index (HAQ-DI) score. HAQ-DI scores were used to predict mortality and resource utilization, and were mapped onto utility values to estimate QALYs. Efficacy and safety data were derived from clinical trials and other secondary sources. Uncertainty in model parameters was explored using one-way deterministic and probabilistic sensitivity analyses.
Results: Patients gained 0.09 more QALYs with second-line tofacitinib plus MTX compared with adalimumab plus MTX (5.13 vs 5.04, respectively) at an additional cost of New Taiwan Dollars (NT$) 12,881. The incremental cost-effectiveness ratio was NT$143,122/QALY. One-way sensitivity analysis confirmed the base-case result was robust.
Limitations: The lack of available clinical data, particularly for HAQ-DI scores, may introduce some bias in the analysis. No patients were in an early stage of RA, which may limit the generalizability of these results. Base-case results from our study are not necessarily generalizable to countries with healthcare systems that differ considerably from Taiwan.
Conclusions: From a payer perspective, second-line treatment with tofacitinib plus MTX is a cost-effective treatment strategy, compared with adalimumab plus MTX, in patients with moderate-to-severe RA in Taiwan.
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease requiring life-long treatment. It is characterized by infiltration of macrophages and T-cells into joints, synovial hyperplasia, cartilage degradation, and bone erosions, leading to functional decline and disabilityCitation1. In 2010, RA was estimated to affect ∼0.24% of the population worldwideCitation2, although the incidence and prevalence vary by regionCitation3. In Taiwan, RA is the most common autoimmune rheumatic diseaseCitation4, with an estimated prevalence of 0.05–0.1%Citation4,Citation5. Data from epidemiologic studies have shown that the prevalence of RA in Taiwan has increased over timeCitation5,Citation6, highlighting a growing disease burden.
Due to its chronic and debilitating nature, RA is associated with reduced quality-of-life (QoL)Citation7 and premature mortalityCitation6, resulting in a substantial burden in terms of cost and lost productivityCitation8,Citation9. In Taiwan, estimated healthcare costs for the RA population were New Taiwan Dollars (NT$) 6.8 billion (including NT$2.6 billion direct costs and NT$4.2 billion indirect costs), based on data from the 2011 Taiwan National Health Insurance Research Database (NHIRD)Citation10.
The aim of RA treatment is to maintain physical functioning and QoL by achieving sustained remission, or low disease activity when remission is not possibleCitation11. Disease-modifying anti-rheumatic drugs (DMARDs) form the cornerstone of treatment for RA in Taiwan. Conventional synthetic DMARDs (csDMARDs), such as methotrexate (MTX), either as monotherapy or as combination therapy, are recommended as first-line therapy for RACitation11. If treatment efficacy is not sustained with these regimens, patients may initiate treatment with biological DMARDs (bDMARDs), in combination with a stable dose of MTX (i.e. 7.5–15 mg weekly), in accordance with the Asia Pacific League of Associations for Rheumatology (APLAR) RA treatment recommendationsCitation11. In Taiwan, bDMARDs that are currently approved for use in patients with RA include the tumor necrosis factor inhibitors (TNFi) adalimumab, etanercept (both approved in 2002), and golimumab (approved in 2012), the B-cell depleting anti-CD20 antibody rituximab (approved in 2011), the interleukin-6 receptor antibody tocilizumab, and the T-cell co-stimulatory modulator abatacept (both approved in 2012)Citation12. The TNFi certolizumab pegol was approved in 2016 and was, therefore, not available in Taiwan at the time of this analysis. Of these, adalimumab and etanercept are the most commonly prescribed bDMARDs for second-line treatment (i.e. after failure of csDMARDs) in TaiwanCitation12. Although the introduction of bDMARDs has substantially improved outcomes for many patients with RA, there remains an unmet need for new agents that allow a greater proportion of patients to reach treatment goals and produce longer-lasting responses than currently available therapies, and that are easily administered and cost-effectiveCitation13,Citation14.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The efficacy and safety of tofacitinib 5 and 10 mg twice daily (BID), as monotherapy or in combination with csDMARDs, in patients with moderate-to-severe RA, have been demonstrated in global Phase 2Citation15–19, Phase 3Citation20–25, and Phase 3b/4Citation26 trials of up to 24 months in duration, and in long-term extension studies of up to 114 months of observationCitation27. In Taiwan, tofacitinib is approved for the treatment of adult patients with moderate-to-severe active RA who have had an inadequate response or are intolerant to MTX. Tofacitinib may be used as monotherapy or in combination with csDMARDs, including MTX, at a recommended dose of 5 mg BID.
To date, several economic analyses have demonstrated the cost-effectiveness of tofacitinib in RACitation28–33. Findings from an analysis of a South Korean patient population with moderate-to-severe RA and an inadequate response to csDMARDs suggested that incorporating tofacitinib into a treatment sequence was a cost-effective alternative to the current standard of care from a societal perspectiveCitation28. Modeling costs and outcomes after failure of MTX for the treatment of moderate-to-severe RA in the US demonstrated that second-line tofacitinib was a cost-effective alternative vs comparative therapy without tofacitinibCitation31. In Taiwan, new treatments must undergo a Health Technology Assessment, which evaluates comparative clinical effectiveness, cost-effectiveness, and budgetary impactCitation34. To our knowledge, there are no published reports of the economic evaluation of tofacitinib in its approved indication for RA in the Taiwanese population. Previous clinical trials of tofacitinib have included adalimumab as an active comparatorCitation24,Citation26. The objective of this analysis was to determine the cost-effectiveness and cost-utility of introducing tofacitinib plus MTX, compared with adalimumab plus MTX, as a second-line treatment (i.e. after csDMARDs) for adult patients with moderate-to-severe RA and an inadequate response to csDMARDs in Taiwan, from a National Health Insurance Administration (NHIA) perspective.
Methods
Model overview
A patient-level simulation cost-effectiveness model, designed in MS Excel, was used to estimate the outcomes of patients with moderate-to-severe RA who received either tofacitinib or adalimumab and had previously failed first-line MTX. A large cohort (100,000) of individual patients and their disease progression over time were simulated to determine economic and QoL outcomes, which provided an estimation of the incremental cost per life-year (LY) and quality-adjusted life-year (QALY; derived from differences in efficacy, and incidence of adverse events [AEs], and serious AEs [SAEs]) of second-line treatment with tofacitinib vs adalimumab, both in combination with MTX, over a lifetime analysis time horizon (remaining lifetime, i.e. based on mean age at MTX failure until death). The perspective of this analysis focused on direct medical costs (which are not considered societal costs for the purposes of this analysis), and did not include societal costs or any other indirect costs.
Patient population
The target patient population comprised adult patients (aged ≥ 18 years) with moderate-to-severe RA and an inadequate response or intolerance to previous therapy with csDMARDs. Simulated baseline characteristics for individual patients entering the model were based on data from the randomized, double-blind, Phase 3 ORAL Standard tofacitinib clinical trial (ClinicalTrials.gov number, NCT00853385)Citation24 and the Taiwan NHIRD (2007–2011). Patients were identified primarily using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM)Citation35. In addition, availability of a Registry of Catastrophic Illness Database of the NHIRDCitation36, which includes RA certified by two rheumatologists, ensured that the classification of RA (ICD-9-CM code 714.0) was in accordance with the American College of Rheumatology (ACR) 1987 revised criteriaCitation37.
Patient sex, age, Health Assessment Questionnaire-Disability Index (HAQ-DI) score, and disease duration at baseline (entry into the model) were accounted for within the model (). The mean patient age was 52.9 years (range = 18–83)Citation24, and the mean HAQ-DI score was 1.5 (range = 0–3)Citation24. The percentage of male patients (22%) and the mean duration of RA (5.6 years; standard error [SE] = 2.73) at MTX failure were obtained from the NHIRD dataset in order to be representative of the real-world situation in Taiwan.
Table 1. Key model parameters, assumptions, and cost variables.
Comparators
The base-case analysis compared second-line treatment with oral tofacitinib 5 mg BID plus MTX vs adalimumab 40 mg once every 2 weeks plus MTX in patients with moderate-to-severe RA who had an inadequate response to first-line treatment with MTX (). MTX dosage in local practice ranges from 7.5–15 mg in accordance with the APLAR RA treatment recommendationsCitation11; MTX dosage used in the model was 10 mg/week. Adalimumab was selected as the comparator, as it is a commonly prescribed bDMARD for the second-line treatment of this patient population in TaiwanCitation12.
Figure 1. Model structure showing (a) the treatment sequence for the base-case scenario, (b) patient progress in the model, and (c) the mechanism for estimating costs, mortality, and quality-of-life based on disease severity, measured by the HAQ-DI. Abbreviations. BID, twice daily; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; Q2W, every 2 weeks; SAE; serious adverse event.
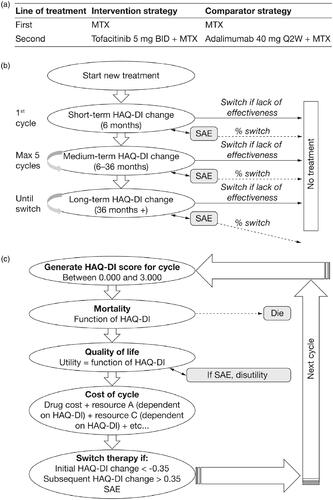
Model structure
The model utilized cycles of 6 months as this was considered to be an appropriate timescale for representing disease pathways and treatment monitoring in RACitation38. HAQ-DI score was used as a proxy for disease severity. Treatment switching was assessed after every 6-month cycle. Once patients entered the model and received one 6-month cycle of treatment, the treatment response was assessed based on changes in HAQ-DI score and the occurrence of a SAE was calculated based on its probability. Patients achieving a clinically significant change in HAQ-DI score (≥0.35 decrease) at 6 months were maintained on their current treatment until either treatment efficacy diminished or an SAE was experienced, at which point patients may discontinue (). A probability of discontinuation was assigned to either loss of effectiveness or SAE. Thus, discontinuation was a two-stage event, where a patient had a probability of experiencing an event (SAE or loss of effectiveness) followed by a probability of discontinuation. In addition, patients could transition to death based on the probability of mortality calculated during each cycle. Half-cycle correction was applied assuming that death of patients occurred mid-cycle. Thus, half a cycle length (3 months) was subtracted from the patient’s age at death.
The model estimated mortality, QoL, and costs based on the individual patient’s disease severity over the course of their lifetime (). HAQ-DI scores were used to predict resource utilization, which in turn predicted the lifetime cost of treatment. HAQ-DI scores were also mapped onto utility values to calculate outcomes in terms of QALYs. Utility was based on HAQ-DI score for each cycle. The model employed half-cycle correction, and the mid-point between the cycle and the subsequent cycle was used to calculate a patient’s utility. As the model assumed that any deaths occurred mid-cycle, the number of QALYs gained in the final cycle was halved.
A first-order model incorporated individual patient variation, and simulated patients using the following input parameters from distributions: starting characteristics (age, weight, and HAQ-DI score) and clinical data (initial effects [first 6 months], medium effects [>6 to 36 months], and long-term effects [>36 months]). Occurrences of events such as treatment switching, death, and SAEs were also modeled stochastically. A second-order model allowed for parameter uncertainty, including uncertainty around the relationship between HAQ-DI score and patient events, by drawing input parameters from distributions including: HAQ-DI to utility mapping, HAQ-DI to mortality mapping, HAQ-DI to resource-use mapping, and ACR functional class to HAQ-DI mapping and efficacy.
The main outcome from the model was the incremental cost-effectiveness ratio (ICER), which was presented as the incremental cost per QALY. Net monetary benefit was calculated using a willingness-to-pay (WTP) threshold of NT$1,500,000/QALYCitation39.
Continuation rules and efficacy
In the model, the first continuation rule was applied at 6 months. Responders were defined as patients with a clinically significant change in HAQ-DI score at 6 months. Patients who were not responders discontinued treatment and did not receive active treatment. The minimum improvement in Disease Activity Score in 28-joints (DAS28) expected for a new therapy in Taiwanese clinical practice (a decrease of 1.2) was converted to a corresponding HAQ-DI score (HAQ-DI threshold). An analysis of data from TEMPO, a trial comparing etanercept and MTX with each treatment alone in patients with RACitation40, was used to establish the relationship between DAS28 and HAQ-DI: ΔHAQ-DI = 0.289 × ΔDAS28. Using this equation, a decrease in DAS28 of −1.21 was converted to an initial decrease in HAQ-DI score of −0.35.
Previous studies have shown that patients with RA who experience a secondary response failure to one TNFi may benefit from switching to another TNFiCitation41,Citation42. The APLAR RA treatment recommendations advocate that patients who fail to achieve remission or who have low disease activity after 6 months of bDMARD therapy switch to another bDMARD agentCitation11. Based on this evidence and also on the expert opinion of two rheumatologists (authors of this manuscript), the probability of switching treatment due to loss of initial effect at 6 months was estimated as 0.8, irrespective of treatment regimen ().
In the model, the second continuation rule was applied for every 6-month cycle following the initial 6 months of treatment. The HAQ-DI change threshold for subsequent 6-month cycles following the first cycle was 0 (i.e. maintenance of initial response to treatment).
HAQ-DI score was used to monitor a patient’s disease severity over time within the model. HAQ-DI changes per cycle were based upon treatment and time spent on treatment. The change per cycle was assumed to be normally distributed. HAQ-DI was modeled for three stages of therapy: initial-, medium-, and long-term efficacy (). Clinical effectiveness data for the initial response to treatment were derived from a mixed treatment comparison (MTC) study that was conducted to estimate the relative efficacy and safety of tofacitinib vs bDMARDs in combination with MTX in patients with RA who had an inadequate response to csDMARDs ()Citation43. Data for medium-term HAQ-DI change (per 6-month cycle) were identified from two literature sourcesCitation44,Citation45 and are presented in . There is a paucity of data for long-term HAQ-DI progression in RA and an assumption was made that there was no disease progression in the long-term (>36 months on treatment) for tofacitinib and adalimumab therapy (i.e. HAQ-DI change was 0). For patients who discontinue treatment, HAQ-DI progression was associated with an absolute change of 0.03 per cycle.
Adverse events
As tofacitinib has a different mode of action to biologic agentsCitation46, such as adalimumab, SAEs were included in the base-case of the model to allow for differences in SAE rates between drug classes. The majority of SAEs observed in patients with RA treated with DMARDs consist of serious infectionsCitation47; thus, SAEs were defined here as a severe infection (such as meningitis, encephalitis, pneumonia, hepatitis, septicemia, bacteremia, etc.) or tuberculosis. The event rates of serious infections used in the model were obtained from a meta-analysis of interventional randomized controlled trials and long-term extension studiesCitation47 (). Serious infections were defined as those requiring hospitalization, anti-infective treatment, or biologics deferral; based on this definition, herpes zoster was included in the meta-analysis.
Based on patient data from a selection of clinical trials of bDMARDs and also on expert opinion, the probability of switching treatment due to an SAE (i.e., serious infection) was 0.5, irrespective of treatment regimen ().
Mortality
In the base-case model, mortality after each cycle was modeled using the association between the HAQ-DI score and mortality rate reported by Wolfe et al.Citation48. The mortality risk-adjustment equation was calculated by applying a multiplier to the general population mortality (adjusted mortality = general mortality rate × 1.33HAQ). Gender- and age-specific mortality rates for the general population for Taiwan in 2012 (the most recently available at the time of the analysis) were obtained from the Ministry of the Interior, Department of Statistics, Taiwan. The combined age-specific mortality rate was calculated, weighting for each gender (assuming that 22% of patients with RA were male). The model used half-cycle correction, and it was assumed that death of patients occurred mid-cycle.
QoL
Patients’ health-related QoL for each cycle was calculated by mapping HAQ-DI scores onto EuroQoL 5 D (EQ-5D) utility values. HAQ-DI to utility mapping was based on the conversion of HAQ-DI scores to utilities using regression of HAQ-DI vs EQ-5D-derived utility from the analysis of data from five tofacitinib Phase 3 clinical trialsCitation20–24. HAQ-DI to EQ-5D mapping was assessed using data derived from patients who received at least one dose of study treatment and had at least one post-baseline HAQ-DI value. A linear regression model was used to predict EQ-5D scores based on demographic variables (age, sex, and disease duration) and HAQ-DI. The HAQ-utility relationship used in the model was: utility = 0.7793 + (HAQ-DI × −0.2529) + (HAQ-DI2 × −0.038) + (age × 0.0013) + (RA duration in years × 0.0010) + (female × 0.0310).
No studies were identified that evaluated the disutility of treatment-specific SAEs in RA. Serious infections, including lower respiratory tract infections (LRTIs), make up the majority of SAEs in RA. In this analysis, it was assumed that the disutility for LRTI could be used as a proxy for SAEs in the model, in the absence of any other data identified from the literatureCitation49. LRTI was associated with a disutility of 0.157 over 4 weeks, measured by EQ-5DCitation49, which was equivalent to a 0.012 QALY loss.
Resource use and costs
Direct medical costs in the economic evaluation were estimated from a Taiwan NHIA perspective in Taiwanese NT$ currency. All health outcomes and costs were discounted at an annual rate of 3%, as recommended by local pharmacoeconomic guidelinesCitation50. The treatment cost for each drug was obtained from the NHIA, and the costs for each 6-month cycle were calculated using the recommended dosage for each drug ()Citation51.
Healthcare resource utilization was calculated per cycle according to patients’ HAQ-DI scores during that cycle, and was based on a cost-of-illness study in South KoreaCitation28, which had similar average costs per patient to Taiwan. The costs in Korean Won (KRW) were converted to NT$ (NT$1 = 0.028 KRW). The costs were stratified according to ACR functional class, using data from the study by Diamantopoulos et al.Citation52 to estimate HAQ-DI thresholds from ACR functional classes. Healthcare resource utilization costs in NT$ by ACR functional class are shown in . The cost of an SAE was assumed to be NT$170,261, estimated from the NHIRD 2007–2011 datasetCitation53.
Sensitivity analysis
A one-way sensitivity analysis and a probabilistic sensitivity analysis (PSA) were performed to assess the robustness of the model by varying key parameters and assumptions. For the one-way sensitivity analysis, the model was run for 10,000 iterations to produce a mean output for an upper limit and a lower limit around the mean input for each scenario, based on the 95% confidence interval around the parameter. For the PSA, key parameters were varied to allow for parameter uncertainty. The model was run with 1,000 iterations to generate a mean estimate of cost-effectiveness for a given parameter set, and this process was repeated to obtain 100 estimates with different parameter sets.
Results
Base-case analyses
The results of the base-case analysis for each treatment strategy are shown in . The estimated total lifetime cost per patient was slightly higher with second-line tofacitinib plus MTX (NT$1,526,413) than for second-line adalimumab plus MTX ($1,513,532; ). This was attributed to higher drug costs associated with the tofacitinib regimen, as adalimumab plus MTX treatment had higher health resource costs and SAE costs vs second-line tofacitinib plus MTX (). Patients in the tofacitinib plus MTX arm had a life expectancy of 31.95 life-years (LYs) compared with 31.92 LYs in the adalimumab plus MTX arm (). The total QALYs accrued per patient were 5.13 for tofacitinib plus MTX, compared with 5.04 for adalimumab plus MTX. Thus, tofacitinib plus MTX resulted in the incremental gain of 0.03 LY and 0.09 QALYs, while increasing the cost by NT$12,881. This produced an estimated ICER of NT$143,122/QALY in the base-case analysis (), suggesting that the combination of tofacitinib plus MTX was cost-effective, as second-line therapy, given a WTP threshold of NT$1,500,000/QALY. Moreover, the net monetary benefit was also positive with second-line tofacitinib plus MTX treatment, and was estimated as NT$122,125 ().
Table 2. Base-case cost-effectiveness results for tofacitinib plus MTX vs adalimumab plus MTX as second-line therapy in patients with RA.
Sensitivity analyses
The results of the one-way sensitivity analysis, showing the effect of varying one parameter in the model at a time, are shown in ( presents the incremental costs, QALYs, and ICERs for this analysis). Adjusting the short-term HAQ-DI score from −0.2 to −0.4 resulted in the greatest effect on net benefit of second-line tofacitinib plus MTX therapy vs adalimumab plus MTX, indicating that a lower short-term HAQ-DI score leads to greater health benefits (). Other parameters with a notable effect on net benefit included medium-term HAQ-DI score, the probability of switching due to initial loss of effect, and the switching threshold. Changing the cost of tofacitinib from NT$504 to NT$618 had a relatively small impact on the health benefit for tofacitinib with MTX over adalimumab with MTX ().
Figure 2. Tornado diagram representing the net benefit in one-way sensitivity analyses with changing baseline parameters. The width of the bars represents the range of results when variables were changed. aHAQ-DI – utility was calculated using the following parameters (lower boundary, upper boundary): constant term (0.800, 0.758), linear term (−0.247, −0.259), quadratic term (−0.031, −0.045), age covariate (0.002, 0.001), gender covariate (0.042, 0.020), RA duration covariate (0.002, 0.000). bHAQ-DI – cost was calculated using the following parameters (lower boundary, upper boundary): Class I (NT$50,568, NT$33,712), Class II (NT$65,453, NT$43,635), Class III (NT$120,019, NT$80,013), Class IV (NT$75,197, NT$50,131). Abbreviations. HAQ-DI, Health Assessment Questionnaire-Disability Index; LOE, loss of effect; LT, long-term; MT, medium-term; N/A, not applicable; NT$, New Taiwan Dollars; QALY, quality-adjusted life-year; RA, rheumatoid arthritis; SAE, serious adverse event; ST, short-term.
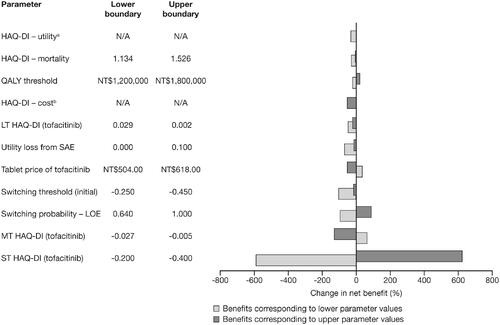
Table 3. One-way sensitivity analysis of cost-effectiveness results for tofacitinib in combination with methotrexate vs adalimumab plus methotrexate as second-line therapy in rheumatoid arthritis.
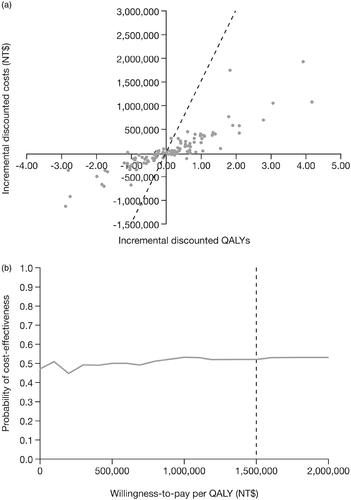
The results of the PSA are presented in . The cost-effectiveness scatter plane () shows that the majority of simulations fell below the NT$1,500,000/QALY WTP threshold. The cost-effectiveness acceptability curve demonstrates that, at a WTP threshold of NT$1,500,000/QALY, second-line tofacitinib plus MTX had a 52% likelihood of being cost-effective compared with adalimumab plus MTX ().
Figure 3. Cost-effectiveness planes for (a) the probabilistic sensitivity analyses, and (b) cost-effectiveness acceptability curve for tofacitinib plus MTX vs adalimumab plus MTX as second-line therapy of patients with RA. The dotted line represents the cost-effectiveness threshold per QALY gained. Abbreviations. MTX, methotrexate; NT$, New Taiwan Dollars; QALY, quality-adjusted life-year; RA, rheumatoid arthritis.
Discussion
This model represents the first economic analysis of tofacitinib as a treatment for patients with moderate-to-severe RA in a Taiwanese setting. In the model, tofacitinib plus MTX was compared with adalimumab plus MTX as a second-line treatment for patients who had an inadequate response to MTX. In the base-case analysis, treatment with tofacitinib plus MTX increased both costs and QALYs gained compared with adalimumab plus MTX, resulting in an ICER of NT$143,122/QALY. In Taiwan, the threshold for cost-effectiveness is three times the gross domestic product (GDP) per capitaCitation54. In our analysis, ICER was low and within 1 GDP; evaluated against an NT$1,500,000/QALY WTP threshold and, over a lifetime horizon, these results indicate that treatment with tofacitinib plus MTX is a cost-effective alternative to adalimumab plus MTX from a Taiwan NHIA perspective.
The cost-effectiveness of tofacitinib for the treatment of patients with moderate-to-severe RA and an inadequate response to csDMARDs has been previously demonstrated in an economic evaluation performed in South KoreaCitation28. This analysis, from a societal perspective, found that the inclusion of tofacitinib was a cost-effective treatment option vs the standard-of-care treatment sequence, irrespective of the position of tofacitinib in the treatment sequence. Initiation of tofacitinib as a first-line therapy was the most cost-effective option compared with its use as second-, third-, and fourth-line therapy. Furthermore, incorporating tofacitinib into the treatment sequence for patients with moderate-to-severe RA and an inadequate response to MTX was found to be cost-effective in the USCitation31 and CanadaCitation33 when considering a third-party payer perspective. Although a global comparison of results is difficult due to differences in methodology between studies, these findings are consistent with the results of our analysis in Taiwan, which found tofacitinib was cost-effective after failure of first-line MTX.
In our study, the one-way sensitivity analysis showed that the cost-utility of tofacitinib plus MTX vs adalimumab plus MTX was robust to changes in the majority of variables analyzed, although ICERs were most sensitive to changes in short-term HAQ-DI score. The one-way sensitivity analysis demonstrated that variation in tofacitinib drug costs resulted in a minimal effect on net benefit. The PSA provided some support for the robustness of the base-case analysis, showing that, at a WTP threshold of NT$1,500,000, the likelihood of second-line tofacitinib plus MTX being cost-effective compared with adalimumab plus MTX was 52%. It is important that the cost included in the model for treating SAEs is estimated accurately; this was based on a recent analysis of the NHIRD dataset in the period between 2007 and 2011Citation53.
This analysis is subject to several potential limitations. In the model, clinical effectiveness was primarily based on HAQ-DI score. However, a lack of completed data for HAQ-DI changes, in terms of disease severity over time, may affect the nature of the model. In addition, several calculations were necessary to map the relationship between HAQ-DI score and other measurements. Nevertheless, published evidence supports HAQ-DI as the primary clinical measure for economic evaluations in RA, as it is closely correlated to health utilities, mortality, and costsCitation55. For our analysis, the initial change in HAQ-DI score at 6 months was derived from an MTC study. For clinical and safety data a single source was preferred, so that consistent endpoints were included in the analysis. The MTC study was selected, rather than a single head-to-head study, because the data were considered to be more robust. Inadequate clinical data for long-term HAQ-DI progression (>36 months on treatment) led to an assumption that the change in HAD-QI score was 0. The lack of available clinical data may introduce some bias in the analysis; however, clinically reasonable assumptions were incorporated into the model. No patients were in an early stage of RA, which may limit the generalizability of these results to the whole population. Although efficacy data and some patient characteristics for the modeled population were derived from studies conducted outside Taiwan, these were assumed to have the most appropriate similarities in clinical setting to our model. Finally, the Taiwan NHIRD does not contain detailed information about lifestyle factors or individual health status (e.g. body mass index, malnutrition) that may influence SAEs or mortality; nor does it include the results of laboratory examinations.
This evaluation was performed from the Taiwan NHIA perspective, and only direct costs were considered in the model. Therefore, the analysis excluded indirect (non-health) costs, such as productivity losses and costs of informal care, which are important in RA, given the disabling nature of the diseaseCitation10. Analyses from a public payer perspective, as described herein, are useful for supporting reimbursement decisions. Moreover, variability in the organization, administration of care, and costs between varying healthcare settings means that the base-case results from our study are not necessarily generalizable to countries with healthcare systems that differ considerably from Taiwan.
Currently, the Taiwanese national reimbursement guidelines for patients with RA receiving bDMARDs or tofacitinib apply to patients with severe RA onlyCitation56. Although the criteria for patients entering the ORAL Standard study, on which the simulated baseline characteristics for this modeling analysis was based, included patients with both moderate and severe RA, the majority of enrolled patients had severe RA. Therefore, our cost-effectiveness analysis reflects the licensed indication of tofacitinib for the treatment of RA, and we believe that the results are relevant and can provide helpful information for health policy decision-makers in Taiwan.
Conclusions
In conclusion, this first analysis of cost-effectiveness from a Taiwanese NHIA perspective demonstrated that tofacitinib 5 mg BID plus MTX as second-line therapy in patients with moderate-to-severe RA who were inadequate responders to MTX is a cost-effective treatment strategy.
Transparency
Declaration of funding
This study was funded by Pfizer Inc.
Declaration of financial/other interests
LC was an employee of York Health Economics Consortium at the time of the analysis, who were paid consultancy fees by Pfizer Inc to develop the analysis. SV is a shareholder of Pfizer Inc and was an employee of Pfizer Inc at the time of the analysis. RAG is a shareholder and employee of Pfizer Inc. The JME peer reviewers and remaining authors of this manuscript have no relevant financial or other relationships to disclose.
Ethical approval/informed consent
This article does not contain any studies with human participants performed by the authors.
Acknowledgements
Medical writing support, under the guidance of the authors, was provided by Jennifer Stewart, PhD, MBA, at CMC Connect, a division of McCann Health Medical Communications Inc, Radnor, PA, and Tracey Warren, PhD, on behalf of McCann Health Medical Communications Inc, and was funded by Pfizer Inc, New York, NY, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464). Gabriel Yu and Ya-Wen Yang each provided a critical review of this manuscript.
Data availability statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- McInnes I, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219.
- Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322.
- Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35:10–14.
- Yu KH, See LC, Kuo CF, et al. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res. 2013;65:244–250.
- Lai CH, Lai MS, Lai KL, et al. Nationwide population-based epidemiologic study of rheumatoid arthritis in Taiwan. Clin Exp Rheumatol. 2012;30:358–363.
- Kuo CF, Luo SF, See LC, et al. Rheumatoid arthritis prevalence, incidence, and mortality rates: a nationwide population study in Taiwan. Rheumatol Int. 2013;33:355–360.
- Chiu YM, Lai MS, Lin HY, et al. Disease activity affects all domains of quality of life in patients with rheumatoid arthritis and is modified by disease duration. Clin Exp Rheumatol. 2014;32:898–903.
- Chang J, Lang H. The economic cost of rheumatoid arthritis in Taiwan [Abstract]. Value Health. 2014;17:A773.
- Wang BCM, Tang CH, Furnback W, et al. Disease burden of rheumatoid arthritis in Taiwan: a population-based analysis [Abstract]. Value Health. 2014;17:A45.
- Panés J, Chan G, Maller E, et al. Effects of oral tofacitinib on patient-reported outcomes in patients with moderate to severe Crohn's disease: results of two Phase 2B randomized placebo-controlled trials [Abstract]. Gastroenterology. 2016;150(S1):S1003.
- Lau CS, Chia F, Harrison A, et al. APLAR rheumatoid arthritis treatment recommendations. Int J Rheum Dis. 2015;18:685–713.
- Liao TL, Lin CH, Chen YM, et al. Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: a nationwide retrospective cohort study in Taiwan. PLoS One. 2016;11:e0153217.
- Campbell J, Lowe D, Sleeman MA. Developing the next generation of monoclonal antibodies for the treatment of rheumatoid arthritis. Br J Pharmacol. 2011;162:1470–1484.
- Pope J, Combe B. Unmet needs in the treatment of rheumatoid arthritis. OJRA. 2013;3:65–78.
- Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–629.
- Kremer JM, Cohen S, Wilkinson BE, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–981.
- Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–1905.
- McInnes IB, Kim HY, Lee SH, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis. 2014;73:124–131.
- Tanaka Y, Suzuki M, Nakamura H, et al. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken). 2011;63:1150–1158.
- Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised Phase 3 trial. Lancet. 2013;381:451–460.
- Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507.
- Kremer J, Li Z-G, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–261.
- van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–570.
- van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519.
- Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386.
- Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457–468.
- Wollenhaupt J, Lee EB, Curtis JR,et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21:89.
- Lee MY, Park SK, Park SY, et al. Cost-effectiveness of tofacitinib in the treatment of moderate to severe rheumatoid arthritis in South Korea. Clin Ther. 2015;37:1662–1676.
- Claxton L, Jenks M, Taylor M, et al. An economic evaluation of tofacitinib treatment in rheumatoid arthritis: modeling the cost of treatment strategies in the United States. J Manag Care Spec Pharm. 2016;22:1088–1102.
- Claxton L, Taylor M, Gerber RA, et al. Modelling the cost-effectiveness of tofacitinib for the treatment of rheumatoid arthritis in the United States. Curr Med Res Opin. 2018;34:1991–2000.
- Claxton L, Taylor M, Moynagh D, et al. Modeling the costs and outcomes associated with sequence of treatment with and without tofacitinib for the treatment of moderate to severe rheumatoid arthritis in the US [Abstract]. Ann Rheum Dis. 2015;74:319–320.
- Claxton L, Taylor M, Soonasra A, et al. An economic evaluation of tofacitinib treatment in rheumatoid arthritis after methotrexate or after 1 or 2 TNF inhibitors from a U.S. payer perspective. J Manag Care Spec Pharm. 2018;24:1010–1017.
- Woolcott JC, Blackhouse G, Claxton L, et al. The economic value of tofacitinib 5 mg BID in the treatment of moderate to severe rheumatoid arthritis: a Canadian analysis [Abstract]. Ann Rheum Dis. 2015;74:785.
- Taiwan Center for Drug Evaluation. Health Technology Assessment cost-benefit analysis methodology guidelines. 2013 [cited 2019 Mar 4]. Available from: http://nihta.cde.org.tw/ReadFile/?p=SRule&n=5c4de492-3c50-4349-940c-d494f0cecc87.pdf
- Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2015 [cited 2019 Feb 18]. Available from: http://www.cdc.gov/nchs/icd/icd9cm.htm
- NHIRD. National Health Insurance Research Database Data Files. 2015 [cited 2016 Oct 22]. Available from: http://nhird.nhri.org.tw/en/Data_Subsets.html#S4
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324.
- Deighton C, Hyrich K, Ding T, et al. BSR and BHPR rheumatoid arthritis guidelines on eligibility criteria for the first biological therapy. Rheumatology (Oxford). 2010;49:1197–1199.
- Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–437.
- Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–681.
- Hansen KE, Hildebrand JP, Genovese MC, et al. The efficacy of switching from etanercept to infliximab in patients with rheumatoid arthritis. J Rheumatol. 2004;31:1098–1102.
- Jamnitski A, Bartelds GM, Nurmohamed MT, et al. The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann Rheum Dis. 2011;70:284–288.
- Bergrath E, Gerber RA, Gruben D, et al. Tofacitinib versus biologic treatments in moderate-to-severe rheumatoid arthritis patients who have had an inadequate response to nonbiologic DMARDs: systematic literature review and network meta-analysis. Int J Rheumatol. 2017;2017:8417249.
- Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411.
- Wollenhaupt J, Silverfield J, Lee EB, et al. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: open-label, long-term extension studies up to 36 months [Abstract]. Arthritis Rheum. 2011;63:S152–S153.
- Lundquist LM, Cole SW, Sikes ML. Efficacy and safety of tofacitinib for treatment of rheumatoid arthritis. World J Orthop. 2014;5:504–511.
- Strand V, Ahadieh S, French J, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther. 2015;17:362.
- Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494.
- Oppong R, Kaambwa B, Nuttall J, et al. The impact of using different tariffs to value EQ-5D health state descriptions: an example from a study of acute cough/lower respiratory tract infections in seven countries. Eur J Health Econ. 2013;14:197–209.
- Taiwan Chapter of International Society for Pharmacoeconomics and Outcomes Research (TaSPOR). Guidelines of methodological standards for pharmacoeconomic evaluation. Version 1.0 ed. Taipei, Taiwan; 2006.
- NHIA. National Health Insurance Administration. 2016 [cited 2017 Nov 4]. Available from: http://www.nhi.gov.tw/Query/query1.aspx?menu=20&menu_id=712&WD_ID=831
- Diamantopoulos A, Benucci M, Capri S, et al. Economic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in Italy. J Med Econ. 2012;15:576–585.
- Kuo H-L, Cherh HK, Hua WC. Health care utilization associated with adverse events among RA patients treated by TNF-alpha in Taiwan [Abstract]. Mod Rheumatol. 2016;26:S50. (ICW-C3-2).
- Lang HC, Chen HW, Chiou TJ, et al. The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu-positive early breast cancer in Taiwan. J Med Econ. 2016;19:923–927.
- Bansback N, Ara R, Karnon J, et al. Economic evaluations in rheumatoid arthritis: a critical review of measures used to define health States. Pharmacoeconomics. 2008;26:395–408.
- Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford). 2016;55:523–534.

