Abstract
Objectives
To provide the most current assessment of real-world healthcare resource utilization (HRU) and costs among patients with non-valvular atrial fibrillation (NVAF) who newly initiated rivaroxaban and apixaban using a large US database.
Material and methods
A retrospective weighted cohort design was used with healthcare insurance claims from the Optum Clinformatics Data Mart databases (January 2012–December 2018). The index date was defined as the first dispensing of rivaroxaban or apixaban. Adult NVAF patients with an index date on or after 1 January 2016, ≥ 12 months of continuous eligibility before the index date and ≥ 1 month after, and without prior use of oral anticoagulant were included. The observation period spanned from the index date to the earliest of the end of data availability, end of insurance coverage, or death. Inverse probability of treatment weighting (IPTW) was used to adjust for differences in baseline characteristics between cohorts. All-cause healthcare resource utilization (HRU), including hospitalization, emergency room, and outpatient visits, and healthcare costs, including medical and pharmacy costs, were evaluated from the payer’s perspective during the observation period up to 18 and 24 months, separately.
Results
In total, 23,822 rivaroxaban and 53,666 apixaban users were included. After weighting, all baseline characteristics were well balanced between cohorts (mean age: 73.8 years, female: 46.6% in both cohorts). Up to 18 months of follow-up, rivaroxaban users incurred significantly lower total healthcare costs compared to apixaban users (cost difference = −$1,121; p = 0.020), driven by significantly lower rates of outpatient hospital visits and associated costs (cost difference = −$1,579; p < 0.001). Similar results were found in the analysis conducted for up to 24 months of follow-up (total cost difference = ‒$1,111; p = 0.020).
Conclusions
In this large retrospective analysis, patients with NVAF initiated on rivaroxaban incurred significantly lower healthcare costs compared to those initiated on apixaban, which were primarily driven by significantly lower outpatient visits and costs during the 18- and 24-month follow-up periods.
Background
Atrial fibrillation (AF) is the most common type of cardiac arrhythmic disorder, which often occurs with no signs or symptomsCitation1,Citation2. In 2010, AF was estimated to affect between 2.7 and 6.1 million people in the USCitation3, a figure projected to increase to more than 12 million people by 2030Citation4. This disorder is associated with a considerable increase in mortalityCitation5–8 and a risk of strokeCitation2,Citation9. Consequently, AF results in a significant economic burdenCitation10,Citation11, a considerable portion of which is attributable to cardiovascular complications such as stroke and systemic embolismCitation12,Citation13.
Anticoagulants are routinely used as prophylactic antithrombotic therapy in patients with AF, with direct oral anticoagulants (DOACs) now recommended by guidelines as the preferred treatment option for non-valvular atrial fibrillation (NVAF) over vitamin K antagonists (VKA), such as warfarinCitation14,Citation15. Although the efficacy of DOACs to prevent stroke and systemic embolism in patients with NVAF is well-recognizedCitation14–16, they are also associated with an increased risk of bleedingCitation17,Citation18. DOACs are preferred to VKAs for their rapid onset of action, fixed dosing regimens, fewer food and drug interactions, and limited need for laboratory monitoringCitation15. The factor Xa inhibitors, rivaroxaban and apixaban, are two DOACs that were approved by the US Food and Drug Administration (FDA) in 2011 and 2012, respectively, for the reduction of stroke and systemic embolism in patients with NVAFCitation19,Citation20, and are the most preferred treatment choices in the US.
Several studies showed that patients initiated on DOACs incur lower healthcare costs than those initiated on warfarinCitation21–24. However, studies that directly compared healthcare costs between patients treated with rivaroxaban and apixaban report mixed resultsCitation25–28. For example, a retrospective study that included commercially-insured patients who received rivaroxaban or apixaban prior to 2015 found no significant difference in all-cause healthcare costs between patients initiated on apixaban versus rivaroxabanCitation25. Since these studies evaluated a period shortly following FDA approval of both medications (i.e. data from 2012 to 2015), such analyses may also not be representative of current cost trends in the US. Furthermore, some studies evaluated specific subpopulations (e.g. using US Department of Defense data)Citation26, thereby reaching conclusions that may not be generalizable. Finally, studies comparing healthcare resource utilization (HRU) in patients with NVAF treated with rivaroxaban or apixaban often restrict their analyses to hospitalization eventsCitation25,Citation28, overlooking important factors such as emergency room (ER) and outpatient visits. The present study thus aims to fill a knowledge gap by providing an updated comprehensive assessment of the real-world HRU and costs among patients with NVAF who were treatment-naïve to oral anticoagulants and newly-initiated on rivaroxaban or apixaban treatments using a large claims US database.
Methods
Data source
Healthcare insurance claims from the Optum Clinformatics Data Mart database were used, with data ranging from 1 January 2012 to 31 December 2018. Optum Clinformatics Data Mart covers 13 million annual lives of UnitedHealth Group members in all census regions in the US. It contains historical data on patient demographics, insurance coverage (i.e. commercial and Medicare), dates of eligibility and death, claims for inpatient and outpatient visits, costs of services, and laboratory tests and results. Data are de-identified and comply with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Study design
A retrospective weighted cohort design was used. The index date was defined as the date of the first dispensing of rivaroxaban or apixaban (i.e. the index medication). The baseline period was defined as the 12 months prior to the index date. The observation period (follow-up period) spanned from the index date to the end of data availability, end of insurance coverage, or death, whichever occurred first. Study outcomes were evaluated over two distinct follow-up periods, censored at 18 months and 24 months of observation, separately.
Study population
In order to evaluate the most recent trends in HRU and costs, only patients with an index date on or after 1 January 2016 were included in the study population. Additional inclusion criteria were ≥ 1 diagnosis of AF during the baseline period or on the index date, ≥ 18 years of age at the index date, and ≥ 12 months of continuous eligibility prior to the index date and ≥ 1 month of continuous eligibility after the index date.
Patients were also required to be newly-initiated on oral anticoagulants (i.e. treatment-naïve to oral anticoagulants); therefore, patients with ≥ 1 pharmacy claim for an oral anticoagulant other than the index medication on the index date or during the baseline period were excluded. Patients were also excluded if they had ≥ 1 claim for mitral stenosis, mechanical heart-valve procedure, or organ/tissue transplant during the baseline period, ≥ 1 diagnosis of VTE during the baseline period, or ≥ 1 diagnosis for pregnancy at any time during the study period.
Included patients were classified into two mutually exclusive cohorts based on the index anticoagulant received.
Study outcomes
Study outcomes included all-cause HRU and costs. HRU was stratified into hospitalizations, ER visits, outpatient visits, and other visits (e.g. home services and hospice). Outpatient visits were further broken down into outpatient hospital visits and office/physician visits. Healthcare costs were stratified into medical and pharmacy costs, and medical costs were further broken down into hospitalization, ER visit, outpatient visit, and other visit costs. Healthcare costs were evaluated from the payer’s perspective (i.e. excluding patient-paid costs) during the observation period, and were inflation-adjusted to 2019 US dollars based on the medical care component of the Consumer Price Index.
Statistical analysis
To adjust for differences in baseline characteristics between the rivaroxaban and apixaban cohorts, inverse probability of treatment weighting (IPTW) based on the propensity score was used. The propensity score was derived from a multivariable logistic regression model conditional on baseline covariates, including age, sex, year of index date, US region, type of insurance plan, Quan-Charlson comorbidity index score, CHA2DS2-VASc score, HAS-BLED score, selected NVAF-related comorbidities (i.e. those with a prevalence of ≥ 5%), baseline risk factors for stroke/systemic embolism and bleeding events (i.e. those with a prevalence of ≥ 5%), all-cause baseline HRU, and all-cause baseline healthcare costs (hospitalization, ER visit, outpatient hospital visit, office/physician visit, other visit, and pharmacy costs).
Patient demographics and baseline clinical characteristics for the rivaroxaban and apixaban cohorts were summarized using frequencies and proportions for categorical variables, and means, standard deviations (SDs), and medians for continuous variables. Baseline characteristics were compared for unweighted and weighted cohorts using standardized differences (std. diff.), with a threshold of < 10% considered a negligible imbalance between covariatesCitation29.
All-cause HRU for the rivaroxaban and apixaban cohorts were reported including the frequency and rate (reported per patient per year [PPPY] by dividing the number of HRU events observed over the observation period by the patient-years of observation) of visits. Weighted rate ratios were calculated from Poisson regression models, with 95% confidence intervals (CIs) and p-values generated using non-parametric bootstrap procedures. All-cause healthcare costs were also reported PPPY by dividing the costs incurred over the observation period by the patient-years of observation. Mean cost differences between cohorts were calculated, and 95% CIs and p-values were generated using non-parametric bootstrap procedures.
Results
Baseline characteristics
A total of 23,822 and 53,666 patients newly initiated on rivaroxaban and apixaban on or after 1 January 2016 were included in the study (). Prior to applying IPTW weights, patients in the rivaroxaban cohort were younger than patients in the apixaban cohort (72.3 vs 74.5; std. diff. = 20.8%), and had lower Quan-Charlson comorbidity index (2.50 vs 3.02), CHA2DS2-VASc (2.43 vs 2.75), and HAS-BLED (1.48 vs 1.59) scores. The rivaroxaban cohort also had lower rates of all-cause hospitalizations (0.51 vs 0.66; std. diff. = 17.5%), and lower total healthcare costs ($26,908 vs $34,198; std. diff. = 13.7%). After weighting, all baseline characteristics were well balanced between cohorts (std. diff < 10%; ). Mean age was 73.8 years and 46.6% were female in both cohorts. The Quan-Charlson comorbidity index score was 2.86, CHA2DS2-VASc score was 2.65, and HAS-BLED score was 1.55 in both cohorts. The all-cause total healthcare costs at baseline were well balanced ($32,022 vs $31,970; std. diff. = 0.1%). The most common selected NVAF-related comorbidities were diabetes (35%), renal disease (35%), and congestive heart failure (32%), while the most commonly observed risk factors for stroke/systemic embolism and bleeding events were hypertension (86%), hyperlipidemia (75%), and chronic kidney disease (32%; ).
Figure 1. Patient disposition. AF, atrial fibrillation; VTE, venous thromboembolism. Note. 1. Patients initiated on treatment with both rivaroxaban and apixaban on the index date were classified as apixaban, and subsequently excluded (n = 16).
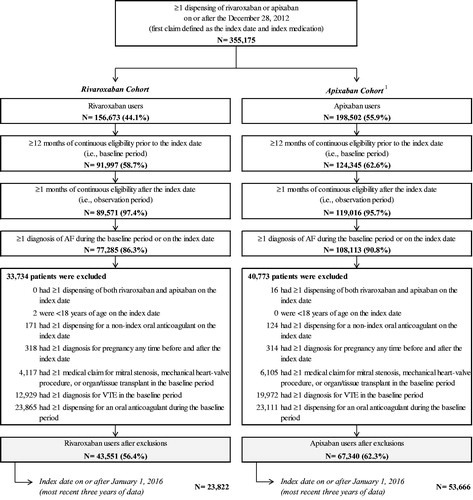
Table 1. Baseline demographics and clinical characteristics – unweighted and weighted rivaroxaban and apixaban cohorts.
Table 2. NVAF-related comorbidities and risk factors for stroke/systemic embolism and major bleeding – unweighted and weighted rivaroxaban and apixaban cohorts.
All-cause HRU
Unweighted analysis
Patients were observed for a mean duration of 368 and 340 days among the unweighted rivaroxaban and apixaban cohorts, respectively, when censoring follow-up at 18 months (). Patients initiated on rivaroxaban had significantly lower rates of hospitalizations PPPY (rate ratio [95% CI] = 0.87 [0.84–0.90]; p < 0.001), ER visits (rate ratio [95% CI] = 0.91 [0.88–0.94]; p < 0.001), and outpatient visits (rate ratio [95% CI] = 0.90 [0.88–0.91]; p < 0.001) compared to patients initiated on apixaban, with similar results observed over 24 months of follow-up.
Table 3. All-cause healthcare resource utilization of the rivaroxaban and apixaban cohorts – up to 18 and 24 months follow-up.
Weighted analysis
Following IPTW, rivaroxaban and apixaban patients had mean observation periods of 351 days and 348 days, respectively, when censoring follow-up at 18 months (). Compared to apixaban, patients initiated on rivaroxaban had significantly lower rates of outpatient visits PPPY (rate ratio [95% CI] = 0.98 [0.96–0.99]; p = 0.008), which were mainly driven by significantly lower rates of outpatient hospital visits (rate ratio [95% CI] = 0.94 [0.90–0.97]; p < 0.001), with no significant differences in rates of hospitalizations, ER visits, office/physician visits, and other visits (). Similar results were also observed up to 24 months of follow-up.
To further describe outpatient hospital visits between weighted rivaroxaban and apixaban cohorts during follow-up, the distribution of physician specialties associated with outpatient hospital visits was compared and found to be similar between cohorts (i.e. all std. diff. < 10%; Supplementary Table S1). The distribution of primary diagnoses associated with outpatient hospital visits was also similar between cohorts with the exception of chronic kidney disease, which was more frequent in the apixaban cohort than the rivaroxaban cohort (5.2% vs 1.6%; std. diff. = 20.0%; Supplementary Table S2).
All-cause healthcare costs
Unweighted analysis
In the analysis conducted up to 18 months of follow-up, patients initiated on rivaroxaban incurred significantly lower total (medical and pharmacy) healthcare costs PPPY compared to patients initiated on apixaban (cost difference: −$4,607; p < 0.001; ). The healthcare cost difference was mainly driven by significantly lower medical costs (cost difference: −$4,118; p < 0.001), which accounted for 89% of the total cost difference. The primary driver of the medical cost difference was outpatient hospital visit costs (cost difference: −$1,861; p < 0.001).
Table 4. Healthcare cost components of the rivaroxaban and apixaban cohorts – up to 18 and 24 months follow-up.
In the analysis conducted up to 24 months of follow-up, similar trends were observed whereby patients initiated on rivaroxaban incurred significantly lower total healthcare costs PPPY compared to patients initiated on apixaban (cost difference: −$4,538; p < 0.001; ). This difference was also largely attributable to the lower medical costs associated with rivaroxaban (cost difference: −$4,070; p < 0.001).
Weighted analysis
Following IPTW, the analysis conducted up to 18 months of follow-up showed that patients initiated on rivaroxaban incurred significantly lower total healthcare costs PPPY compared to patients initiated on apixaban (cost difference: −$1,121; p = 0.020). Similar to the unadjusted analysis, the difference in healthcare costs was mainly driven by significantly lower medical costs (cost difference: −$949; p = 0.036), which accounted for 85% of the total cost difference. The primary driver of the medical cost difference was outpatient hospital visit costs (cost difference: −$1,579; p < 0.001; ). All other healthcare cost components (i.e. hospitalization, ER, office/physician visit, other visit, and pharmacy costs) did not significantly differ between NVAF patients initiated on rivaroxaban and apixaban (all p-values > 0.05).
In the weighted 24-month follow-up analysis, the total cost of care was slightly lower compared to that observed in the 18-month follow-up analysis, but the difference between cohorts remained similar. Patients initiated on rivaroxaban had significantly lower total healthcare costs compared to patients initiated on apixaban (cost difference: −$1,111; p = 0.020). Similar to the 18-month analysis, the difference in healthcare costs was primarily driven by significantly lower outpatient hospital visit costs (cost difference: −$1,522; p < 0.001; ).
Discussion
This retrospective study used claims data from a large nationally representative database to compare recent trends in cost of care between oral anticoagulant-naïve patients with NVAF who were initiated on rivaroxaban or apixaban. Unweighted results showed that patients initiated on rivaroxaban had lower HRU and corresponding healthcare costs following treatment initiation, although rivaroxaban patients seemed to have less severe disease than apixaban patients, indicated by lower Quan-Charlson comorbidity index, CHA2DS2-VASc, and HAS-BLED scores. After balancing differences in baseline characteristics between the rivaroxaban and apixaban cohorts, the rivaroxaban users had significantly fewer outpatient hospital visits. Furthermore, rivaroxaban users incurred significantly lower all-cause total healthcare costs than apixaban users; the difference in costs was driven by significantly lower outpatient hospital visit costs.
A previous real-world study by Amin et al.Citation25 used the OptumInsight Research Database from 2013 to 2015 in order to compare the total all-cause healthcare costs associated with rivaroxaban and apixaban treatments in NVAF patients. The study reported similar costs between cohorts and a higher risk of all-cause hospitalization among patients with rivaroxaban use. However, given that the study evaluated outcomes over a short follow-up period soon after the approval of apixaban in December 2012, the results may not be representative of the now established direct oral anticoagulants in the market. The study also did not evaluate other measures of HRU, such as outpatient visits, which the current study identified as the main contributor of cost differences between the two cohorts. Furthermore, the current study focused on oral anticoagulant-naïve patients with NVAF who were newly initiated on rivaroxaban or apixaban, while the previous study included both oral anticoagulant-naïve and treatment-experienced patients. Moreover, differences in patients’ characteristics, such as comorbidity scores, may account for some discrepancy in results between the two studies, as the current study includes patients with descriptively higher Quan-Charlson comorbidity index scores (2.9 vs 2.3), but lower CHA2DS2-VASc (2.7 vs 3.8) and HAS-BLED (1.6 vs 2.8) scores compared to those of patients in the Amin et al.Citation25 study.
Other studies conducted to evaluate the difference in the economic burden associated with rivaroxaban and apixaban observed higher costsCitation26–28 and a higher risk of all-cause hospitalization for rivaroxaban compared with apixabanCitation26–28. However, several differences in study population and design could explain the differences between the results of our study and those of previous studies. First, similarly to the study conducted by Amin et al.Citation25, these studies evaluated healthcare costs over relatively short follow-up periods (mean follow-up: 4.5–9 months) compared to the current study. Second, some patient populations included in these studies may have limited generalizability (i.e. using US Department of Defense data) compared to our studyCitation26. Finally, even though propensity score matching was used in previous studies, some of them only accounted for baseline hospitalizations without considering other HRU components (e.g. ER and outpatient visits), nor considering baseline healthcare costs, when balancing the patient cohortsCitation26,Citation28. Not adjusting for these variables could have led to healthcare resource use and cost differences that were carried forward in the follow-up. Conversely, the weighting procedure used in the current study ensured that all study outcomes (i.e. HRU and their corresponding costs) were well-balanced between cohorts in the baseline before performing comparisons in the follow-up.
The rate of hospitalizations and their associated costs observed in this study were not significantly different between rivaroxaban and apixaban users, which may indicate that patients with NVAF initiated on rivaroxaban or apixaban incur similar costs associated with stroke and SE, the major contributors of hospitalization costs within this populationCitation12,Citation25. Indeed, previous studies showed that patients using these treatments had similar risk of hospitalization due to stroke and systemic embolismCitation25, and incurred similar medical costs associated with stroke and systemic embolismCitation25,Citation26. This study suggests that, despite similar hospitalization costs, the rate of outpatient hospital visits and corresponding costs (which may not differ significantly shortly after treatment initiationCitation27,Citation30) can in the long-term drive the healthcare costs as observed in patients initiating apixaban compared to rivaroxaban. Outpatient hospital visits, which are more costly than office visits and are typically observed among patients with more severe diseaseCitation31,Citation32, may be driven by complications associated with AF that could be managed in an outpatient settingCitation33,Citation34, such as transient ischemic attacks and clinically-relevant non-major bleedingCitation35,Citation36. Together with the present results, this may indicate that patients using rivaroxaban achieve better disease control than those using apixaban, thus requiring fewer outpatient hospital visits to manage complications and subsequently incurring lower medical costs. Moreover, considering that AF affects millions of people in the US each year, the small difference in total healthcare costs associated with rivaroxaban use could translate into a significant reduction in economic burden of AF patients in the US.
A previous study assessing the cost of non-adherence to oral anticoagulants showed that total medical costs for patients who adhere to medication were significantly lower compared to those who were non-adherentCitation37, and another study suggested that higher adherence to once-daily DOAC resulted in significant cost savings compared to twice-daily regimens in the real-worldCitation37,Citation38. It is possible that the lower healthcare costs observed among rivaroxaban users relative to apixaban users may be attributed to improved adherence, though this was not explored in the current study. Further research is warranted to evaluate the reasons that may underlie the cost differences found in the present study.
Limitations
While this study has several design strengths, it is subject to certain limitations. First, since claims databases record diagnostic and procedure codes, clinical information, such as disease severity or reasons for change in treatment, is not available. Second, in spite of the large size of the present study population and its nationwide geographical representation, results may not be generalizable to patients with health insurance plans that substantially differ from those of the population analyzed in this study. Lastly, while IPTW is used to account for potential differences between the rivaroxaban and apixaban cohorts, the possibility of residual confounding due to unmeasured confounders cannot be excluded. As seen in the unweighted baseline characteristics, patients initiated on apixaban tended to have more severe disease states, as indicated by the higher baseline risk scores, rates of hospitalization, and healthcare costs. Although observed differences have been balanced, there may be unobserved confounders that influence the final results and could not be accounted for. Despite this, retrospective, observational studies that control for confounding factors through weighting techniques provide quality results generalizable to real-life scenarios.
Conclusions
In this large retrospective claims analysis of NVAF patients, those newly initiated on rivaroxaban incurred significantly lower total all-cause healthcare costs compared to patients initiated on apixaban up to 18 and 24 months post-treatment initiation. The majority of the total cost difference was driven by a significantly lower rate of outpatient hospital visits and their associated costs for patients treated with rivaroxaban. Considering that AF affects millions of people in the US each year, the total healthcare cost difference reported in the current study may translate into substantial savings at the national level.
Transparency
Declaration of funding
This study was funded by Janssen Scientific Affairs, LLC.
Declaration of interest
GG, FL, SDM, JT, and PL are employees of Groupe d’analyse, Ltée, a consulting company that provided paid consulting services to Janssen Scientific Affairs, LLC. for the conduct of the present study. DM and BKB are employees of Janssen Scientific Affairs and shareholders of Johnson & Johnson.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors were responsible for the study design and the interpretation of the study results. GG, FL, SDM, and JT were responsible for the data analysis. All authors reviewed the manuscript for intellectual content and approved the final draft.
Supplemental Material: Table 2
Download MS Word (13.3 KB)Supplemental Material: Table 1
Download MS Word (13.2 KB)Acknowledgements
Medical writing assistance was provided by Loraine Georgy, PhD, an employee of Groupe d’analyse, Ltée., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript.
References
- National Heart, Lung, and Blood Institute. Atrial Fibrillation: National Heart, Lung, and Blood Institute; 2019. [cited 12 May 2020]. Available from: https://www.nhlbi.nih.gov/health-topics/atrial-fibrillation
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006;48(4):854–906.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147.
- Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925.
- Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case-control study. Eur Heart J. 2013;34(14):1061–1067.
- Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980-1998. Am J Epidemiol. 2002;155(9):819–826.
- Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952.
- Bordignon S, Chiara Corti M, Bilato C. Atrial fibrillation associated with heart failure, stroke and mortality. J Atr Fibrillation. 2012;5(1):467.
- Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. Am J Manag Care. 2004;10(14 Suppl):S451–S458.
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011; 4(3):313–320.
- Li X, Tse VC, Au-Doung LW, et al. The impact of ischaemic stroke on atrial fibrillation-related healthcare cost: a systematic review. Europace. 2017;19(6):937–947.
- Chatterjee NA, Lubitz SA. Systemic Embolic Events (SEE) in Atrial Fibrillation: SEEing Embolic Risk More Clearly. Circulation. 2015;132(9):787–789.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;140(2):e125–e151.
- Saraiva JFK. Stroke prevention with oral anticoagulants: summary of the evidence and efficacy measures as an aid to treatment choices. Cardiol Ther. 2018;7(1):15–24.
- Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933–2944.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet (London, England). 2014;383(9921):955–962.
- Holster IL, Valkhoff VE, Kuipers EJ, et al. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145(1):105–112.e15.
- Xarelto® (rivaroxaban) Highlights of Prescribing Information. Janssen Pharmaceuticals, Inc.; 2019. p. 1–19. [cited 12 May 2020]. Available from: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf
- Eliquis® (apixaban) Highlights of Prescribing Information. Bristol-Myers Squibb Company; 2018. p. 1–15. Available from: https://packageinserts.bms.com/pi/pi_eliquis.pdf
- Deitelzweig S, Luo X, Gupta K, et al. Effect of apixaban versus warfarin use on health care resource utilization and costs among elderly patients with nonvalvular atrial fibrillation. JMCP. 2017;23(11):1191–1201.
- Laliberte F, Cloutier M, Crivera C, et al. Effect of rivaroxaban versus warfarin on health care costs among nonvalvular atrial fibrillation patients: observations from rivaroxaban users and matched warfarin users. Adv Ther. 2015;32(3):216–227.
- Laliberte F, Pilon D, Raut MK, et al. Is rivaroxaban associated with lower inpatient costs compared to warfarin among patients with non-valvular atrial fibrillation. Curr Med Res Opin. 2014;30(8):1521–1528.
- Amin A, Keshishian A, Trocio J, et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr Med Res Opin. 2017;33(9):1595–1604.
- Amin A, Keshishian A, Vo L, et al. Real-world comparison of all-cause hospitalizations, hospitalizations due to stroke and major bleeding, and costs for non-valvular atrial fibrillation patients prescribed oral anticoagulants in a US health plan. J Med Econ. 2018;21(3):244–253.
- Gupta K, Trocio J, Keshishian A, et al. Real-world comparative effectiveness, safety, and health care costs of oral anticoagulants in nonvalvular atrial fibrillation patients in the U.S. Department of Defense Population. JMCP. 2018;24(11):1116–1127.
- Lin J, Trocio J, Gupta K, et al. Major bleeding risk and healthcare economic outcomes of non-valvular atrial fibrillation patients newly-initiated with oral anticoagulant therapy in the real-world setting. J Med Econ. 2017;20(9):952–961.
- Amin A, Keshishian A, Trocio J, et al. A real-world observational study of hospitalization and health care costs among nonvalvular atrial fibrillation patients prescribed oral anticoagulants in the U.S. Medicare Population. JMCP. 2018;24(9):911–920.
- Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17(12):1202–1217.
- Mehta PA, Grocott-Mason R, Dubrey SW. Adherence to anticoagulation guidelines for atrial fibrillation: a district general hospital survey. Br J Cardiol. 2004;11:474–477.
- Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9(5):348–356.
- Lion J, Malbon A, Henderson MG, et al. A comparison of hospital outpatient departments and private practice. Health Care Financ Rev. 1985;6(4):69–81.
- Franco L, Becattini C, Vanni S, et al. Clinically relevant non-major bleeding with oral anticoagulants: non-major may not be trivial. Blood Transfus. 2018;16(4):387–391.
- Majidi SL, Guerrero CR, Burger KM, et al. Inpatient versus outpatient management of TIA or minor stroke: clinical outcome. J Vasc Interv Neurol. 2017;9(4):49–53.
- Hart RG, Pearce LA, Koudstaal PJ. Transient ischemic attacks in patients with atrial fibrillation: implications for secondary prevention: the European Atrial Fibrillation Trial and Stroke Prevention in Atrial Fibrillation III trial. Stroke. 2004;35(4):948–951.
- O’Brien EC, Holmes DN, Thomas L, et al. Therapeutic strategies following major, clinically relevant nonmajor, and nuisance bleeding in atrial fibrillation: findings from ORBIT-AF. J Am Heart Assoc. 2018;7(12):e006391.
- McHorney CA, Peterson ED, Ashton V, et al. Modeling the impact of real-world adherence to once-daily (QD) versus twice-daily (BID) non-vitamin K antagonist oral anticoagulants on stroke and major bleeding events among non-valvular atrial fibrillation patients. Curr Med Res Opin. 2019;35(4):653–660.
- Stephenson JJ, Shinde MU, Kwong WJ, et al. Comparison of claims vs patient-reported adherence measures and associated outcomes among patients with nonvalvular atrial fibrillation using oral anticoagulant therapy. PPA. 2018;12:105–117.
