Abstract
Aims: The objective of this feasibility study was to determine the extent to which data from randomized controlled trials (RCTs) may serve as a useful source for collecting health care resource use (HCRU) for the purposes of estimating costs of managing adverse events (AEs), specifically, grade 3–4 nausea and thrombocytopenia, which may be experienced during chemotherapy treatment.
Materials and Methods: The feasibility study was conducted in four steps: (1) HCRU data were extracted from patient narratives in four phase 3 RCTs in non–small cell lung cancer; (2) missing HCRU data were imputed; (3) unit costs were applied to the resulting HCRU data set and costs of managing AEs were estimated; and (4) the overall utility of using RCT data as a source for estimating costs of AEs was evaluated.
Results: 33 nausea and 68 thrombocytopenia AEs met eligibility criteria and were evaluated in this study. Medication usage was recorded as a treatment in 76% of nausea AEs, although only 14% of the instances of medication usage included the minimum data elements required for costing. Platelet transfusions were provided in 24% of thrombocytopenia AEs; however, in only one instance were the minimum data elements recorded. Of nausea and thrombocytopenia AEs, 18% and 72%, respectively, required no missing data assumptions or imputation.
Limitations: Only two AEs were considered, and they may not be representative of all AEs in terms of suitability for use in estimating HCRU and costs of managing AEs. Not all grade 3–4 AEs met the criteria for requiring a patient narrative. HCRU data in the narratives were incomplete.
Conclusions: The usefulness of RCTs for estimating the costs of AEs may be improved by using a standardized form to collect HCRU data for key AEs, including an appropriate level of detail required to estimate costs of managing the AEs.
Introduction
Cancer treatments commonly cause adverse events (AEs). Newer treatments that have a lower frequency of severe treatment-related AEs provide opportunities to improve patient quality of life and reduce costs, which is a reason AEs often are included in economic evaluations of cancer treatments. The Second Panel on Cost-Effectiveness in Health and Medicine included AEs in its impact inventory as an example of other health effects that should be considered in a cost-effectiveness analysisCitation1. The National Institute for Health and Care Excellence (NICE) notes that technology appraisals should identify health outcomes that are important to patients, such as health benefits and adverse effectsCitation2, although NICE does not specifically mention the costs of adverse effects. Using lung cancer as an example, many economic evaluations of treatments incorporated the costs of AEsCitation3–9. In some economic analyses, AEs are a cost driverCitation8,Citation9; in others, AEs are not a substantial component of costsCitation3–7. This may reflect the actual distribution of costs, but it also may reflect the methods and data sources used to estimate AE costs.
In modeled economic evaluations of new treatments, often the incidence rates of individual AEs are obtained from randomized controlled trials (RCTs). Generally, in a model, the incidence rate is treated like a probability and multiplied by the cost of managing an instance of the AE to get a treatment-specific expected cost of managing the AE. In RCTs, typically there are prespecified criteria and processes for defining, identifying, and reporting AEs; however, in modeled economic evaluations, the sources of data and approaches for estimating the cost to manage an AE vary. These include identifying the cost to manage an AE from the published literature and, if applicable, inflating and/or converting it so that it reflects the desired cost-year, currency, and health care system, sometimes without consideration of changes in practice patterns over time or differences across countries that may affect the underlying assumptions about health care resource use (HCRU). Another approach involves identifying the HCRU required to manage an instance of the AE and applying unit costs or reference costs to generate a total expected cost to manage the AE. The estimates of the HCRU data sometimes are based on assumptions, perhaps informed by one or more expert clinicians, or developed using a more formal approach such as a Delphi panel to derive consensus-based estimates of HCRU. In other models, a separate database analysis may be conducted to generate real-world estimates of the HCRU and costs to treat an AE, although these estimates may not necessarily be directly applicable to the target patient population for a new treatment, or relevant to the payer or provider evaluating the new treatment’s economic value. Although clinical trial data are not generally used for the purpose of estimating costs of AEs, there may be an opportunity to leverage the significant amount of information collected in association with AEs during RCTs to estimate the HCRU and costs of AEs.
The objective of this feasibility study was to determine the extent to which clinical trial data may serve as a useful source for collecting HCRU for the purposes of estimating costs of managing AEs (specifically, grade 3–4 nausea and thrombocytopenia) during cancer treatment. Specifically, we sought to use patient narratives in relation to applicable adverse events coded using the United States (US) Department of Health and Human Services Common Terminology Criteria for Adverse Events, Version 5.0Citation10, to estimate such costs.
Methods
The feasibility study was conducted in four steps: (1) HCRU data were extracted from patient narratives; (2) missing HCRU data were assessed and imputed; (3) unit costs were applied to the resulting HCRU data set and costs of managing AEs were estimated; and (4) the overall utility of using RCT data as a source for estimating costs of AEs was evaluated. HCRU data were extracted from patient narratives, regardless of treatment arm, documented in a convenience set of four large phase 3 RCTs in patients with non–small cell lung cancer (NSCLC) sponsored by Eli Lilly and Company (Lilly): PARAMOUNTCitation11, REVELCitation12, PRONOUNCECitation13, and PROCLAIMCitation14. Information from the patient narratives used in this feasibility study was de-identified and coded. The data were not collected for the purposes of this feasibility study and did not allow the feasibility study personnel to ascertain the identity of the subjects for whom the patient narratives were developed. Therefore, this feasibility study was considered by the Institutional Review Board of RTI International to not constitute human subject research.
Nausea and thrombocytopenia were selected a priori for this feasibility study because they are relatively common AEs in cancer care that may be expected to require different resources for their management. HCRU data were extracted for those nausea and thrombocytopenia AEs that required submission of patient narratives, which were a subset of all nausea and thrombocytopenia AEs. Patient narratives were included in this feasibility study if at least one HCRU associated with an AE was reported.
All HCRU data, including medications, emergency department (ED) visits, inpatient hospitalizations, laboratory tests, medical procedures, and blood products, were extracted from the patient narratives. Specific rules for the extraction of HCRU variables were applied to ensure consistency between reviewers. All narratives underwent double data extraction; particularly complex narratives were reviewed by a medical oncologist to confirm appropriate interpretation of information.
To assess the suitability for costing purposes of the data extracted from patient narratives, the percentage of AEs eligible for costing that contained HCRU was reported for each AE. Essential information required for costing each HCRU was pre-specified. Essential information required for costing medications used for nausea were defined as medication strength, frequency, formulation, and prescription duration. Essential information required for costing platelet transfusions included number of units transfused and type of platelets transfused. For the purposes of costing hospitalizations, essential information required for costing purposes included an admission date and a discharge date. Essential information for the purposes of costing other resources mentioned in the narratives (e.g. ED visit) were similarly pre-specified. For medications, platelet transfusions, and inpatient hospitalizations, which were the most commonly described HCRU in the patient narratives, the extent of missing data was documented.
Once the data were extracted from the narratives and the extent of missingness was evaluated, all missing data were addressed using a series of rules, assumptions, and imputation approaches. For example, in the case of partial HCRU data (e.g. hospital admission date but no discharge date), efforts were made to obtain the missing data based on other information in the patient narrative (e.g. date of discharge assumed equal to date of death if a patient died while in the hospital) to ensure completeness of data. If medication details were not reported, desktop research was conducted to determine from the package insert the appropriate formulation, strength, frequency, and duration of use. If number of platelet units per transfusion was not recorded, the mean number of units for those AEs that did provide number of units was assumed. Detailed rules, assumptions, and data imputation methods are presented in the Supplemental Material (Exhibit 1).
Once a complete data set of HCRU was generated, unit costs were applied to generate cost estimates for each AE. Unit costs were drawn from publicly available data sources and adjusted for medical inflation to 2017 US dollars (USD) using the consumer price indexCitation15. Specifically, medications were costed using data from RedBookCitation16, inpatient hospitalizations from Health Care Cost and Utilization ProjectCitation17; ED visits from a special report from the Centers for Disease Control and PreventionCitation18; and outpatient visits and procedures from the Centers for Medicare and Medicaid Services, including the Physician Fee ScheduleCitation19, Medicare Fee-for-Service Hospital Outpatient Prospective Payment System Ambulatory Payment CodesCitation20, and the Clinical Laboratory Fee ScheduleCitation21 (Supplementary Table S1). Costs were estimated for each AE for each patient, and AE costs were summarized using descriptive statistics for each AE in total and by grade. Finally, to form the basis of an evaluation of the effect of imputation on the costs of the AEs, a comparison was made of the costs of hospitalizations with complete data required for costing available from the patient narratives and costs of hospitalizations estimated using the dataset including imputed HCRU.
Results
HCRU data from patient narratives and extent of missingness
Of all grade 3–4 AEs in all four RCTs, 34% met inclusion criteria for this feasibility study (). Of the eligible AEs, 14% were excluded because the patient narrative failed to mention any HCRU to manage the AE. A total of 101 events were included in this study (n = 33 for nausea and n = 68 for thrombocytopenia). All nausea AEs were recorded as grade 3. Of thrombocytopenia AEs, 42 were grade 3; 26 were grade 4.
Figure 1. Flow diagram for number of grade 3–4 adverse events costed, by adverse event. HCRU: health care resource use.
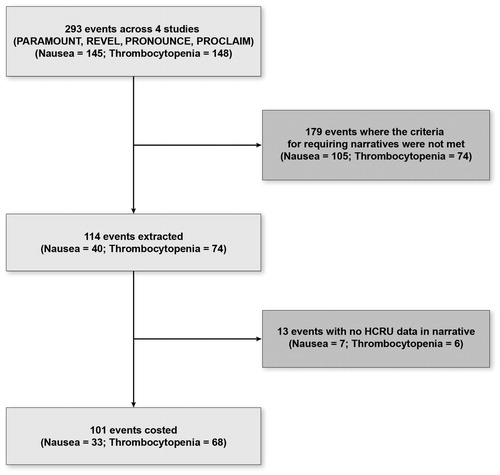
Hospitalizations were recorded among 79% of grade 3 nausea AEs () and 19% of grade 3–4 thrombocytopenia AEs (). Medication usage was recorded as a treatment in 76% of the grade 3 nausea AEs, although in those instances only 14% of narratives included the minimum data elements (medication formulation and prescription duration) required for medication costing (). Although the medication start date was nearly always provided, the end date was missing in 21% of instances (Supplementary Table S2). Platelet count assessments were recorded in 97% of narratives for grade 3–4 thrombocytopenia (). Platelet transfusions were provided in 24% of AEs; however, in only one instance were the minimum data elements (number of units and type of platelets) required for transfusion costing recorded (). Of the nausea AEs, 6 (18%) required no missing data assumptions or imputation. None of the six included were narratives where medication use was recorded. Of the thrombocytopenia AEs, 49 (72%) required no imputation for missing data. Four included HCRU other than only laboratory assessments for platelet count.
Table 1. Percentages of AEs eligible for costing with information recorded in patient narratives pertaining to key HCRU: nausea.
Table 2. Percentages of AEs eligible for costing with information recorded in patient narratives pertaining to key HCRU: thrombocytopenia.
Table 3. Extent of known missing data for medication use: nausea.
Table 4. Extent of known missing data for platelet transfusions: thrombocytopenia.
The average number of medications prescribed for a grade 3 nausea AE was 2.3 (77 medications for 33 AEs). Of the 77 records of medications prescribed for nausea, only one record indicated strength, and only one indicated frequency. Of the 25 unique medications, 16 (64%) are available, per the RedBookCitation16, in multiple formulations (e.g. oral, intravenous, patch). Duration of the prescription (noted by start and end date) for 57% (44 of 77) of instances was available (). The percentage of records of medications where formulation and duration were both available (i.e. a semi-complete set of data to facilitate costing) was 14% (11 of 77).
The most commonly recorded HCRU for grade 3–4 thrombocytopenia was a laboratory assessment for platelet count (208 assessments for 68 AEs, average 3.06 per AE). Of the 16 grade 3-4 thrombocytopenia AEs that required a platelet transfusion, 27 transfusions were provided (average of 1.69 transfusions per AE requiring a transfusion).
Three patients received medication for thrombocytopenia. Of those three patients, medication start date and end date were provided for two patients, and only medication end date was provided for the other patient. For medications prescribed for thrombocytopenia, no other information was provided (e.g. strength, frequency).
The average number of hospitalizations incurred for a grade 3 nausea AE was 0.8 (27 hospitalizations for 33 AEs). For grade 3–4 thrombocytopenia, the average number of hospitalizations was 0.2 (13 hospitalizations for 68 AEs). Of the 27 unique hospitalizations for grade 3 nausea, 22 (81.5%) included both admission and discharge dates, which facilitated the calculation of length of stay (LOS) without requiring the use of imputed data (). For the 18 unique hospitalizations for grade 3–4 thrombocytopenia, 8 (61.5%) included both admission and discharge dates.
Table 5. Extent of missing data for duration of hospitalization.
Costs of nausea and thrombocytopenia from this feasibility study
Based on descriptive analyses of the complete HCRU data set, with unit costs applied to counts of each HCRU, the mean cost to treat an event of grade 3 nausea was $1,364.36 (standard deviation [SD] = $2,615.50) (), excluding hospitalization costs, and $13,684.11 (SD = $12,745.16) including hospitalization costs. The mean costs to treat an event of grade 3 thrombocytopenia, excluding and including hospitalization costs, were $78.40 (SD = $227.90) () and $3,192.22 (SD = $10,003.50), respectively. For grade 4 thrombocytopenia events, excluding and including hospitalization costs, costs were $1,480.33 (SD = $2,071.41) and $5,690.64 (SD = $8,636.67). Thrombocytopenia grade 4 events were on average $1,402 more costly than grade 3 events excluding hospitalizations and $2,498 more costly than grade 3 events including hospitalization costs. The mean costs to treat an event of grade 3–4 thrombocytopenia combined, excluding and including hospitalization costs, were $614.44 (SD = $1,450.47) () and $4,147.50 (SD = $9,516.61), respectively.
Table 6. Costs to treat nausea (all events recorded as Grade 3), per adverse event (2017 USD), by resource use component.
Table 7. Costs to treat grade 3–4 thrombocytopenia per event (2017 USD), by resource use component.
Hospitalization HCRU and cost estimates before and after addressing missing data
The LOS per hospitalization for grade 3 nausea including imputed data (n = 27) was 6.2 days (range, 2–22); excluding imputed data (n = 22), the average LOS per hospitalization was 6.9 days (range, 2–22). The average LOS per hospitalization for grade 3–4 thrombocytopenia including imputed data (n = 13) was 7.6 days (range, 3–22); excluding imputed data (n = 8), the average LOS per hospitalization was 7.4 days (range, 3–22). For nausea, the expected cost of hospitalization including imputed data was $15,057 and excluding imputed data was $16,680 (). Conversely, for thrombocytopenia, the cost estimate including imputed data was higher ($18,481) than the cost estimate excluding imputed data ($17,861).
Table 8. Impact of imputing missing data for hospitalization.
Discussion
Of the publications that included costs of AEs mentioned previouslyCitation3–9, only Hess et al.Citation8 provided costs for managing nausea and thrombocytopenia, which were used in a modeled cost-effectiveness analysis. Hess et al.Citation8 assumed that grade 3–4 AEs required hospitalization and assumed a cost per nausea event of $6,225 and for thrombocytopenia of $18,354 (inflated to 2017 US dollarsCitation15) based on hospitalization cost data from HCUP.netCitation17.
In this study using patient narratives, based on descriptive analyses of the complete HCRU data set, with unit costs applied to counts of each HCRU, the mean cost to treat an event of grade 3 nausea was $13,684 and the mean cost to treat an event of grade 3–4 thrombocytopenia was $4,148 when including hospitalization costs. It is important to note that the set of AEs considered in this feasibility study does not reflect the full set of all AEs, being limited to the subset of those with narratives, which reflect the more severe grade 3–4 AEs. These results should not be assumed to be cost estimates for all AEs. Our cost for a hospitalization for nausea is higher and for thrombocytopenia was similar to the estimate provided in Hess et al.Citation8; however, there was documentation of hospitalization in only 79% of patients who experienced nausea and 19% of patients who experienced thrombocytopenia in our study.
Patient narratives from RCTs provide a data source potentially useful as a basis for estimating HCRU and costs of treating AEs; however, substantial limitations exist with the use of RCT data as a source for HCRU required to manage AEs. A limitation of using RCT-based HCRU to estimate costs, generally, is that RCT-based costs are often protocol-driven. The extent to which RCT protocols influence the management of AEs experienced in clinical trials is unknown. In addition, although predefined close follow-up of AEs in an RCT in principle could be useful to estimate the costs of AEs, RCT protocols rarely prespecify collection of adequate information to estimate such costs. Furthermore, similar to HCRU data collected in phase 3 RCTs, attributing drug or other resource use to a particular AE from narratives is complicated and cannot always be done with certainty, making it difficult to develop cost estimates for specific AEs.
There are several limitations in this feasibility study. First, only two AEs were considered, and they may not be representative of all AEs in terms of the suitability of information recorded in patient narratives for use in estimating HCRU and associated costs of managing AEs. Additionally, not all grade 3–4 AEs met the criteria for requiring a patient narrative; therefore, not all AEs could be considered when assessing AE HCRU and costs. According to guidelines developed by the International Council on Harmonisation (ICH), a brief patient narrative should be developed describing each death, other serious adverse event, and other significant adverse event that is judged to be of special interest because of clinical importance. Events that were clearly unrelated to the test drug/investigational product may be omitted or described very briefly. In general, the narrative should describe the following: the nature and intensity of event; the clinical course leading up to the event, with an indication of timing relevant to test drug/investigational product administration; relevant laboratory measurements; whether the drug was stopped, and when; countermeasures; post-mortem findings; investigator’s opinion on causality and sponsor’s opinion on causalityCitation22.
Second, for those AEs for which a narrative was developed, perhaps because estimating HCRU and costs generally are not clinical trial objectives, HCRU data in the narratives were incomplete or not well documented; although, we found that the inclusion of imputed data made little difference to the results. Third, there were a few records (five for thrombocytopenia and eight for nausea) with no HCRU recorded. As it seems unlikely that AEs would require no HCRU, these AEs were excluded from the analysis and the decision was made to include in the analysis only patient narratives if at least one HCRU associated with an AE was reported. Additionally, an extensive amount of missing data was observed for medications used to treat nausea. For example, dosing frequencies (e.g. twice daily) were not recorded in the patient narratives (other than for one record, which indicated dosing should occur as needed). This may be because dose frequency might be regarded as unnecessary detail by someone writing the narrative given that many medications are prescribed on a particular schedule routinely. The same rationale would seem to be less compelling in explaining why 43% of records did not indicate the duration of the prescription for the medication, which is essential for costing purposes. Finally, our cost estimates assumed a cost per day based on the AE as the primary discharge diagnosis but there could have been additional reasons why patients were hospitalized. A larger study may be required to look at effects on hospital costs from additional diagnoses. Similarly, although the patient narratives pertained to AEs, an assumption was made that any HCRU recorded in the narrative should be attributed to the AE, when that may not have been the case. For example, in some instances, a medication not approved for treatment of nausea was recorded in the narrative for a nausea AE. Although each of these instances was reviewed by a clinical advisor, a decision was taken not to exclude any medication recorded in the narrative for an AE.
In international, multisite RCTs, there may be substantial differences in local strategies for treating AEs, practices for deciding when to admit a patient to the hospital, and availability of medications, any of which limit the generalizability of HCRU across regions. These same differences, as well as different cost approaches, such as costing inpatient hospitalization length of stay, can contribute to differences in costs across markets beyond differences in HCRU used for similar AEs. Furthermore, questions of generalizability of clinical trial data to a real-world population are applicable to HCRU data recorded in a clinical trial.
Systematically collected AE-related HCRU data in a structured format, such as a HCRU questionnaire for key AEs, may be a more fruitful approach than relying on HCRU data that may be recorded in patient narratives. Additionally, the questionnaire would need to address attribution of HCRU (e.g. whether the AE led to or intensified an inpatient hospitalization). In particular, the study questionnaire would need to note when AEs were co-occurring, recognizing that use of an HCRU (e.g. an inpatient hospitalization) may have been attributed to multiple AEs. If a standard reporting form, including clear start and end dates for use of HCRU for a particular AE, were incorporated into clinical trial data collection forms, it may provide the research team with a more complete dataset.
Conclusion
The usefulness of RCTs for collecting HCRU data to better understand the costs of AEs may be improved by using a standardized form to collect HCRU data for key AEs, including an appropriate level of detail required to estimate costs of managing the AEs (e.g. start and end date of drugs used to treat the AE, inpatient hospitalization LOS, and number of follow-up visits required until AE resolution).
Transparency
Declaration of funding
RTI Health Solutions received funding under a research contract with Eli Lilly and Company to conduct this study.
Declaration of financial/other relationships
D.M., C.L.B., and A.N. have disclosed that they are employees of RTI International, with whom Eli Lilly and Company contracted to complete this research. L.H., Y.-J.H., Z.L.C., and L.B. have disclosed that they are employees of Eli Lilly and Company. J.M.E. peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have made substantial contributions to the study design, analysis, and interpretation of the results of this feasibility study and have contributed to and are wholly responsible for the content presented herein.
Acknowledgements
The authors would like to thank Rebecca Conroy, Matthew Lyall, Mackenzie Neighbors, and Weyinmi Nuabor for their aid in data extraction and James A. Kaye, MD, DrPH, for his guidance in interpreting complex patient narratives.
Supplemental Material
Download MS Word (29.4 KB)References
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal National Institute for Health and Care Excellence. 2013. [cited 2019 Mar 11]. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
- Zuluaga-Sanchez S, Hess LM, Wolowacz SE, et al. Cost-effectiveness of olaratumab in combination with doxorubicin for patients with soft tissue sarcoma in the United States. Sarcoma. 2018;2018:1.
- Graham C, Knox H, Hess LM, et al. Cost-effectiveness in the second-line treatment of non-small cell lung cancer (NSCLC) in the US. Value Health. 2015;18(7):A457–A458.
- Kumar G, Woods B, Hess LM, et al. Cost-effectiveness of first-line induction and maintenance treatment sequences in non-squamous non-small cell lung cancer (NSCLC) in the US. Lung Cancer. 2015;89(3):294–300.
- Wen F, Zheng H, Zhang P, et al. OPTIMAL and ENSURE trials-based combined cost-effectiveness analysis of erlotinib versus chemotherapy for the first-line treatment of Asian patients with non-squamous non-small-cell lung cancer. BMJ Open. 2018;8(4):e020128.
- Huang M, Lou Y, Pellissier J, et al. Cost-effectiveness of pembrolizumab versus docetaxel for the treatment of previously treated PD-L1 positive advanced NSCLC patients in the United States. J Med Econ. 2017;20(2):140–150.
- Hess LM, Rajan N, Winfree K, et al. Cost analyses in the US and Japan: a cross-country comparative analysis applied to the PRONOUNCE trial in non-squamous non-small cell lung cancer. Adv Ther. 2015;32(12):1248–1262.
- Lewis G, Peake M, Aultman R, et al. Cost-effectiveness of erlotinib versus docetaxel for second-line treatment of advanced non-small-cell lung cancer in the United Kingdom. J Int Med Res. 2010;38(1):9–21.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. November 27, 2017. [cited 2019 Apr 18]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247–255.
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673.
- Zinner RG, Obasaju CK, Spigel DR, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients with advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2015;10(1):134–142.
- Senan S, Brade A, Wang LH, et al. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. JCO. 2016;34(9):953–962.
- Bureau of Labor Statistics (BLS). Inflation & prices: medical care services in U.S. city average, all urban consumers, not seasonally adjusted. 2017. [cited 2017 Oct 12]. Available from: https://www.bls.gov/data/.
- Red Book Online [database on the Internet]. Ann Arbor, MI: Truven Health Analytics; 2017. [cited 2017 Dec 20]. Available from: http://www.micromedexsolutions.com/. Subscription required to view.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. [cited 2018 Feb 20]. Available from: https://hcupnet.ahrq.gov/.
- Centers for Disease Control and Prevention (CDC). Health, United States, with special feature on emergency care (Data table for Figure 29), 2012. [cited 2017 Dec 27]. Available from: http://www.cdc.gov/nchs/data/hus/hus12.pdf.
- Centers for Medicare & Medicaid Services (CMS). Physician fee schedule. 2017. [cited 2017 Dec 27]. Available from: https://www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- Centers for Medicare & Medicaid Services (CMS). Addendum B. 2018a. [cited 2017 Dec 27]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates.html?DLSort=2&DLEntries=10&DLPage=1&DLSortDir=descending.
- Centers for Medicare and Medicaid Services (CMS). Clinical Laboratory Fee Schedule. 2018b. v1. [cited 2017 Dec 28]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html.
- ICH E3. Guideline for Industry: Structure and Content of Clinical Study Reports. 1996. [cited 2018 Nov 19]. Available from: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073113.pdf.
