Abstract
Background: Adults admitted to hospital with community-acquired pneumonia (CAP) impose significant burden upon limited hospital resources. To achieve early response and possibly early discharge, thus reducing hospital expenditure, the choice of initial antibiotic therapy is pivotal.
Methods: A cost-consequences model was developed to evaluate ceftaroline fosamil (CFT) as an alternative to other antibiotic therapies (ceftriaxone, co-amoxiclav, moxifloxacin, levofloxacin) for the empiric treatment of hospitalized adults with moderate/severe CAP (PORT score III–IV) from the perspective of the Spanish National Health System (NHS).
Findings: Compared with ceftriaxone, the model predicted an increase in the number of CFT-treated patients discharged early (PDE) (30.6% vs. 26.1%) while decreasing initial antibiotic failures (3.8% vs. 7.6%). For patients with pneumococcal pneumonia, CFT was cost-saving vs. ceftriaxone (by 1.2%) and significantly increased PDE (32.1% vs. 24.6%). CFT resulted in cost-saving vs. levofloxacin, due lower initial antibiotic therapy costs and increased PDE (30.6% vs. 14.9%). Moxifloxacin and co-amoxiclav early response rate of 53.63% and 54.24% resulted in cost neutrality vs. CFT, with direct comparison hampered by the significantly different early response criteria utilized in the literature.
Conclusions: Despite a higher unit cost, CFT is a reasonable alternative to other agents for adults hospitalized with moderate/severe CAP, given the projected higher PDE achieved with similar or lower total costs.
Introduction
Community-acquired pneumonia (CAP) remains an acute infection with significant associated morbidity, mortality and economic burdenCitation1–4, particularly prevalent in the elderly. Indeed, within an estimated European incidence of 0.2–242 per 1,000 adults/year, significantly higher rates are observed in the elderly populationCitation3,Citation4. Accordingly, in the US, 1.3 million cases of CAP were estimated to have occurred in adults ≥65 years in 2012Citation5. With the globally aging population, the incidence and burden of CAP is therefore set to increase even furtherCitation3,Citation6,Citation7. One study in primary care showed an incidence of CAP in Spain that was in the range 4.63/1,000 people/yearCitation4. The incidence in people older than 65 years was higher in men, with a range of 7.06–36.93 compared to 5.43–19.62 in women.
Although mild disease can be managed in the community, a significant proportion of patients with comorbidities, such as metabolic or cardiovascular disease, or more severe CAP will require hospitalizationCitation8. The medical costs associated with each episode of inpatient care for CAP was estimated to exceed $18,000 in the US in 2012, which equated to an overall annual burden above $13 billionCitation5,Citation6. Similarly, in Europe, the estimated inpatient care direct costs amount to €5.7 billion per year with a further estimated €3.6 billion of indirect costs due to productivity lossCitation3. Given that the main determinants of overall costs are in-patient treatment, hospital length of stay and the need for intensive careCitation3,Citation9, effective treatment which can achieve rapid clinical cure and enable early discharge are therefore highly desirable. Furthermore, the rising concern of antibiotic resistance, which has been observed in all pathogens associated with CAPCitation3, also necessitates alternative effective treatments.
A series of well-established guidelines govern the treatment strategy, with the aim of reducing unnecessary hospitalization and readmissions, length of hospital stays, mortality and in turn costCitation10–12. Upon admission, most patients are treated empirically with antibiotics selected according to disease severity, local patterns of bacterial resistance and safety profilesCitation1. As initial antibiotic treatment failure has been shown to prolong hospital stay, increase antibiotic usage and add to hospital expenditure, this is a pivotal decisionCitation13. The most common antibiotic approach involves monotherapy with a respiratory fluoroquinolone or combination therapy with a macrolide plus a β-lactamCitation14,Citation15.
Ceftaroline fosamil (CFT) is a fifth-generation cephalosporin with a broad spectrum of antibacterial activity. In an integrated analysis of the two pivotal, multicenter, phase III RCTs trials (FOCUS 1 and FOCUS 2), CFT treatment was non-inferior to ceftriaxone in adults with CAP (Pneumonia Outcomes Research Team (PORT) risk class III or IV)Citation16. Specifically, CFT demonstrated numerically higher clinical cure rates than ceftriaxone in the clinically evaluable population both in the individual trials (FOCUS 1: 86.6% vs. 78.2%, FOCUS 2: 82.1% vs. 77.2%) and in the combined analysis (84.3% vs. 77.7%)Citation16. Furthermore, when the trials data were analyzed focusing on the early response (Day 4, 72–96 h, CFT appeared to provide clinical benefit over ceftriaxone at Day 4, with Day 4 clinical response rates of 69.5% vs. 59.4%Citation17. In addition, real-world studies have shown CFT to be effective, irrespective of whether it was used as monotherapy or concurrent therapyCitation18, first- or second-line therapyCitation18,Citation19, in elderly patientsCitation18,Citation20 or in patients with significant comorbiditiesCitation20.
As newer agents, such as CFT, become available, the number of treatment options open to clinicians, will increase. However, given the rising concern of national health system expenditures, decisions need to be made based on economic value as well as clinical benefit. Focusing on Spain, between 2004 and 2013, the hospitalization rates for CAP in Spain increased significantly from 142.4 to 163.87 cases/100,000 inhabitantsCitation21. This study was therefore undertaken to evaluate intravenous (IV) CFT (600 mg q12hr) as an alternative to available IV antibiotic therapies for the empiric treatment of hospitalized patients with moderate/severe CAP from the perspective of the Spanish National Health System.
Methods
Model structure
A decision-tree model was developed in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA), to simulate the clinical pathway of a hypothetical cohort of adult patients admitted to hospital in Spain with moderate/severe CAP (PORT score III–IV) tracking the costs and consequences of treatments as shown in . Upon admission, the initial decision was to either treat with CFT 600 mg every 12 h (q12hr) or an alternative antibiotic. The alternative antibiotics included in the analysis were ceftriaxone 1–2 g every 24 h (q24h), co-amoxiclav 1.2 g every 8 h (q8h), moxifloxacin 400 mg q24h or levofloxacin 0.5–1 g q24h, based on CAP guidelines and clinical expert consultationCitation10–12.
Figure 1. Schematic visualization of the decision-tree model. Over each branch, a brief description of the corresponding patient pathway has been summarized. Abbreviations. CAP, community-acquired pneumonia; ICU, intensive care unit; IV, intravenous.
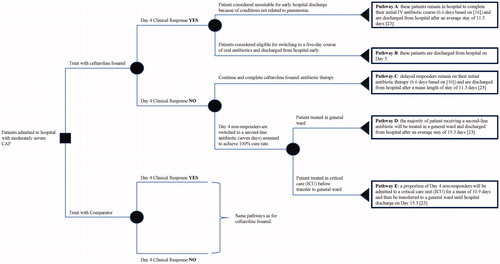
Patients initially receive intravenous (IV) antibiotic therapy for four days before clinical and microbiological assessment. After 4 days, responding patientsCitation17 will either switch to a 5-day course of oral antibiotics and be discharged early, or remain in hospital due to conditions unrelated to pneumonia (e.g. unable to swallow), and continue to receive IV antibiotic therapyCitation22. Non-responders after 4 days will either remain on IV therapy until clinical cure and discharge or switch to a second-line antibiotic (e.g. ceftriaxone) until clinical cure and dischargeCitation23. The non-responders switched to a second-line antibiotic could either be treated in the general ward or in an intensive care unit (ICU) and then switched to the general ward.
This model structure was considered most appropriate since costs and clinical outcomes (resolution of signs and symptoms of the infection) occur over a relatively short period of time, typically <3 weeks from hospital admission to dischargeCitation24.
Model inputs
Clinical efficacy inputs
The clinical inputs used in the cost-effectiveness analyses are summarized in . The clinical cure rate for CFT was obtained from an integrated analysis of phase III trialsCitation16, and in the absence of head-to-head data, the clinical cure rates for the other antibiotics were estimated from a published network meta-analysis (NMA)Citation25. Accordingly, the relative risk (RR) of CFT versus ceftriaxone, co-amoxiclav, levofloxacin and moxifloxacin were reported to be 1.10 (95% confidence intervals [Cl] 1.04–1.16), 1.18 (95% Cl 1.06–1.3), 1.05 (95% Cl 0.96–1.15) and 1.08 (95% Cl 0.99–1.16) respectively.
Table 1. Clinical inputs for the cost-effectiveness analysis.
Day 4 clinical response was based on criteria set by the FDA and was defined as meeting criteria for both clinical stability and clinical improvement on Day 4:
Clinical stability was defined according to the Infectious Diseases Society of America/American Thoracic Society guidelines as temperature <37.8 °C, heart rate <100 beats/min, respiratory rate <24 breaths/min, systolic blood pressure >90 mmHg, oxygen saturation >90% and confusion/disorientation (recorded as absent).
Clinical improvement was defined as improvement of at least one of four symptoms present at baseline (i.e. cough, dyspnea, pleuritic chest pain, sputum production) with worsening of none.
Patients who did not meet both criteria were considered non-responders.
Day 4 clinical response rates, sourced from a post hoc analysis of the FOCUS trials in the CE populationCitation17, were 69.5% for CFT and 59.4% for the ceftriaxone. Focusing on the pneumococcal pneumonia confirmed group, the clinical response rates at Day 4 were 73.0% in the CFT group and 56.0% in the ceftriaxone group. The values for the other comparators are summarized in along with the corresponding references.
It is important to notice that the moxifloxacin early response criteria utilized in the literature were significantly different to those utilized for CFT, with early response defined as just body temperature <37.5 °CCitation27. Similarly, early response criteria for co-amoxiclav were less stringent than the criteria used for CFT (improvement in two out of four CAP symptoms, clinical stability not required)Citation28. Therefore, comparison against moxifloxacin and co-amoxiclav was hampered by the lack of comparable early response data in the literature. Nevertheless, threshold analysis was performed for both co-amoxiclav and moxifloxacin to assess the level of early response rate at which cost-neutrality could be achieved with respect to CFT.
Economic inputs
Health care resource use and costs are shown in . The average hospital length of stay (LOS) for patients both responding to and failing on initial antibiotic therapy, as well as the associated admission rates to and duration of stays in ICU for the latter patients was obtained from a large observational study of CAP management in hospitalized patientsCitation23. A prospective observational study of 200 patients with CAP admitted to hospital provided the proportion of treatment responders (44%) eligible for iv-to-oral switch and early hospital dischargeCitation22. The duration of first-line antibiotic treatment was assumed as 6.6 days for CFT and all comparators, consistent with the duration observed in the CFT trialsCitation16. Ceftriaxone monotherapy was selected as the second line antibiotic of choice for patients treated in general wards, following clinical experts’ consultation, while a combination therapy (ceftriaxone + azithromycin) was deemed more suitable for ICU patients. The duration of second line antibiotic treatment was assumed to be 7 days, due to lack of reliable sources found in the literature but varied during the sensitivity analysis.
Table 2. Economic inputs for the cost-consequences analysis.
Ceftriaxone 1 g q24hr and levofloxacin 500 mg q24hr dosages were used for the base case as these were the doses used in the source clinical trials. However, higher doses are routinely needed in real life to achieve similar efficacy to that observed in clinical trials. Additional scenarios with ceftriaxone 2 g q24hr and levofloxacin 1 g q24hr were simulated to reflect the doses most commonly used in clinical practice. Drug and hospital resource costs were sourced from the Spanish national price list 2017.
Model assumptions
When building the decision-tree model, multiple assumptions had to be made to simplify the clinical pathways and allow data from multiple sources to be integrated. Firstly, the early response rate across trials was assumed to be comparable. Secondly, the duration of IV treatment in patients remaining in hospital beyond 4 days was assumed to be the same for CFT and all the comparators considered, due to the lack of treatment specific data published in the literatureCitation16. Thirdly, as the costs of initial microbiological testing, and concomitant antibiotics providing coverage against atypical pathogens were assumed to be the same in both treatment arms, these were not considered in the model in the base case (consistent with the clinical trial protocols from which the efficacy inputs have been derived). Nevertheless, one additional scenario was tested adding concomitant macrolide therapy (azithromycin 500 mg q24h – first 3 days IV then oral formulation for a daily cost of €7.31) to each beta-lactam treatment (ceftaroline fosamil, ceftriaxone, co-amoxiclav) as recommended in the latest clinical guidelines and likely happening in clinical practiceCitation14,Citation15.
Model analyses
The model predicted health outcomes such as percentage of patients discharged at Day 4, percentage of patients experiencing antibiotic treatment failure, and percentage of patients treated in the ICU, as well as incremental costs of CFT versus each of the comparators were included in the analysis. Furthermore, sub-group analysis focusing on the pneumococcal pneumonia confirmed group was performed for CFT versus ceftriaxone, given the high-mortality associated with this specific causative organismCitation3. Due to lack of data a pneumococcal subgroup analysis was not possible for the other comparators.
Deterministic one-way sensitivity analysis was performed to identify the primary sources of uncertainty in the model’s estimates of treatment costs and clinical outcomes. The model base-case inputs were varied within their 95% CIs, obtained from assigned statistical distributions as specified in and Citation2 (beta-distribution for probabilities, and lognormal distribution for relative risks) while the costs were varied by ±25%.
In additional, probabilistic sensitivity analysis (PSA) was undertaken to assess the impact of simultaneously varying clinical outcomes and health care resource use with respect to the model outcomes, using Monte Carlo simulation (1,000 simulations were performed).
Results
Compared to ceftriaxone-treated patients, a higher percentage of CFT-treated patients were predicted by the model to be eligible for early discharge (30.6% vs. 26.1%; ), and fewer were projected to experience initial antibiotic failure (3.8% vs. 7.6%) or require ICU treatment (0.9% vs. 1.9%) (see ). The predicted total average costs of CFT were €286.10 per patient higher than ceftriaxone (1 g q24hr), resulting in a 5% increase ( and ). Specifically, CFT patients were projected to have higher initial antibiotic drug costs and oral step-down costs versus ceftriaxone. Similar results were obtained when ceftriaxone 2 g q24hr dosage was compared, but the marginal increase in total costs between CFT and ceftriaxone was reduced to 4.6%. Simulating the combination of CFT and ceftriaxone with a macrolide marginally increased the total costs per patient (€5,917.13 and €5,684.98 respectively) compared to the base case results (see ) without impacting the incremental total costs per patient (since the same macrolide treatment was added to both comparators).
Figure 2. Comparison of total costs per patient (left side) and the percentage of patients discharged early (right side) for CFT, Ceftriaxone, and Levofloxacin. Abbreviations. CFT, ceftaroline fosamil; q12hr, every 12 hours; q24hr, every 24 hours
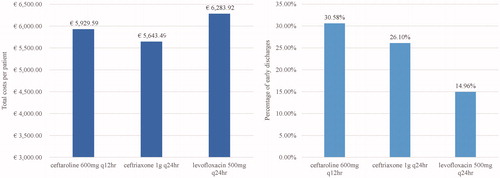
Table 3. Total and incremental percentage of patients, for each treatment pathway (according to ), predicted by the model.
Table 4. Total and incremental hospital costs per patient by antibiotic regimen and pneumonia type.
When the analysis was restricted to patients with pneumococcal pneumonia, CFT was estimated to be cost-saving versus ceftriaxone (1 g q24hr), with a total cost reduction of €54.63 per patient (). Despite higher projected initial antibiotic and oral antibiotic step-down costs, those associated with hospitalization and second-line antibiotics were predicted to be lower with CFT. More patients achieved initial antibiotic treatment success with CFT than ceftriaxone (63.97% versus 61.54%), and more CFT patients would be early discharged than ceftriaxone (32.1% versus 24.6%). Considering a higher dosage of ceftriaxone (2 g q24hr) resulted in higher cost-savings of €74.62 per patient while adding macrolide concomitant therapy to CFT and ceftriaxone did not impact the per-patient costs savings.
CFT was also predicted to be cost-saving overall versus levofloxacin 500 mg q24hr (€354.33 per patient), mainly ascribable to the relatively high Day 4 response rate which resulted in reduced length of hospital stay (). Indeed, in comparison with levofloxacin, CFT increased the percentage of early discharges (30.6% vs. 14.96%) and decreased initial antibiotic failures (4.8% vs. 13%) and number of patients treated in ICU (0.9% vs. 2.6%) (see ). Increasing levofloxacin dosage (1 g q24hr) increased total cost savings per patient, predicted by the model, to €425.68 while adding concomitant macrolide therapy to CFT reduced the cost savings per patient to €312.79.
Threshold analysis indicated that total cost neutrality with respect to CFT was achieved with an early response rate of 53.63% and 54.24% for moxifloxacin and co-amoxiclav respectively. Considering concomitant macrolide therapy with the beta-lactams (CFT and co-amoxiclav) marginally reduced moxifloxacin early response rate for achieving cost neutrality to 52.1% while had no impact on CFT/co-amoxiclav comparison.
Finally, consistently with the literature, the model demonstrated that antibiotic costs represent a small proportion of overall hospital costs (<11% of total costs), the main driver being hospital length of stay (>85% of total costs).
Sensitivity analyses
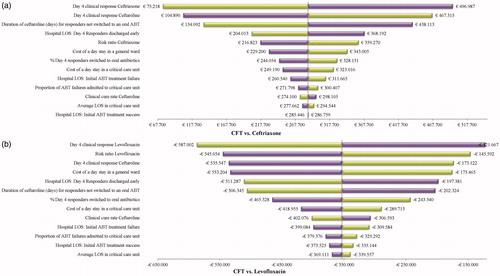
The deterministic sensitivity analyses, where each input parameter was varied separately as the lower and upper bound of the corresponding confidence interval (see ), demonstrated that day 4 responses, clinical cure rates and antibiotic treatment duration were the key drivers of total costs, when comparing CFT with ceftriaxone (see ). Similarly, the top-three parameters generating the most variation in total costs when comparing CFT with levofloxacin were day 4 responses and the risk ratio of clinical cure rates, as highlighted in . The full list of per patient incremental cost obtained setting each model parameter to the lower and upper bound of the confidence interval is presented in with respect to ceftriaxone and in with respect to levofloxacin.
Figure 3. Deterministic sensitivity analysis results: CFT 600 mg q12hr versus Ceftriaxone 1 g q24hr (a); CFT 600 mg q12hr versus Levofloxacin 500 mg q24hr (b). Abbreviations. ABT, antibiotic therapy; CFT, ceftaroline fosamil; LOS, length of stay; q12hr, every 12 hours; q24hr, every 24 hours.
Threshold analysis was repeated for moxifloxacin and co-amoxiclav using the lower and upper bounds of the risk ratio confidence intervals (see ). Varying the moxifloxacin and co-amoxiclav risk ratios to the lower and upper bounds of the confidence interval resulted in an early response rate of 48–57.2% and 48.3–58.1%, respectively, for achieving cost-neutrality with respect to CFT.
The probabilistic sensitivity analysis, during which the cost and clinical inputs were simultaneously varied by random sampling from the input distributions (see and Citation2) demonstrated that CFT reduced the total cost in 90.9% of the cases compared with levofloxacin and in 3% of the cases compared with ceftriaxone.
Discussion
With an aging population, the incidence of CAP and in turn the associated economic burden will continue to rise in the coming yearsCitation3. Given the rising amount of treatments available to clinicians and the fact that treatment decisions will be based upon both clinical and economic factors, this modelling study was undertaken to compare the cost and consequences of using CFT in hospitalized adults with moderate to severe CAP with respect to alternative antibiotics commonly used.
The greater early treatment success with CFT compared with ceftriaxone was associated with a reduction in costs for second line antibiotic and hospitalization. However, these cost savings were insufficient to offset the higher CFT drug acquisition and infusion costs; resulting in a marginal (<5%) increase in total costs versus ceftriaxone (1–2 g q24hr). Nevertheless, the model predicted that in responding patients more would be discharged early with CFT than ceftriaxone. Furthermore, in the pneumococcal pneumonia subgroup analysis, CFT treatment resulted cost-saving while significantly increasing the number of patients achieving initial antibiotic treatment success and early discharge. This would suggest that CFT may be preferred to ceftriaxone in hospitalized adults with suspected pneumococcal pneumonia, which is one of the main causative organisms of CAPCitation3.
When compared against levofloxacin, the model demonstrated that CFT would result in significant cost savings per patient, due to greater clinical efficacy resulting in decreased initial antibiotic treatment failures and increased percentage of early discharges.
The sensitivity analysis demonstrated that early response, treatment duration and length of hospitalization are the key drivers of total costs, which is consistent with what has been previously described in the literatureCitation13. Furthermore, the model demonstrates that antibiotic costs represent a small proportion of overall hospital costs, the main driver being hospital LOSCitation3,Citation13. This may be of benefit in focusing payers’ attention away from pharmacy spend alone towards a review of the overall economic burden of managing patients with moderately severe CAP in their hospital.
The developed model has multiple limitations. Firstly, except for ceftriaxone, there are no head to head clinical trials comparing CFT with the other antibiotics; thus, the clinical inputs were based on available literature (see ) and indirect comparison (NMA)Citation25 validated by clinical expert opinion. Furthermore, significantly different criteria used to measure early response data (Day 4) were observed in the literature, which hampered comparisons with moxifloxacin and co-amoxiclav (see Section 2.2.1). Secondly, the treatment length has been assumed equal between CFT and all the comparators (see ), given the lack of evidence in literature. Given that this parameter is one of the key driver of total costs, especially when comparing CFT with ceftriaxone (see ), further studies leveraging real-world evidence regarding average length of treatment for each of the considered comparators might be of interest. Thirdly, antibiotic dosage used in the clinical trials differs to the dosage used in clinical practice. Multiple scenarios with increased antibiotic dosage demonstrated a limited impact of the dosage variations with respect to the model outcomes and conclusions. However, the clinical efficacy was assumed to remain constant due to the lack of literature evidence. Fourthly, the unit costs used in the analysis (see ) could change in the future due to the introduction of generics and do not take into considerations confidential discounts provided by the manufacturers. Therefore, these two aspects along with other considerations (e.g. changes in recommended dosages) will dictate modified cost-consequences results. Finally, susceptibility rates were not considered in the analysis, which might be a critical factor in countries with high levels of emerging antibiotic resistanceCitation3,Citation29. Specifically, different types of causative organisms for CAP would have different susceptibility patterns with respect to each of the antimicrobial agents commonly usedCitation29, in turn affecting the clinical efficacy of each treatment. On one hand, this would require additional data regarding country-specific susceptibility patterns along with organism-specific clinical inputs for each comparator included in the analysis, thus significantly increasing the complexity of the analysis. On the other hand, this could be of significant interest for future analyses, given that the diverse nature of resistance across Europe highlights the need of developing national guidelines to provide optimal treatmentCitation3.
Conclusion
In conclusion, despite a higher unit cost, ceftaroline fosamil is a reasonable alternative to other agents in the treatment of adults hospitalized with moderate/severe CAP, given the projected higher rate of patients discharged early achieved with similar or lower total costs than the alternative treatments considered.
Transparency
Declaration of funding
Funding for this project was provided by Pfizer International Operations, Paris, France.
Declaration of financial/other interests
Carmen Peral, Claudie Charbonneau, Jennifer Hammond, and Wajeeha Ansari are employees of Pfizer. Evidera received consulting fees from Pfizer for undertaking this economic analysis. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have made substantial contributions to the development of the manuscript and have approved the final version submitted.
Acknowledgements
We thank Julia Donnelly (on behalf of Evidera) for medical writing assistance.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions see (https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- Remington LT, Sligl WI. Community-acquired pneumonia. Curr Opin Pulm Med. 2014;20(3):215–224.
- Wunderink RG, Waterer G. Advances in the causes and management of community acquired pneumonia in adults. BMJ. 2017;358:j2471.
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79.
- Rivero-Calle I, Pardo-Seco J, Aldaz P, et al. Incidence and risk factor prevalence of community-acquired pneumonia in adults in primary care in Spain (NEUMO-ES-RISK project). BMC Infect Dis. 2016;16(1):645.
- Yu H, Rubin J, Dunning S, et al. Clinical and economic burden of community-acquired pneumonia in the medicare fee-for-service population. J Am Geriatr Soc. 2012;60:n/a–n/a.
- Wroe PC, Finkelstein JA, Ray GT, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. 2012;205(10):1589–1592.
- Torres A. Community-acquired pneumonia: changing paradigms about mortality. Community Acquir Infect. 2014;1:1.
- Reyes S, Martinez R, Vallés JM, et al. Determinants of hospital costs in community-acquired pneumonia. Eur Respir J. 2008;31(5):1061–1067.
- Brown JD, Harnett J, Chambers R, et al. The relative burden of community-acquired pneumonia hospitalizations in older adults: a retrospective observational study in the United States. BMC Geriatr. 2018;18:92.
- Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011;17:1.
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society Consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Supplement_2):S27–S72.
- Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–iii55.
- Ott SR, Hauptmeier BM, Ernen C, et al. Treatment failure in pneumonia: impact of antibiotic treatment and cost analysis. Eur. Respir. J. 2012;39(3):611–618.
- Cillóniz C, Rodríguez-Hurtado D, Rodríguez-Hurtado D, et al. Characteristics and management of community-acquired pneumonia in the era of global aging. Med Sci. 2018;6:35.
- Menéndez R, Torres A, Aspa J, et al. [Community-acquired pneumonia. New guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR)]. Arch Bronconeumol. 2010;46:543–558.
- File TM Jr, Low DE, Eckburg PB, et al. Integrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled‐blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community‐acquired pneumonia. Clin Infect Dis. 2010;51:1395–1405.
- Eckburg PB, Friedland HD, Llorens L, et al. Day 4 clinical response of ceftaroline fosamil versus ceftriaxone for community-acquired bacterial pneumonia. Infect Dis Clin Pract. 2012;20(4):254–260.
- Ramani A, Udeani G, Evans J, et al. Contemporary use of ceftaroline fosamil for the treatment of community-acquired bacterial pneumonia: CAPTURE study experience. J Chemother. 2014;26(4):229–234.
- Guervil DJ, Kaye KS, Hassoun A, et al. Ceftaroline fosamil as first-line versus second-line treatment for acute bacterial skin and skin structure infections (ABSSSI) or community-acquired bacterial pneumonia (CABP). J. Chemother. 2016;28(3):180–186.
- Udeani G, Evans J, Cole P, et al. Ceftaroline fosamil for the treatment of community-acquired bacterial pneumonia in elderly patients. Hosp Pract. 2014;42(3):109–115.
- de Miguel-Díez J, Jiménez-García R, Hernández-Barrera V, et al. Trends in hospitalizations for community-acquired pneumonia in Spain: 2004 to 2013. Eur J Intern Med. 2017;40:64–71.
- Ramirez J. A, Vargas S, Ritter GW, et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community-acquired pneumonia. Arch Intern Med. 1999;159(20):2449–2454.
- Ostermann H, Blasi F, Medina J, et al. Health economic analysis of current clinical management of patients hospitalised with community-acquired pneumonia across Europe (2010–2011) (REACH study). 22nd European Congress of Clinical Microbiology and Infectious Diseases, London, UK. 2012.
- Garau J. Current management of patients hospitalised with community acquired pneumonia across Europe (2010–2011): assessment of clinical practice patterns and real-life effectiveness of antibiotics from the REACH study. 22nd ECCMID, London, UK. Presentation; 2012.
- Rao N, Gibson E, Lawson R, et al. Zinforo (Ceftaroline Fosamil) versus other empiric antibiotics for moderateto-severe community acquired Pneumonia (CAP) in adults: A network meta-analysis. ISPOR 2016 – Poster, Vienna, Austria. 2016.
- Shorr AF, Khashab MM, Xiang JX, et al. Levofloxacin 750-mg for 5 days for the treatment of hospitalized Fine Risk Class III/IV community-acquired pneumonia patients. Respir Med. 2006;100(12):2129–2136.
- Welte T, Petermann W, Schurmann D, et al. Treatment with sequential intravenous or oral moxifloxacin was associated with faster clinical improvement than was standard therapy for hospitalized patients with community-acquired pneumonia who received initial parenteral therapy. Clin Infect Dis. 2005;41(12):1697–1705.
- File TM, Rewerska B, Vucinić-Mihailović V, et al. SOLITAIRE-IV: a randomized, double-blind, multicenter study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumonia. Clin Infect Dis. 2016;63(8):1007–1016.
- Leprince C, Desroches M, Emirian A, et al. Antimicrobial Susceptibility Studies Distribution and antimicrobial susceptibility of bacteria from adults with community-acquired pneumonia or complicated skin and soft tissue infections in France: the nationwide French PREMIUM study. Diagn Microbiol Infect Dis. 2015;83(2):175–182.
- Botplusweb.portalfarma.com. BOT Plus 2. Base de Datos de Medicamentos [Internet]. [cited 2018 Sep 10]. Available from: https://botplusweb.portalfarma.com/botplus.aspx/.
