Abstract
Aims: This analysis evaluated the cost-effectiveness of once-weekly semaglutide vs glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in patients with type 2 diabetes (T2D) uncontrolled on metformin or basal insulin in Sweden.
Materials and methods: This cost-effectiveness analysis (CEA) was conducted using the Swedish Institute of Health Economics (IHE) Diabetes Cohort Model. Analyses were conducted from the Swedish societal perspective over a time horizon of 40 years. For patients uncontrolled on metformin, dulaglutide was the comparator, and data from the SUSTAIN 7 clinical trial was used. For patients uncontrolled on basal insulin, lixisenatide was chosen as the comparator and data was obtained from a network meta-analysis (NMA).
Results: The results show that, in patients with inadequate control on metformin, semaglutide 1.0 mg dominated (i.e. provided greater clinical benefit, and was less costly) dulaglutide 1.5 mg. In patients with inadequate control on basal insulin, semaglutide 1.0 mg dominated lixisenatide. The reduction in costs is largely driven by the reduction in complications seen with once-weekly semaglutide.
Limitations and conclusions: It is likely that this analysis is conservative in estimating the cardiovascular (CV) cost benefits associated with treatment with once-weekly semaglutide. In patients inadequately controlled on basal insulin, the analyses vs lixisenatide were based on results from an NMA, as no head-to-head clinical trial has been conducted for this comparison. These CEA results show that once-weekly semaglutide is a cost-effective GLP-1 RA therapy for the treatment of T2D in patients inadequately controlled on metformin or basal insulin, addressing many current clinician, patient, and payer unmet needs in Sweden.
Introduction
Type 2 diabetes (T2D) is a progressive disease characterized by rising glycated hemoglobin (HbA1c) levels, and the consequences of failing to maintain glycemic control are substantial. Suboptimal glycemic control for prolonged periods of time is associated with an increased risk of microvascular (retinopathy, neuropathy, and nephropathy) and macrovascular (myocardial infarction [MI], stroke, and heart failure) complicationsCitation1. Obesity is the most potent risk factor for developing T2D, accounting for 80–85% of the overall risk of developing the conditionCitation2. The burden of T2D is significant in Sweden. In 2016, there were 410,528 patients (4% of the Swedish population) with diabetes registered with the Swedish national diabetes register (NDR), thought to represent ∼90% of the total number of people diagnosedCitation3. Of these, ∼90% have T2DCitation3. A Swedish register study showed that 34% of patients with T2D also have cardiovascular disease (CVD)Citation4. By linking the Swedish NDR with the hospitalization register from the National Board of Health and Welfare, it was shown that 20% of people with T2D in Sweden had been treated for coronary heart disease (CHD), 8% for heart failure, and 8% for stroke, compared with 10%, 4% and 5% in a matched cohort without T2DCitation3. Furthermore, CVD is accountable for 52% of deaths in patients with T2DCitation5. Overall, T2D is associated with a 15% increase in risk of mortality in SwedenCitation6.
Diabetes is associated with a significant economic burdenCitation7,Citation8. The estimated total cost of T2D in Sweden in 2013 was SEK 16 billion, and is expected to increase to SEK 18 billion by 2020Citation9. The economic burden of T2D is increased in patients who are overweight or obeseCitation10. These patients are at increased risk of co-morbidities and, consequently, have significantly greater medical expenditures than individuals with diabetes and normal weightCitation11–13.
Current treatment guidelines for T2DCitation14–16 recommend that patients achieve and maintain glycemic levels as close to normal (generally HbA1c <7% [53 mmol/mol]) as possible. However, despite the obvious health benefits, cost savings, and clear guidelines, many patients do not achieve HbA1c treatment targets. In 2016, only 51% of patients with T2D in Sweden reached the treatment target HbA1c < 52 mmol/molCitation17. Only 22% of patients with T2D treated with oral anti-diabetic drugs (OADs) and insulin reached the same targetCitation17. Several landmark studies, such as the UK Prospective Diabetes Study (UKPDS)Citation1, have demonstrated the long-term benefits of maintaining good glycemic control. The UKPDS 10-year follow-up study reported that the significant risk reductions in MI persisted or emerged over timeCitation18.
Optimal treatments for T2D would enable patients to achieve and maintain good glycemic control, whilst managing their weight effectively, thereby reducing the risk of cardiovascular complications. A Swedish report based on data from the NDR estimates that a treatment regimen following national treatment guidelines in order to decrease glycemic levels would be cost neutral, while it would save 4,000 life years; prevent 800 MIs, 500 strokes, 450 persons from having end stage renal disease requiring dialysis, and 200 persons from becoming blind by the year 2030Citation9.
Semaglutide is a human GLP-1 RA suitable for once-weekly (QW) subcutaneous administration. The efficacy and safety of once-weekly semaglutide over 30 weeks to 2 years has been extensively studied in patients with T2D along the diabetes continuum of care through the SUSTAIN clinical trial programCitation19–25. The SUSTAIN trials demonstrated that once-weekly semaglutide (at a dose of 1.0 mg, once-weekly) is associated with superior and sustained improvements in HbA1c and body weight, compared with all active control and placebo treatments assessed in the clinical trial program. Furthermore, the long-term safety and cardiovascular outcomes trial, SUSTAIN 6, showed that once-weekly semaglutide, in addition to standard-of-care, significantly reduced the risk of CV outcomes compared with placebo (standard-of-care alone) by 26% in patients with T2D at high CV riskCitation25.
The objective of the current study was to assess the long-term cost-effectiveness of once-weekly semaglutide vs GLP-1 RAs in patients uncontrolled on metformin or uncontrolled on basal insulin in Sweden.
Materials and methods
These CEAs were conducted using the IHE Diabetes Cohort ModelCitation26, which has been used in other CEAs in SwedenCitation27–29. Briefly, this model uses cohort simulation to estimate the cost-effectiveness of competing interventions for T2D. It is constructed from Markov health states representing important microvascular and macrovascular complications, and uses a fixed cycle length of 1 year. Analyses were conducted from the Swedish societal perspective using macrovascular risk equations from the Swedish NDR to reflect the Swedish population, a time horizon of 40 years, and a discount rate of 3% for costs and outcomes. Deterministic and probabilistic sensitivity analyses were run to account for parameter uncertainty. Pharmaceutical and medical device prices were taken from the Swedish Dental and Pharmaceutical Benefits Agency (Tandvårds-och Läkemedelsförmånsverket, TLV) database, April 2018. All other costs were identified from the literature and inflated using the inflation rate from the consumer price index for healthCitation30.
Comparator choice for these CEA analyses was based on TLV requirements. Prior to reimbursement of once-weekly semaglutide, TLV considered dulaglutide to be the most effective and cost-effective once-weekly GLP-1 RA in SwedenCitation31. Dulaglutide was also the most recently launched GLP1 RA in Sweden, and, similar to semaglutide, administered once-weekly. Therefore, dulaglutide 1.5 mg was used as the comparator in the CEA in the once-weekly semaglutide reimbursement application. The analysis of patients inadequately controlled on metformin was based on SUSTAIN 7, the randomized clinical trial comparing once-weekly semaglutide and dulaglutide head-to-head in patients inadequately controlled on metforminCitation24. The higher dose of dulaglutide (1.5 mg) was chosen as it is used by 81% of patients, and the flat pricing strategy makes it more cost-effective than the low dose (0.75 mg). Furthermore, the 0.75 mg dose is recommended by the European Medicines Agency (EMA) for use as a monotherapy, or patients >75 years of ageCitation32. In Sweden, the reimbursement of dulaglutide is restricted to patients who are not using insulin. Therefore, in analyses of patients inadequately controlled on basal insulin, TLV considered lixisenatide to be the most relevant comparator to once-weekly semaglutide, and it has consequently been used as the comparator for this analysis. The analysis is based on an NMACitation33, as there is no clinical trial directly comparing once-weekly semaglutide with lixisenatide in patients inadequately controlled on basal insulin. Baseline characteristics and treatment effects for both analyses are defined in the Supplementary Appendix. Base case analyses used statistically significant treatment effects; where differences were not statistically significant, once-weekly semaglutide values were used for both treatment arms.
In line with previous Swedish CEAsCitation29 and the UKPDSCitation34,Citation35, it was assumed that HbA1c increased at 0.15%-point per year, and that treatment was intensified when HbA1c had reached baseline values (HbA1c 8.22% in patients inadequately controlled on metformin, and HbA1c 8.31% in patients inadequately controlled on basal insulin). For the analysis of patients inadequately controlled on metformin, treatment was first intensified by switching from once-weekly semaglutide or dulaglutide to basal insulin, and then further intensified by the addition of bolus insulin (i.e. to a basal-bolus regimen). For the analysis of patients inadequately controlled on basal insulin, treatment was intensified by switching from once-weekly semaglutide or lixisenatide to bolus insulin (i.e. a basal-bolus regimen). Insulin doses and treatment effects following intensification were taken from an analysis by Willis et al.Citation36, and are defined in the Supplementary Appendix. Body mass index (BMI) annual drift of 0.08 kg/m2 was taken from Chaudhry et al.Citation37.
Patients treated with once-weekly semaglutide or dulaglutide, in addition to metformin, were assumed to use one self-monitored blood glucose (SMBG) test per week. Patients treated with basal insulin were assumed to use one SMBG test per day. Patients treated with a basal-bolus insulin regimen were assumed to use four SMBG tests per day. The average daily resource use is presented in the Supplementary Appendix. Total costs are presented in .
Table 1. Total costs.
The cost of long-term diabetes-related complications was based on a literature review conducted for the cost-effectiveness analysis of liraglutide vs sitagliptin and sulphonylurea in SwedenCitation28 (see Supplementary Appendix).
The cost per severe hypoglycemic event from a Swedish societal perspective was based on a study by Jönsson et al.Citation38 and calculated to be SEK 1,993 per event (SEK 1,469 for healthcare costs and SEK 524 for societal costs). The cost per mild hypoglycemic event was based on data from Brod et al.Citation39 and Geelhoed-Duijvestijn et al.Citation40, and calculated to be SEK 59 per event (SEK 44 for healthcare costs and SEK 15 for societal costs).
As analyses were conducted from the societal perspective, indirect costs of diabetes-related complications were also included, using the human capital approach. People were assumed to be in the work force from 20–65 years of age, with an average monthly salary of SEK 32,800 in 2016Citation41. Sick leave due to diabetes-related complications was taken from an analysis of Danish register data of 34,882 patients with diabetesCitation42 (see Supplementary Appendix). For complications where data on sick leave could not be found, we conservatively assumed that the number of sick leave days was zero.
Utility values associated with patient demographics, treatment effects (including HbA1c, BMI, systolic blood pressure [SBP], cholesterol, and hypoglycemia), and long-term complications are based on published data (see Supplementary Appendix).
One-way and probabilistic sensitivity analyses were conducted to test the robustness of the base case analyses.
Results
The greater efficacy observed with once-weekly semaglutide over comparator treatments in clinical trials and indirect analyses results in a prolonged time on-treatment before intensification is required.
In the base case analyses of patients uncontrolled on metformin, treatment switching occurred after 12 years with once-weekly semaglutide and after 10 years with dulaglutide. The greater efficacy results in a reduction in the cumulative incidence of diabetes related complications, primarily driven by the reduced incidence of retinopathy (), and increased quality-adjusted life expectancy. Treatment with semaglutide 1.0 mg in patients inadequately controlled on metformin reduced treatment costs, costs related to complications and indirect costs, leading to a total cost reduction of SEK 21,740 (). Furthermore, treatment with once-weekly semaglutide resulted in a health gain of 0.28 quality-adjusted life years (QALYs) compared with dulaglutide 1.5 mg (). Once-weekly semaglutide 1.0 mg, therefore, dominated (i.e. provided greater clinical benefit and was less costly) dulaglutide 1.5 mg in patients inadequately controlled on metformin.
Table 2. Complications.
Table 3. Differences in Costs.
Table 4. Base case cost-effectiveness results.
The results were stable across sensitivity analyses, showing that the base case analysis in patients inadequately controlled on metformin are robust. The scatterplot shows that the probabilistic sensitivity analyses (PSA) produced similar mean results to the base case (). The cost-effectiveness acceptability curve (CEAC) shows a 99% probability that once-weekly semaglutide 1.0 mg is cost-effective against dulaglutide 1.5 mg at a willingness-to-pay threshold of SEK 500,000 per QALY gained (). In one-way sensitivity analyses (see for list of parameters varied) of patients with inadequate control on metformin, once-weekly semaglutide 1.0 mg was dominant vs dulaglutide 1.5 mg in all analyses conducted, except when treatment was intensified at HbA1c 7.5% (). To test the efficacy variables, the upper and lower 95% confidence intervals of the HbA1c and BMI treatment effects were assessed, and once-weekly semaglutide remained dominant in all four analyses. To test the assumption of patients intensifying treatment when HbA1c returns to baseline values, a sensitivity analysis was conducted where patients intensify at HbA1c 7.5%. In this analysis, patients stayed on once-weekly semaglutide for 8 years and dulaglutide for 5 years. Removing the utility gain associated with reduction in HbA1c had little effect on the cost, but reduced the QALY gain with once-weekly semaglutide from 0.28 QALYs to 0.20 QALYs, meaning that once-weekly semaglutide remained dominant.
Figure 1. Cost-effectiveness acceptability curves and probabilistic sensitivity analysis plots in patients inadequately controlled on metformin or basal insulin. QALYs, quality adjusted life years.
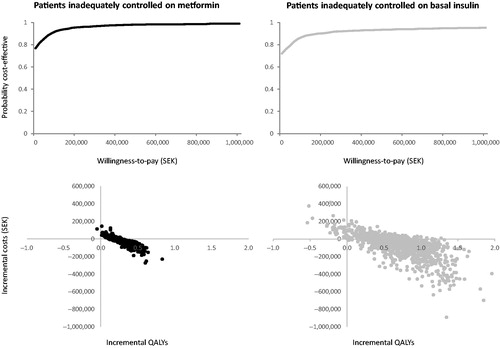
Table 5. One-way sensitivity analysis parameters.
Table 6. One-way sensitivity analysis results.
In the base case analyses of patients uncontrolled on basal insulin, treatment switching occurred after 13 years with once-weekly semaglutide and 3 years with lixisenatide. The greater efficacy results in a reduction in the cumulative incidence of diabetes related complications, primarily driven by the reduced incidence of retinopathy and nephropathy (), and increased quality-adjusted life expectancy. While once-weekly semaglutide lead to increased treatment costs, the treatment costs were fully offset by reductions in complication costs and indirect costs resulting in a total reduction of SEK 25,169 (). Treatment with once-weekly semaglutide reduced both direct healthcare costs and total costs to society, and resulted in a gain of 0.71 QALYs (). Once-weekly semaglutide 1.0 mg, therefore, dominated lixisenatide in patients inadequately controlled on basal insulin.
The results were stable across sensitivity analyses, showing that the base case analyses in patients inadequately controlled on basal insulin are also robust. The scatterplot shows that the PSA produced similar mean results to the base case (). The CEAC shows a 95% probability that once-weekly semaglutide is cost-effective against lixisenatide in patients uncontrolled on basal insulin at a willingness-to-pay threshold of SEK 500,000 per QALY gained (). In one-way sensitivity analyses in patients with inadequate control on basal insulin, once-weekly semaglutide dominated lixisenatide in the majority of analyses, and was cost-effective in the remaining analyses (20-year time horizon SEK 22,219 per QALY gained; 0.1% annual HbA1c drift SEK 12,353 per QALY gained; smoker SEK 18,701 per QALY gained) (). When testing the efficacy variables (utilizing upper and lower 95% confidence intervals for the HbA1c and BMI treatment effects), once-weekly semaglutide remained dominant in all four analyses. Removing the utility gain associated with reduction in HbA1c had little effect on the costs, but reduced the QALY gain with once-weekly semaglutide from 0.71 QALYs to 0.63 QALYs, meaning that once-weekly semaglutide remained dominant. In the test of the assumption that patients intensify treatment when HbA1c returns to baseline values, when the parameters were set so that patients intensified at HbA1c 7.5%, patients remained on once-weekly semaglutide for 7 years and lixisenatide for 1 year.
Discussion
Therapeutic inertia is common amongst patients with diabetesCitation43,Citation44, with factors such as fear of weight gainCitation45, fear of hypoglycemiaCitation46, and reluctance to initiate a more complex regimenCitation47 contributing. There is, therefore, an unmet clinical need for antidiabetic therapies which can address these issues, but at minimal additional cost to existing treatments.
The results show that, in Sweden, once-weekly semaglutide is cost-saving compared with dulaglutide in patients with inadequate control on metformin and compared with lixisenatide in patients with inadequate control on basal insulin. Treatment with once-weekly semaglutide reduced costs by SEK 21,740, and lead to a gain of 0.28 QALY in patients with inadequate control on metformin, meaning that it dominated dulaglutide. SEK 710 of this cost saving is attributable to the reduced lost productivity associated with dulaglutide. Further CEAs of once-weekly semaglutide vs dulaglutide have been conducted in the CanadianCitation48 and UKCitation49 settings. In the Canadian base case analysisCitation48, the HbA1c drift was set to zero, and both treatment arms were intensified after 3 years, resulting in a 0.05 QALY gain with once-weekly semaglutide vs dulaglutide. The authors conducted a scenario analysis, referred to as the “more clinically relevant scenario analysis” with inputs in line with our base case analysis. In this analysis, there was a gain of 0.4 QALYs, in line with the 0.28 QALYs observed in our analysis in the Swedish setting. In the UK analysisCitation49, in which the IQVIA CORE Diabetes Model was used, gains of 0.17 QALYs were observed with once-weekly semaglutide vs dulaglutide. All three analyses (in the Swedish, Canadian, and UK settings) show that once-weekly semaglutide remains dominant vs dulaglutide.
In patients with inadequate control on basal insulin, once-weekly semaglutide was associated with a SEK 25,169 cost saving, and a 0.71 QALY gain, meaning that once-weekly semaglutide dominated lixisenatide. SEK 2,342 of this cost saving is attributable to the reduced lost productivity associated with dulaglutide. The sensitivity analyses show that these results are robust.
These cost-effectiveness analyses were conducted using the IHE Diabetes Cohort Model, using risk equations from the Swedish NDR to reflect the Swedish population. The external validity of the IHE Diabetes Cohort Model has previously been tested according to the recommendations of ISPOR/MSDM, including dependent and independent external validation exercisesCitation26. The validation indicated that the model has a predictive accuracy in line with other models of T2D. This model is applicable to CEAs of diabetes in Sweden as it is based on Swedish risks, healthcare system, and costsCitation27–29.
In line with the improved clinical efficacy associated with semaglutide 1.0 mg compared with dulaglutide 1.5 mg in patients uncontrolled on metformin, and with semaglutide 1.0 mg compared with lixisenatide in patients uncontrolled on basal insulin, there are cost savings associated with the reduced incidence of diabetes-related microvascular and macrovascular complications observed with once-weekly semaglutide. This cost saving adds to the saving in treatment costs vs dulaglutide in patients uncontrolled on metformin, and completely offsets the increase in treatment costs vs lixisenatide in patients uncontrolled on basal insulin.
It is likely that this analysis under-estimates the CV benefits of once-weekly semaglutide. Analyses of other antidiabetic drugs such as liraglutide and empagliflozin which have also shown statistically significant improvements in major adverse cardiovascular events (MACE) show that the reduction in CV events is only partially driven by reduction in HbA1cCitation50–52. The IHE Diabetes Cohort Model (consistent with the CORE diabetes modelCitation53) calculates the reduction in the incidence of complications based on statistically significant treatment effects including HbA1c and BMI reduction. Treatment with once-weekly semaglutide is also associated with a statistically significant reduction in MACECitation25. In patients inadequately controlled on basal insulin, the analysis is likely to be conservative, as the cardiovascular outcomes trial for lixisenatideCitation54 did not show a significant reduction in MACE.
In patients inadequately controlled on metformin, the analyses vs dulaglutide were based on a head-to-head clinical trial of patients uncontrolled on metforminCitation24. As there is no clinical trial in patients inadequately controlled on basal insulin comparing once-weekly semaglutide with lixisenatide, the cost-effectiveness analysis in this population is based on results from a previously-published NMACitation33. The methodology of the NMA aligns with guidance from NICE, ISPOR, and the Cochrane Institute. All the trials included within the NMA were derived from a systematic literature review, ensuring all available evidence was captured.
This health economic analysis based on comprehensive clinical evidence suggests that, compared with other GLP-1 RAs, once-weekly semaglutide is a cost-saving treatment for patients with T2D in Sweden, in line with its licensed indication. Further clinical and economic research could be conducted to investigate the cost-effectiveness of once-weekly semaglutide compared with other anti-diabetes treatments such as sodium-like glucose co-transporter-2 inhibitors (SGLT-2is).
Conclusion
Once-weekly semaglutide is a cost-effective GLP-1 RA for the treatment of T2D in patients inadequately controlled on metformin or basal insulin, addressing many current clinician, patient, and payer unmet needs in Sweden.
Transparency statement
Declaration of funding
Funding for this study and article processing charges was provided by Novo Nordisk. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Declaration of financial/other interests
ÅE is an employee and shareholder of Novo Nordisk. AF is an employee at IHE and declares no conflict of interest. A reviewer on this manuscript discloses their relationship as a consultant to Eli Lilly. Another reviewer discloses that they have participated in advisory boards for, and their department has received grant income from, Novo Nordisk, the sponsor of this study. This reviewer also discloses participating in advisory boards for, and their department receiving grant income from, companies that produce competitive products, including Sanofi, Eli Lilly, Bayer, Abbvie, Merck, Takeda, Astellas, Daiichi-Sankyo, Novartis, Servier, and AstraZeneca. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material Appendix 1
Download MS Word (83.9 KB)Acknowledgements
Editorial assistance in the preparation of this article was provided by Deb Burford of DRG Abacus. Support for this assistance was funded by Novo Nordisk.
References
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412.
- Diabetes UK. Facts and stats. 2016. Available from: https://www.diabetes.org.uk/Documents/Position%20statements/DiabetesUK_Facts_Stats_Oct16.pdf. [Accessed 2018 April 12].
- Gudbjörnsdottir S, Eliasson B, Cederholm J, et al. Nationella diabetesregistret, Årsrapport, 2016 års resultat. 2016. https://wwwndrnu/pdfs/Arsrapport_NDR_2016pdf. [Accessed 2018 April 12].
- Norhammar A, Bodegard J, Nystrom T, et al. Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia. 2016;59:1692–1701.
- Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44:S14–S21.
- Läkemedelsverket. Läkemedelsbehandling för glukoskontroll vid typ 2-diabetes – behandlingsrekommendation. 2017. Available from: https://lakemedelsverket.se/diabetestyp2. [Accessed 2018 April 12].
- Bolin K, Gip C, Mork AC, et al. Diabetes, healthcare cost and loss of productivity in Sweden 1987 and 2005–a register-based approach. Diabet Med. 2009;26:928–934.
- Ringborg A, Martinell M, Stalhammar J, et al. Resource use and costs of type 2 diabetes in Sweden – estimates from population-based register data. Int J Clin Pract. 2008;62:708–716.
- Steen Carlsson K, Andersson E, Lundquist A, et al. Påverkbara kostnader för typ 2-diabetes år 2020 och år 2030 i Sverige. Prognoser med IHE Cohort Model of Type 2 Diabetes. 2015. Available from: https://ihe.se/publicering/paverkbara-kostnader-typ-2-diabetes-ar-2020-och-ar-2030-sverige-prognoser-med-ihe-cohort-model-type-2-diabetes/ [Accessed 2018 April 12].
- Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590.
- DiBonaventura M, Lay AL, Kumar M, et al. The association between body mass index and health and economic outcomes in the United States. J Occup Environ Med. 2015;57:1047–1054.
- Li Q, Blume SW, Huang JC, et al. The economic burden of obesity by glycemic stage in the United States. Pharmacoeconomics. 2015;33:735–748.
- Sullivan PW, Ghushchyan V, Ben-Joseph RH. The effect of obesity and cardiometabolic risk factors on expenditures and productivity in the United States. Obesity. 2008;16:2155–2162.
- International Diabetes Federation (IDF) Clinical Guidelines Task Force. Global guideline for type 2 diabetes. 2012. Available from: https://www.idf.org/e-library/guidelines/79-global-guideline-for-type-2-diabetes. [Accessed 2018 April 12].
- American Diabetes Association. Standards of medical care in diabetes - 2019. Diabetes Care. 2019;2:S1–S186.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701.
- National diabetes register. Available from: https://www.ndr.nu/#/knappen. [Accessed 2018 April 12].
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589.
- Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endo. 2017;5:251–260.
- Ahren B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endo. 2017;5:341–354.
- Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Dia Care. 2018;41:258–266.
- Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endo. 2017;5:355–366.
- Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291–2301.
- Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endo. 2018;6:275–286.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844.
- Lundqvist A, Steen Carlsson K, Johansen P, et al. Validation of the IHE cohort model of type 2 diabetes and the impact of choice of macrovascular risk equations. PLoS One. 2014;9:e110235.
- Kiadaliri AA, Gerdtham UG, Eliasson B, et al. Cost-utility analysis of glucagon-like peptide-1 agonists compared with dipeptidyl peptidase-4 inhibitors or neutral protamine hagedorn basal insulin as add-on to metformin in type 2 diabetes in Sweden. Diabetes Ther. 2014;5:591–607.
- Steen Carlsson K, Persson U. Cost-effectiveness of add-on treatments to metformin in a Swedish setting: liraglutide vs sulphonylurea or sitagplitin. J Med Econ. 2014;17:658–669.
- Ericsson A, Lundqvist A. Cost effectiveness of insulin degludec plus liraglutide (IDegLira) in a fixed combination for uncontrolled type 2 diabetes mellitus in Sweden. Appl Health Econ Health Policy. 2017;15:237–248.
- SCB. Consumer price index for health. Available from: http://www.scb.se/en_/Finding-statistics/Statistics-by-subject-area/Prices-and-Consumption/Consumer-Price-Index/Consumer-Price-Index-CPI/Aktuell-Pong/33779/Consumer-Price-Index-CPI/33907/. [Accessed 2018 April 12].
- TLV. 2015. Available from: https://www.tlv.se/download/18.467926b615d084471ac3367e/1510316392137/bes150522-trulicity.pdf. [Accessed 2018 April 12].
- European Medicines Agency. Dulaglutide (Trulicity®) summary of product characteristics. 2014. Available from: https://www.ema.europa.eu/documents/product-information/trulicity-epar-product-information_en.pdf. [Accessed 2018 April 12].
- Witkowski M, Wilkinson L, Webb N, et al. A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving basal insulin. Diabetes Ther. 2018;9:1233–1251.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124:136–145.
- Ward AJ, Salas M, Caro JJ, et al. Health and economic impact of combining metformin with nateglinide to achieve glycemic control: comparison of the lifetime costs of complications in the U.K. Cost Eff Resour Alloc. 2004;2:1–9.
- Willis M, Asseburg C, Nilsson A, et al. Multivariate prediction equations for HbA1c lowering, weight change, and hypoglycemic events associated with insulin rescue medication in type 2 diabetes mellitus: informing economic modeling. Value Health. 2017;20:357–371.
- Chaudhry ZW, Gannon MC, Nuttall FQ. Stability of body weight in type 2 diabetes. Diabetes Care. 2006;29:493–497.
- Jonsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with Type 2 diabetes in Sweden. Value Health. 2006;9:193–198.
- Brod M, Christensen T, Thomsen TL, et al. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health. 2011;14:665–671.
- Geelhoed-Duijvestijn PH, Pedersen-Bjergaard U, Weitgasser R, et al. Effects of patient-reported non-severe hypoglycemia on healthcare resource use, work-time loss, and wellbeing in insulin-treated patients with diabetes in seven European countries. J Med Econ. 2013;16:1453–1461.
- Statistics Sweden. Salary structures, whole economy, Average monthly salary 2014–2016. Available from: http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__AM__AM0110__AM0110C/LonSNIKon/table/tableViewLayout1/?rxid=11a7e405-b69f-406a-8271-a0159f766f9d. [Accessed 2018 April 12].
- Sorensen J, Ploug UJ. The cost of diabetes-related complications: registry-based analysis of days absent from work. Econ Res Int. 2013; Article ID 618039. Available from: http://dx.doi.org/10.1155/2013/618039
- Yu S, Schwab P, Bian B, et al. Use of add-on treatment to metformin monotherapy for patients with Type 2 diabetes and suboptimal glycemic control: a U.S. Database Study. JMCP. 2016;22:272–280.
- Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–3417.
- Home PD, Boulton AJM, Jimenez J, et al. Issues relating to the early or earlier use of insulin in type 2 diabetes. Pract Diab Int. 2003;20:63–71.
- Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study. Diabet Med. 2012;29:682–689.
- Dushay J, Abrahamson MJ. Insulin therapy for type 2 diabetes: making it work. J Fam Pract. 2010;59:E1–8.
- Johansen P, Hakan-Bloch J, Liu AR, et al. Cost-effectiveness of once-weekly semaglutide 1.0 mg vs. dulaglutide 1.5 mg as add-on to metformin in the treatment of type 2 diabetes in Canada. Diabetes. 2018;67.
- Viljoen A, Hoxer CS, Johansen P, et al. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21:611–621.
- Marso SP, Lindsey JB, Stolker JM, et al. Cardiovascular safety of liraglutide assessed in a patient-level pooled analysis of phase 2: 3 liraglutide clinical development studies. Diab Vasc Dis Res. 2011;8:237–240.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128.
- Niessner A, Tamargo J, Koller L, et al. Non-insulin antidiabetic pharmacotherapy in patients with established cardiovascular disease: a position paper of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur Heart J. 2018;39:2274–2281.
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20:S5–S26.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257.

