Abstract
Background and aims
The ReFLeCT study demonstrated that switching to insulin degludec from other basal insulins was associated with reductions in glycated hemoglobin and hypoglycemic events in type 1 (T1D) and type 2 diabetes (T2D), and reductions in insulin doses in T1D. The aim of the present analysis was to assess the short- and long-term cost-effectiveness of switching to insulin degludec in Sweden.
Methods
Short-term outcomes were evaluated over 1 year in a Microsoft Excel model, while long-term outcomes were projected over patient lifetimes using the IQVIA CORE Diabetes Model. Cohort characteristics and treatment effects were sourced from the ReFLeCT study. Costs (in 2018 Swedish krona [SEK]) encompassed direct medical expenditure and indirect costs from loss of workplace productivity. In the long-term analyses, patients were assumed to receive insulin degludec or continue prior insulin therapy (primarily insulin glargine U100) for 5 years, before all patients intensified to once-daily degludec and mealtime aspart.
Results
Switching to insulin degludec was associated with improved quality-adjusted life expectancy of 0.04 and 0.02 quality-adjusted life years (QALYs) over 1 year, and 0.16 and 0.08 QALYs over patient lifetimes, in T1D and T2D. Combined costs in T1D and T2D were estimated to be SEK 1,249 lower and SEK 1,181 higher over the short-term, and SEK 157,258 and SEK 2,114 lower over the long-term. Benefits were due to lower insulin doses in T1D, reduced rates of hypoglycemia, and lower incidences of diabetes-related complications. Insulin degludec was associated with an incremental cost-effectiveness ratio of SEK 64,298 per QALY gained for T2D over 1 year and considered dominant for T1D and T2D in all other comparisons.
Conclusions
Insulin degludec was projected to be cost-effective or dominant versus other basal insulins for the treatment of T1D and T2D in Sweden.
Introduction
Diabetes represents a growing clinical and economic burden in Sweden, with prevalence of the disease expected to rise from 4.8% to 6.0% (with type 1 [T1D] and type 2 diabetes [T2D] estimated to account for 10% and 90% of cases, respectively), and diabetes-related expenditure projected to increase from approximately SEK 33 billion to SEK 35 billion (EUR 3.3 billion to EUR 3.5 billion, with SEK 1.00 approximately equal to EUR 0.10) between 2019 and 2045Citation1,Citation2. Healthcare systems are coming under ever-growing pressure with constrained budgets and increasing patient numbers, and payers often need to understand the benefits of investment in novel interventions, considering both the short- and long-term budgetary implications of reimbursement. Short-term cost-effectiveness analyses provide pertinent information for payers when considering annual budgets, while evaluations of interventions through long-term cost-effectiveness analyses are particularly crucial for diabetes as a large proportion of diabetes-related expenditure is associated with the treatment of long-term complicationsCitation3,Citation4. Landmark studies, such as the Diabetes Control and Complications Trial (DCCT) in T1D and the United Kingdom Prospective Diabetes Study (UKPDS) in T2D, have shown that reductions in glycated hemoglobin (HbA1c) result in fewer diabetes-related complications and improved patient outcomes in the long-termCitation5,Citation6. However, hypoglycemia is a common adverse event associated with insulin regimens that has pertinent clinical and economic impacts in the short-termCitation7,Citation8. Several studies have shown that hypoglycemic events not only have an impact on quality-of-life, but are also associated with substantial loss of workplace productivity and healthcare resource use – factors that are crucial in settings such as Sweden, where a societal perspective is often required for health economic evaluationsCitation7–13.
Insulin regimens are essential for survival in T1D to replace lost insulin production, while insulin therapies maintain an important role for the treatment and control of progressive T2D. Based on guidelines published jointly by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA), basal insulin therapies are indicated for the treatment of T2D as a third-line therapy in the majority of patientsCitation14. In patients with T2D with a compelling need to minimize the risk of hypoglycemia, avoid weight gain or promote weight loss, basal insulins are indicated as fourth-line therapy optionsCitation14.
Insulin degludec is a once-daily, long-acting basal insulin with a duration of action of more than 42 h, approved for the treatment of people with T1D and T2DCitation15. Insulin degludec has been associated with lower rates of hypoglycemia versus other basal insulins in several clinical trialsCitation16–18. Recently, insulin degludec was assessed in a real-world setting in the prospective, observational, single-arm ReFLeCT study. ReFLeCT examined the impact of switching from non-degludec basal insulin therapy (with or without bolus insulin) to insulin degludec (with or without bolus insulin) in people with T1D and T2DCitation19. This study showed that insulin degludec was associated with significantly fewer hypoglycemic events than previous insulin therapy, as well as significant reductions in HbA1c, in people with T1D and T2D, and lower insulin doses and slight increases in body weight in people with T1D.
The aim of the present analysis was to assess the impact of changes in hypoglycemic event rates on initiation of insulin degludec on short-term cost-effectiveness outcomes, and changes in HbA1c, body weight, and hypoglycemic events on long-term cost-effectiveness outcomes in patients with T1D and T2D in Sweden.
Methods
Modeling approach over 1 year
Short-term projections of cost-effectiveness were performed using a model built in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA), an older version of which was used in a previous short-term cost-effectiveness analysis of insulin degludec in Sweden (Supplementary Material, Figure S1)Citation20. The model captured non-severe (sub-categorized into diurnal and nocturnal) and severe hypoglycemic event rates with insulin degludec and continuation of previous insulin therapy, as well as the costs of treating hypoglycemia, acquisition costs of included medications and consumables, and costs arising from loss of workplace productivity due to hypoglycemic events. Resource use captured basal and bolus insulin doses, routine needle use, self-monitoring of blood glucose (SMBG) tests, and additional blood glucose (BG) tests required following a hypoglycemic event. The model reported health outcomes of quality-adjusted life expectancy (expressed in quality-adjusted life years [QALYs]), direct and indirect costs, and, where applicable, incremental cost-effectiveness ratios (ICERs), which describe the cost per additional unit of effectiveness gained for the intervention versus the comparator. When an intervention is associated with improved clinical outcomes and cost savings, no ICER is calculated and it is instead considered dominant versus the comparatorCitation21.
Clinical and cost outcomes were projected over a 1-year time horizon, with disease-specific and background mortality assumed to be zero in both treatment arms. Baseline quality-of-life and non-severe hypoglycemic event disutilities were taken from published sources, while severe hypoglycemic event disutilities were taken from an unpublished Sweden-specific subset of subsequently published data (Supplementary Material, Table S1)Citation22–24.
Long-term modeling approach
Long-term projections of cost-effectiveness were performed using the IQVIA CORE Diabetes Model (version 9.0; IQVIA, Durham, NC), an interactive, internet-based computer model developed to evaluate the long-term health outcomes and economic consequences of implementing interventions in the treatment of T1D and T2D (Supplementary Material, Figure S2)Citation25,Citation26. Previous publications have detailed the architecture, assumptions, features, and capabilities of the model, which have been validated both in 2004 and 2014Citation26,Citation27. Model outputs include time to onset and cumulative incidence of complications, life expectancy, quality-adjusted life expectancy (expressed in QALYs), direct and indirect costs, and, where required, ICERs.
Clinical and cost outcomes were projected over patient lifetimes, as per the guidelines for the assessment of cost-effectiveness for interventions for diabetesCitation28. Background mortality was captured based on Sweden-specific life tables published by the World Health Organization (Supplementary Material, Table S2)Citation29. Health-state utilities and event disutilities relating to quality-of-life were specific to T1D and T2D and were based on published sources (Supplementary Material, Table S3)Citation22,Citation23,Citation25,Citation30–37.
Patients were assumed to switch to insulin degludec (maintaining any prior bolus insulin therapy) or continue previous insulin therapy for 5 years, before all patients in both treatment arms intensified to insulin degludec plus mealtime insulin aspart.
Clinical data
Baseline patient characteristics and treatment effects for insulin degludec were sourced from the ReFLeCT study (approved by the Institutional Review Board [IRB]), where available ( and Supplementary Material, Table S4)Citation19. The ReFLeCT study enrolled 556 insulin-treated adults with T1D and 611 insulin-treated adults with T2D with treatment plans to initiate insulin degludec. No treatment effects were applied for the continuation of previous insulin therapy, with parameters for this treatment arm assumed to remain at baseline, as participants were receiving these medications prior to the study initiation. On initiation of insulin degludec, only treatment effects that reached statistical significance in the ReFLeCT study were applied. HbA1c was measured according to the National Glycohemoglobin Standardization Program (NGSP), based on the DCCTCitation38. Change in HbA1c was not applied in the short-term analysis, as differences in glycemic control would not be expected to drive differences in complication rates over a 1-year time horizon.
Table 1. Insulin doses and treatment effects from the ReFLeCT study applied in the cost-effectiveness analyses of insulin degludec in type 1 and type 2 diabetes in Sweden.
Cost data and resource use
Costs were accounted from a Swedish societal perspective and expressed in 2018 Swedish krona (SEK). In the short-term analysis, direct costs captured costs of medications and consumables and costs of hypoglycemia, while indirect costs captured loss of workplace productivity arising from hypoglycemic events (Supplementary Material, Tables S5 and S6)Citation39,Citation40. In the long-term analysis, direct costs captured medication and consumables costs, costs of treating diabetes-related complications, and the costs of patient management (Supplementary Material, Tables S5 and S7–S11). Indirect costs in the long-term analysis captured loss of workplace productivity from annual days off work estimates estimated by Sørenson and PloughCitation41 and the most recent annual salaries available in Sweden (Supplementary Material, Table S12)Citation42. Indirect costs in both the short- and long-term analyses were only accrued while simulated patients were below the set retirement age (65 years). Costs of included medications and consumables were based on published list prices (sourced in November 2019), while costs of complications were based on published sources and inflated to 2018 SEK where appropriate using the most recently available inflation rate for health published by Statistics Sweden (Supplementary Material, Table S11)Citation39,Citation40,Citation43–48.
The cost of basal insulin therapy in the continuation of a previous therapy arm was based on a weighted average of basal insulin use in the ReFLeCT study. In T1D, 63.8% of people were receiving insulin glargine U100, 22.7% were receiving insulin detemir, and 13.5% were receiving other or unclassified basal insulins. In T2D, 59.1% of people were receiving insulin glargine U100, 20.8% were receiving insulin detemir, and 20.1% were receiving other or unclassified basal insulins. People receiving other or unclassified basal insulins in both the T1D and T2D analyses were assumed to receive insulin NPH, the lowest priced basal insulin in Sweden, to ensure the analyses were conservative from the perspective of insulin degludec. The daily doses of basal insulin (22.8 and 25.0 international units [IU] for insulin degludec and comparator insulin, respectively, in T1D, and 37.5 IU in both arms for T2D) were based on data from the ReFLeCT study. The daily doses of insulin aspart in each treatment arm (23.8 and 27.3 IU for insulin degludec and comparator insulin, respectively, in T1D, and 38.9 IU in both arms for T2D) were weighted according to the proportion of patients receiving bolus insulin.
Sensitivity analyses
The projection of long-term outcomes from short-term data is associated with uncertainty. To evaluate the impact of alternative data inputs and assumptions on long-term cost-effectiveness outcomes, several sensitivity analyses were performed. These included:
Shortening the time horizon of the analysis to 20 years, 10 years, and 1 year (for which it should be noted that not all patients had died, and therefore not all relevant clinical and cost outcomes were captured);
Applying discount rates of 0% and 5% in separate analyses;
Evaluating the key drivers of clinical benefits by applying the differences in HbA1c, body mass index (BMI), and hypoglycemia in turn in separate analyses;
Application of the biosimilar insulin glargine (Abasaglar) price rather than the insulin glargine U100 (Lantus) price in the previous insulin therapy treatment arm (Supplementary Material, Table S5);
Abolishing the dose differences between the two treatment arms; and
Combining the application of the biosimilar insulin glargine price in the previous insulin therapy treatment arm with the abolition of the differences in dosing.
As the projection of short-term outcomes is associated with less uncertainty, only two sensitivity analyses were performed for the short-term approach. In one analysis, the price of biosimilar insulin glargine was applied in the place of insulin glargine U100 in the continuation of the previous insulin therapy arm, while, in the other, people in the T2D cohort were assumed to accrue indirect costs.
Results
Short-term base case analysis
Short-term projections of clinical and cost outcomes over a 1-year time horizon indicated that switching to insulin degludec resulted in improved quality-adjusted life expectancy of 0.04 QALYs in people with T1D and 0.02 QALYs in people with T2D compared with continuation of previous basal insulin therapy (). Clinical benefits were a result of fewer hypoglycemic events with insulin degludec versus continuation of previous insulin therapy.
Table 2. Short-term cost-effectiveness outcomes of insulin degludec versus continuation of previous insulin therapy in type 1 and type 2 diabetes in Sweden.
Direct costs were estimated to be SEK 795 lower with insulin degludec in people with T1D over 1 year, with higher treatment costs entirely offset due to cost savings from avoidance of hypoglycemic events (SEK 1,191 per patient; ). Further indirect cost savings of SEK 453 were achieved through reduced loss of workplace productivity, leading to total combined cost savings of SEK 1,249 for insulin degludec in people with T1D. In people with T2D, insulin degludec was associated with direct cost increases of SEK 1,181 versus continuation of previous insulin therapy, with higher treatment costs partially offset by cost savings due to avoidance of hypoglycemia (SEK 440 per patient). Indirect costs were not accrued in the T2D analyses as patients were beyond the set retirement age (65 years) at baseline.
Figure 1. Short-term mean direct costs over 1 year in the cost-effectiveness analyses of insulin degludec in type 1 and type 2 diabetes in Sweden. SEK, 2018 Swedish krona.
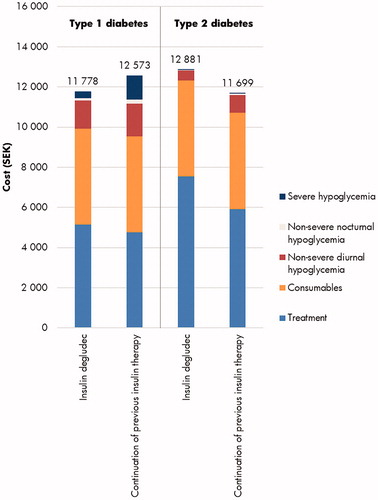
With improved clinical outcomes and cost savings, insulin degludec was considered dominant versus continuation of previous insulin therapy for people with T1D over a 1-year time horizon in Sweden (). For people with T2D, insulin degludec was associated with improved clinical outcomes and increased costs, and was therefore associated with an ICER of SEK 64,298 per QALY gained versus continuation of previous insulin therapy (). Based on a willingness-to-pay threshold of SEK 500,000 per QALY gained in Sweden, insulin degludec was considered a cost-effective treatment option in people with T2D versus continuation of previous insulin therapy over the short-term.
Long-term base case analysis
Long-term projections of clinical outcomes indicated that switching to insulin degludec resulted in improved life expectancy of 0.06 years and improved quality-adjusted life expectancy of 0.16 QALYs versus continuation of previous insulin therapy in people with T1D, and improved life expectancy and quality-adjusted life expectancy of 0.05 years and 0.08 QALYs, respectively, in people with T2D (). Clinical benefits were a result of reduced incidences of diabetes-related complications with insulin degludec in both comparisons, with the mean time to onset of any diabetes-related complication estimated to be 0.3 and 0.2 years longer in the T1D and T2D analyses, respectively.
Table 3. Long-term cost-effectiveness outcomes of insulin degludec versus continuation of previous insulin therapy in type 1 and type 2 diabetes in Sweden.
Over patient lifetimes, direct costs were estimated to be SEK 7,559 and SEK 2,114 lower with insulin degludec in people with T1D and T2D, respectively, with higher treatment costs entirely offset by cost savings due to avoidance of diabetes-related complications (most notably renal complications in T1D, with mean cost savings of SEK 3,492 per patient, and diabetic foot ulcer, amputation, and neuropathy complications in T2D, with mean cost savings of SEK 5,469 per patient; ). Indirect cost savings were only accrued in the T1D analyses and totaled SEK 149,699. As per the short-term analysis, indirect costs were not accumulated in the long-term T2D analyses as patients were above the set retirement age (65 years) at baseline.
Figure 2. Long-term mean direct costs over patient lifetimes in the cost-effectiveness analyses of insulin degludec in type 1 and type 2 diabetes in Sweden. SEK, 2018 Swedish krona.
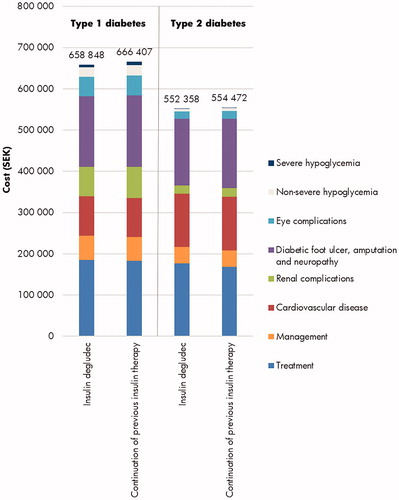
With improved clinical outcomes and cost savings, insulin degludec was considered dominant versus continuation of previous insulin therapy for people with T1D and T2D over a lifetime time horizon in Sweden ().
Sensitivity analyses
Type 1 diabetes
Sensitivity analyses showed that the results of the base case analyses were robust to changes in input parameters and assumptions (). In the long-term T1D analyses, application of shorter time horizons led to reduced quality-adjusted life expectancy for both treatment arms, and reduced incremental benefits with insulin degludec, demonstrating the importance of reductions in the incidence of long-term complications. However, switching to insulin degludec remained dominant versus previous insulin therapy. Lowering the discount rates to 0% led to increased clinical benefits and cost savings with insulin degludec, while increasing the discount rates to 5% had the converse effect, with clinical benefits and cost savings reduced.
Table 4. Sensitivity analysis results of insulin degludec versus continuation of previous insulin therapy in type 1 and type 2 diabetes in Sweden.
Testing for the key drivers of clinical benefits by applying each of the HbA1c, BMI, and hypoglycemic event differences in turn showed that fewer hypoglycemic events with insulin degludec were the biggest driver of improved quality-adjusted life expectancy and cost savings, with benefits of 0.09 QALYs and cost savings of SEK 137,785 per patient when only this difference between the treatment arms was applied. The difference in HbA1c also made a substantial contribution, with improvements in quality-adjusted life expectancy of 0.04 QALYs when only this difference was applied. Differences in BMI made negligible contributions to the clinical benefits observed in the base case analysis.
Applying the cost of biosimilar insulin glargine in the previous insulin therapy arm of the long-term analysis led to slightly decreased cost savings, but no changes to the conclusion that switching to insulin degludec was dominant. A similar outcome was observed in the short-term analysis with this price applied, with reduced cost savings, but insulin degludec remaining dominant versus previous insulin therapy. Abolishing the differences in doses of basal insulins between the treatment arms of the long-term analysis and combining this with the price of biosimilar insulin glargine in separate analyses led to only minor changes in cost outcomes and insulin degludec remained dominant versus continuation of previous insulin therapy.
Type 2 diabetes
Sensitivity analyses around the key inputs and assumptions of the T2D analyses also showed that the results of the base case analyses were robust to changes (). Application of shorter time horizons led to reduced clinical outcomes for both treatment arms – with a 1-year time horizon applied, insulin degludec was associated with an ICER of SEK 156,379 per QALY gained, while use of a 10-year time horizon yielded an ICER of SEK 24,890 per QALY gained. Application of a 20-year time horizon resulted in cost savings and switching to insulin degludec was considered dominant versus previous insulin therapy. Lowering the discount rates to 0% led to greater clinical benefits and cost savings with insulin degludec, while increasing the discount rates to 5% had the converse effect, but insulin degludec remained dominant.
Testing for the key drivers of clinical benefits by applying the HbA1c and hypoglycemic event differences in turn showed that the greater reduction in HbA1c with insulin degludec was the biggest driver of improved outcomes, with clinical benefits of 0.05 QALYs and cost savings of SEK 337 per patient when only these differences between the treatment arms were applied. The difference in hypoglycemic event rates also made a substantial contribution, with improvements in quality-adjusted life expectancy of 0.03 QALYs when only this difference was applied. There was no difference in changes in BMI between the treatment arms in the T2D analyses, as there was no statistically significant difference between the treatment arms for this parameter in people with T2D in the ReFLeCT study.
Applying the cost of biosimilar insulin glargine in the previous insulin therapy arm of the long-term analysis led to smaller cost savings with insulin degludec, but it remained dominant versus continuation of previous insulin therapy. In the short-term analysis, applying this price led to increased incremental costs, and an ICER of SEK 85,035 per QALY gained for insulin degludec versus previous insulin therapy. Assuming people in the T2D cohort accrued indirect costs led to reduced incremental costs with insulin degludec, and it was associated with an ICER of SEK 30,500 per QALY gained.
Discussion
The present analysis has shown that, based on both short- and long-term projections of outcomes from the real-world ReFLeCT study, switching to insulin degludec results in improved clinical outcomes versus continuation of previous insulin therapy in people with T1D and T2D in SwedenCitation19. Over a 1-year time horizon, insulin degludec was associated with cost savings in people with T1D, but cost increases in people with T2D. Over patient lifetimes, cost savings were projected in both T1D and T2D with insulin degludec. Clinical benefits in the short-term analysis were a result of fewer hypoglycemic events. In the long-term analysis, clinical benefits were achieved through projected reduced incidences and delayed time to onset of diabetes-related complications with insulin degludec, due to the greater reductions in HbA1c. Reductions in the incidences of diabetes-related complications also yielded cost savings in both the T1D and T2D long-term analyses, with higher treatment costs entirely offset. Based on a willingness-to-pay threshold of SEK 500,000 per QALY gained in Sweden, insulin degludec was considered a dominant treatment option in people with T1D and a cost-effective treatment option in people with T2D versus continuation of previous insulin therapy over the short-term, and a dominant treatment option in both people with T1D and T2D versus continuation of previous insulin therapy over the long-term.
Insulin degludec has long been associated with comparable glycemic control but fewer hypoglycemic events versus other basal insulins in several clinical trialsCitation16–18. However, evidence suggests that the burden of hypoglycemic events are often underestimated in clinical trials compared with real-world practiceCitation49. Up to now, observational studies that examined insulin degludec with hypoglycemia events prospectively recorded were lacking, but the ReFLeCT study offers a novel source of evidence to fill this data gapCitation19. Using data from the ReFLeCT study, the present analyses have shown that reductions in hypoglycemia are key drivers of improvements in quality-of-life for both people with T1D and T2D, while reducing costs for the healthcare payer over patient lifetimes. These clinical benefits were observed over both the short- and long-term, with results over a 1-year time horizon comparable across the two different models, and insulin degludec remained a cost-effective or dominant treatment option in both T1D and T2D. Additional significant benefits observed with insulin degludec in the ReFLeCT study, such as greater reductions in HbA1c, were also projected to lead to further long-term improvements in life expectancy and quality-adjusted life expectancy, while providing further cost savings from fewer diabetes-related complicationsCitation19.
The use of evidence from a real-world study might also represent a limitation of the present analysis, but these data provide robust evidence of how the included interventions perform in real-world clinical practice and possibly include more accurate rates of hypoglycemia than those reported in clinical trialsCitation49. While confounding factors can often play a role in real-world studies, the present analysis aimed to mitigate some uncertainty by applying only statistically significant differences between the interventions. The results of the present health economic analysis are also in line with previous long-term cost-effectiveness analyses of insulin degludec versus insulin glargine based on real-world data, with insulin degludec representing a dominant treatment option in both T1D and T2D in ItalyCitation50. The use of data from a single-arm study might also be seen as a potential weakness, as treatment effects are inseparable from study effects. Changes in insulin doses, dose intervals, and add-on or removal of bolus insulin and other antihyperglycemic medications were at the discretion of the treating physician during the ReFLeCT study and this could have influenced outcomes. However, the proportion of patients using antidiabetic therapies during the 12-month follow-up period was similar to that of the baseline period. Moreover, results from both the present study and previous analyses based on real-world data match those in several other country settings based on clinical trial data, with the consistency between the two types of data sources lending credence to the conclusions of the present studyCitation51–53.
The projection of long-term outcomes from short-term data represents a limitation of the long-term analysis. This is, however, an essential tenet of all long-term diabetes modeling, and arguably represents the most robust source of evidence in the absence of long-term clinical trial data. The inclusion of the short-term analysis, as well as the performed sensitivity analyses that test the inputs and assumptions of the present study, also mitigate some of the uncertainty around these long-term projections and represent a key strength, with insulin degludec remaining a cost-effective or dominant treatment option throughout.
Conclusions
Compared to previous basal insulin therapy (with insulin glargine U100 at both originator and biosimilar prices), switching to insulin degludec was projected to be dominant in people with T1D and cost-effective in people with T2D over 1 year, and dominant in both people with T1D and T2D over a lifetime time horizon from a Swedish societal perspective.
Transparency
Declaration of funding
This study was supported by funding from Novo Nordisk Scandinavia AB, Malmö, Sweden. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Declaration of financial/other interests
JJ has received speaker’s and or consultant fees from Abbott, Ascensia, AstraZeneca, Boehringer Ingelheim, Eli-Lilly, Medtronic, Nordic Infucare, Novo Nordisk, and Sanofi. ÅE and ACM are employees of Novo Nordisk Scandinavia AB. BE and SS have no relevant or material financial interests that relate to the research described. JG and JDDRF are employees of Novo Nordisk A/S. BH and SJPM are employees of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk Scandinavia AB to support preparation of the analysis. MT has received speaker’s fees from AstraZeneca, Eli-Lilly, Novo Nordisk, and Sanofi. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download TIFF Image (8.3 MB)Supplemental Material
Download TIFF Image (159.9 KB)ReFLeCT_appendix_CLEAN_20_06_17.docx
Download MS Word (596 KB)Acknowledgements
None reported.
Data availability statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- International Diabetes Federation (IDF). IDF Diabetes Atlas – 9th Edition. 2019; [cited 2020 Dec 12]. Available from: https://diabetesatlas.org/en/.
- International Diabetes Federation (IDF). About Diabetes. 2019; [cited 2020 Jan 15]. Available from: https://idf.org/52-about-diabetes.html.
- Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281.
- Ringborg A, Martinell M, Stålhammar J, et al. Resource use and costs of type 2 diabetes in Sweden – estimates from population-based register data. Int J Clin Pract. 2008;62(5):708–716.
- The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–983.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
- Davis RE, Morrissey M, Peters JR, et al. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin. 2005;21(9):1477–1483.
- Lundkvist J, Berne C, Bolinder B, et al. The economic and quality of life impact of hypoglycemia. Eur J Health Econ. 2005;6(3):197–202.
- Polonsky WH, Fisher L, Hessler D. The impact of non-severe hypoglycemia on quality of life in patients with type 2 diabetes. J Diabetes Complicat. 2018;32(4):373–378.
- Marrett E, Stargardt T, Mavros P, et al. Patient-reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. Diabetes Obes Metab. 2009;11(12):1138–1144.
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534.
- O’Reilly DJ, Burke N, Tarride JE, et al. Direct health-care costs and productivity costs associated with hypoglycemia in adults with type 1 and type 2 diabetes mellitus that participated in the Canadian hypoglycemia assessment tool program. Can J Diabetes. 2018;42(6):659–663.
- Brod M, Christensen T, Thomsen TL, et al. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health. 2011;14(5):665–671.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2270.
- European Medicines Agency (EMA). Tresiba (insulin degludec). 2015; [cited 2020 Jan 16]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/tresiba.
- Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine u100 on hypoglycemia in patients with type 1 diabetes: the SWITCH 1 randomized clinical trial. JAMA. 2017;318(1):33–44.
- Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine u100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA. 2017;318(1):45–56.
- Marso SP, McGuire DK, Zinman B, et al. Efficacy and Safety of Degludec versus Glargine in Type 2 Diabetes. N Engl J Med. 2017;377(8):723–732.
- Fadini GP, Feher M, Hansen TK, et al. Switching to degludec from other basal insulins is associated with reduced hypoglycemia rates: a prospective study. J Clin Endocrinol Metab. 2019;104(12):5977–5990.
- Ericsson Å, Pollock RF, Hunt B, et al. Evaluation of the cost-utility of insulin degludec vs insulin glargine in Sweden. J Med Econ. 2013;16(12):1442–1452.
- York Health Economics Consortium. Dominance [online]. York; York Health Economics Consortium; 2016; [cited 2020 Jan 14]. Available from: https://yhec.co.uk/glossary/dominance/.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22(4):340–349.
- Lauridsen JT, Lønborg J, Gundgaard J, et al. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–2650.
- Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11:90.
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(sup1):S5–S26.
- Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(1):S27–S40.
- McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–724.
- American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265.
- World Health Organisation. Global Health Observatory data repository: Life tables by country (Sweden). 2018; [cited 2020 Dec 09]. Available from: http://apps.who.int/gho/data/view.main.61780?lang=en.
- Peasgood T, Brennan A, Mansell P, et al. The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type i diabetes. Med Decis Making. 2016;36(8):1020–1033.
- Li B, Cairns JA, Draper H, et al. Estimating health-state utility values in kidney transplant recipients and waiting-list patients using the EQ-5D-5L. Value Health. 2017;20(7):976–984.
- Coffey JT, Brandle M, Zhou H, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25(12):2238–2243.
- Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–230.
- Wasserfallen JB, Halabi G, Saudan P, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19(6):1594–1599.
- Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ. 1995;311(7020):1595–1599.
- Fenwick EK, Xie J, Ratcliffe J, et al. The impact of diabetic retinopathy and diabetic macular edema on health-related quality of life in type 1 and type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(2):677–684.
- Lee AJ, Morgan CL, Morrissey M, et al. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with Type 1 diabetes, Type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22(11):1482–1486.
- John WG, Mosca A, Weykamp C, et al. HbA1c standardisation: history, science and politics. Clin Biochem Rev. 2007;28(4):163–168.
- Geelhoed-Duijvestijn PH, Pedersen-Bjergaard U, Weitgasser R, et al. Effects of patient-reported non-severe hypoglycemia on healthcare resource use, work-time loss, and wellbeing in insulin-treated patients with diabetes in seven European countries. J Med Econ. 2013;16(12):1453–1461.
- Jönsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with Type 2 diabetes in Sweden. Value Health. 2006;9(3):193–198.
- Sørensen J, Plough UJ. The cost of diabetes-related complications: registry-based analysis of days absent from work. Economics Research International. 2013;2013:1–8.
- Statistics Sweden. Average monthly salary by sector 1992–2018. 2019; [cited 2020 Dec 12]. Available from: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/labour-market/wages-salaries-and-labour-costs/salary-structures-whole-economy/pong/tables-and-graphs/average-monthly-salary-by-sector/.
- Gerdtham UG, Clarke P, Hayes A, et al. Estimating the cost of diabetes mellitus-related events from inpatient admissions in Sweden using administrative hospitalization data. Pharmacoeconomics. 2009;27(1):81–90.
- Ghatnekar O, Carlsson K, Kostnader för insjuknande i stroke år 2009. En incidensbaserad studie. [Costs for stroke incidence in 2009. An incidence-based study]. Institutet för hälsooch sjukvårdsekonomi (IHE), Lund Universitet, Sverige [Institute of Health and Medical Economics (IHE), Lund University, Sweden]. 2012: 2.
- Prompers L, Huijberts M, Schaper N, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia. 2008;51(10):1826–1834.
- Palmer A, Goodall G, Nielsen S, et al. Cost-effectiveness of insulin aspart versus human soluble insulin in type 2 diabetes in four European countries: subgroup analyses from the PREDICTIVE study. Curr Med Res Opin. 2008;24(5):1417–1428.
- Persson U, Willis M, Odegaard K. A case study of ex ante, value-based price and reimbursement decision-making: TLV and rimonabant in Sweden. Eur J Health Econ. 2010;11(2):195–203.
- Statistics Sweden. CPI, Indices for Main Groups, annual averages. 2019; [cited 2020 Dec 12]. Available from: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/prices-and-consumption/consumer-price-index/consumer-price-index-cpi/pong/tables-and-graphs/consumer-price-index-cpi/cpi-indices-for-main-groups-annual-averages/.
- Elliott L, Fidler C, Ditchfield A, et al. Hypoglycemia event rates: a comparison between real-world data and randomized controlled trial populations in insulin-treated diabetes. Diabetes Ther. 2016;7(1):45–60.
- Haldrup S, Lapolla A, Gundgaard J, et al. Cost-effectiveness of switching to insulin degludec from other basal insulins in real-world clinical practice in Italy. J Med Econ. 2020;23(3):271–279.
- Evans M, Mehta R, Gundgaard J, et al. Cost-effectiveness of insulin degludec vs. insulin glargine u100 in type 1 and type 2 diabetes mellitus in a UK setting. Diabetes Ther. 2018;9(5):1919–1930.
- Pollock RF, Valentine WJ, Marso SP, et al. Long-term cost-effectiveness of insulin degludec versus insulin glargine U100 in the UK: evidence from the basal-bolus subgroup of the DEVOTE Trial (DEVOTE 16). Appl Health Econ Health Policy. 2019;17(5):615–627.
- Mezquita-Raya P, Darbà J, Ascanio M, et al. Cost-effectiveness analysis of insulin degludec compared with insulin glargine u100 for the management of type 1 and type 2 diabetes mellitus – from the Spanish National Health System perspective. Expert Rev Pharmacoecon Outcomes Res. 2017;17(6):587–595.

