Abstract
Objectives
Two intravenous (IV) iron formulations, ferric derisomaltose (FDI) and iron sucrose (IS), are currently available for the treatment of iron deficiency anemia (IDA) in China. Clinical studies have demonstrated that FDI has an improved efficacy and safety profile versus IS, while requiring fewer infusions to correct iron deficits. Based on these findings, the present study evaluated the costs and benefits of FDI and IS for the treatment of IDA, from a healthcare system and societal perspective in China.
Methods
A patient-level model was developed to project time to hematological response and incidence of cardiovascular adverse events and hypersensitivity reactions (HSRs) associated with FDI and IS over 5 years. Costs included iron acquisition, administration, and adverse event/HSR treatment costs, based on published studies, fee schedules, and a physician survey. Health state utilities associated with adverse events, HSRs, and the number of infusions were obtained from the literature and a time trade-off survey.
Results
From a healthcare system perspective, FDI was associated with incremental costs of RMB 1,934 (purchasing power parity USD 462) and incremental quality-adjusted life expectancy of 0.078 quality-adjusted life-years (QALYs) versus IS, yielding an incremental cost-utility ratio of RMB 24,901 (USD 5,949) in the base case scenario. From a societal perspective, FDI was associated with reduced total costs and therefore dominant versus IS.
Limitations
Limitations included the absence of clinical data specific to China and insufficient data to model persistence with treatment.
Conclusions
This was the first cost-utility analysis comparing FDI and IS for the treatment of IDA in China. Based on a patient-level model, FDI was found to improve quality of life and reduce administration and adverse events costs relative to IS. Using the 2020 Chinese gross domestic product per capita of RMB 72,447 (USD 17,307) as a cost-effectiveness threshold, FDI would be considered cost-effective in China.
PLAIN LANGUAGE SUMMARY
Ferric derisomaltose (FDI) was approved in February 2021 for the treatment of iron deficiency anemia (IDA) in China and allows for fast iron correction in one visit with a good safety profile. The current standard of care in China is iron sucrose (IS). Clinical and economic decision-making can benefit from having longer-term projections on the benefits and costs of new medications relative to the current standard of care, which is why we conducted the first cost-utility analysis of FDI and IS for China. We developed a patient-level model that captured the effects of the iron formulations on IDA, in addition to incidences of adverse events and hypersensitivity reactions (HSRs) associated with either formulation. Costs of the iron formulations, their administration, and of treatments for adverse events and HSR were modeled alongside the quality of life effects of IDA, adverse events, HSRs, and iron infusions. We used published clinical data and Chinese cost data to inform our model. Our results show that FDI was associated with higher quality-adjusted life expectancy than IS, regardless of the perspective of the analysis, and higher total costs from the healthcare system perspective. From a societal perspective, FDI was associated with lower costs due to reduced travel and waiting time and smaller productivity losses given there were fewer appointments. These results imply that FDI is likely good value for money for the healthcare system and indeed cost-saving for society relative to IS, which has so far been the most widely used IV iron treatment in China.
Introduction
Despite substantial improvements in recent decades, anemia and iron deficiency anemia (IDA) remains prevalent in China, with an estimated 282 million anemia cases, of which approximately 176 million are due to IDACitation1. Chinese women of reproductive age are particularly at risk. A nationwide study of pregnant women in 2016 estimated the prevalence of anemia and IDA to be 19.8% and 13.9%, respectivelyCitation2. In a longitudinal study in southwest China, the overall anemia prevalence in women of reproductive age who had been or intended to become pregnant was 16.4% in 2018 (23.0% in 2014)Citation3. In women of reproductive age living in Shanghai, the prevalence of IDA was 14.8%Citation4.
The burden of anemia and IDA in China is increasingly studied among elderly and chronically ill people. Chronic kidney disease (CKD) and its association with anemia have become a particular concern, given a CKD prevalence of 10.8%Citation5 that is expected to increase further in coming yearsCitation6. The Chinese Chronic Kidney Disease Cohort Study (C-STRIDE), based on patients with pre-dialysis CKD, showed that 10.3% of patients had Hb levels <10 g/dL and that patients’ quality of life (QoL) was reduced as levels of hemoglobin decreasedCitation7. More severe anemia has also been suggested to impair work productivity in Chinese patients with CKDCitation8.
Anemia and IDA also are frequently encountered challenges in managing patients with cancer and those undergoing surgery. Huang et al. showed that, in Chinese patients undergoing treatment for non-small cell lung cancer, pre-treatment anemia was associated with a reduction of 6.9 months in median overall survival and an increase in the risk of death by 60% relative to no pre-treatment anemiaCitation9. Anemia is also a frequent complication following major surgery, for cancer and other diseasesCitation10. Lin et al. reported that 28% of patients had preoperative anemia and that preoperative hemoglobin levels of less than 130 g/L were associated with increased length of hospital stay and hospital costsCitation11.
Iron administration is an important therapeutic option to meet the outlined burden imposed by anemia and IDA. Oral iron is widely used for iron supplementation, including in China, but its frequent association with gastrointestinal side effects and relatively low effectivenessCitation12 make it a suboptimal choice if patients have chronic inflammatory diseases, if an iron deficit is substantial, or if an iron deficit requires rapid correction, including in perioperative settingsCitation13.
In these scenarios, intravenous (IV) iron is likely preferable as IV iron can correct iron deficits safely and rapidlyCitation12, and the benefits of IV iron are documented across conditions and settingsCitation14. IV iron is more effective in resolving pre-treatment anemia and iron deficiency than oral ironCitation15, and there is evidence to suggest that IV iron increases hemoglobin levels relative to placebo and oral iron before major surgery and in the treatment of chemotherapy-induced anemiaCitation16,Citation17.
Despite the availability of effective IV iron treatment, anemia and IDA are often not managed appropriatelyCitation18. In China, only an estimated 20% of patients with mild and 50% of patients with severe anemia receive treatmentCitation19. In C-STRIDE, nearly two-thirds of patients with Hb levels <10 g/dL did not receive anemia treatmentCitation7, while only 1.4% and 2.0% of patients with non-dialysis-dependent and dialysis-dependent CKD, respectively, received intravenous (IV) iron for anemia attributed to iron deficiency (ID)Citation8. Non-dialysis-dependent patients with CKD in Shanghai mostly (73%) received no iron supplementation at all, and only 8.2% achieved the treatment target of 11–12 g/dLCitation20. Similar data were reported by Zhou et al.Citation21, for dialysis-dependent patients with CKD from nine major dialysis facilities across China, of whom 60% failed to reach a target hemoglobin level of 11 g/dL, despite 85% of patients receiving treatment with erythropoietin and 40% receiving oral iron (only 20% received IV iron). This is mirrored in a recent patient survey by Hao et al. Citation22, in which only 26% of patients with CKD and anemia reported receiving injections (either erythropoietin-stimulating agents or intravenous infusions), while 69% reported receiving dietary advice, 64% reported iron supplements, and 53% reported oral iron treatment for their anemia. Overall, 41% of patients with CKD and anemia reported receiving no treatment for anemia.
The treatment of ID and anemia in China could likely be improved by moving from oral to IV iron in a range of clinical contexts. However, as pointed out by Li and ZhangCitation19, the use of traditional IV iron formulations in China is unsatisfactory as multiple infusions are required to correct iron deficits, thereby increasing the risk of patient non-adherence to treatment, and given concerns around the safety of IV iron, including fear of hypersensitivity reactions (HSR).
For IV iron sucrose (IS) – the current standard IV iron formulation used in China – safety concerns limit the administration of IS to 200 mg per infusion and at most three infusions per week, so iron deficit correction requires several visits to a healthcare providerCitation23,Citation24. In contrast, ferric derisomaltose (FDI), which was recently approved in China, is associated with a more rapid and more marked hematological response than iron sucrose, and FDI allows for a single-dose correction of iron deficits as it can be dosed at up to 20 mg per kilogram of body weight. FDI, therefore, reduces the need for repeat visits and the risk of non-adherence associated with longer-term treatmentCitation19,Citation23,Citation25. Relative to iron sucrose, FDI is also associated with a lower incidence of HSRsCitation26.
Differences such as these between FDI and IS, when combined with cost and quality of life data, should be modeled in cost-utility analyses (CUA) to inform clinical and reimbursement decision-making; however, no such analyses have yet been conducted in China. The present study, therefore, aimed to evaluate, in the Chinese setting, the cost-utility of FDI relative to IS, for the treatment of patients with IDA.
Methods
A Consolidated Health Economic Evaluation Reporting Standards 2022 checklist for this study can be found in Supplementary Table 1Citation27.
Supporting studies
Prior to commencing the development of the cost-utility model, two supporting studies were conducted to inform the model development and generate data not otherwise available. The first supporting study was an expert survey of nine senior physicians from disciplines reflecting key etiologies of chronic and acute IDA, including hematology, nephrology, gynecology, obstetrics, and orthopedics. All physicians were affiliated with tertiary (AAA) hospitals, and hospitals were chosen from across China (Supplementary Table 2). Physicians completed a questionnaire covering epidemiological data and the IDA treatment pathway, including patient characteristics, IDA recurrence, and treatment persistence, as well as resource use and costs, including time and cost per infusion, length and cost of inpatient stay, and outpatient procedures and tests.
The second supporting study was a time trade-off (TTO) study, implemented as an online questionnaire in a general Chinese population sample, designed to elicit preferences for and utilities associated with the number, duration, and risk of IV iron infusionsCitation28. Briefly, participants were presented with health state vignettes that combined treatment process attributes (1, 2, 5, or 7 infusions per year; infusion duration of 15–30 min or 30–60 min) and risk (no versus 1/1,000 risk of long-term adverse events). Disutilities for infusion numbers not directly included in vignettes (e.g. four infusions) were derived using non-linear regression analysisCitation28.
Model structure
The model was structured as a patient-level, discrete-time, illness-death model, implemented in Microsoft Excel (Microsoft, Redmond, WA, USA). A patient-level framework for modeling IV iron was used previously in a CUA for the United KingdomCitation29 and is aligned with National Institute for Health and Care Excellence technical guidanceCitation30, which recommends patient-level simulations for non-linear relationships between patient characteristics and model outcomes, as is the case for the relationship between baseline body weight, hemoglobin levels, and iron demand. The framework also facilitated probabilistic modeling to account for stochastic (or first-order) uncertainty, uncertainty around model parameters (second-order uncertainty), and heterogeneity in patient characteristics.
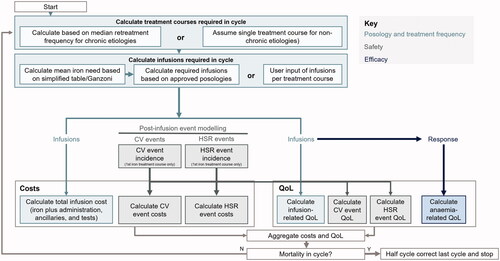
Modeled patients were assigned initial age, body weight, and Hb values sampled from baseline distributions. Based on these characteristics, a mean iron need was determined, and the required number of treatment courses was calculated for each patient in each cycle (). Chronic IDA etiologies were distinguished from non-chronic etiologies, with the latter requiring a single treatment course. From the infusions administered per cycle, the hematologic response (change in Hb levels), anemia-related QoL, and infusion-related costs were modeled. In addition, the model accounted for the incidence of post-infusion events, namely cardiovascular (CV) events and HSRs, as well as the costs and QoL impact of these events. Patient trajectories were evaluated using Monte Carlo methods, with costs and QoL outcomes aggregated per cycle.
Survival and quality-adjusted life expectancy (QALE; expressed as quality-adjusted life-years, QALYs), as well as cost outcomes, were summarized across modeled patients for each formulation and combined to yield an incremental cost-utility ratio (ICUR).
Patient characteristics
Baseline patient age, body weight, and pre-treatment hemoglobin levels were sourced from publicly available data from a randomized controlled trial (RCT; NCT03591406) comparing ferric carboxymaltose (FCM) with IS for the treatment of IDA in China (). These data had been validated in the above-described physician survey to reflect the target population, including broad eligibility criteria covering a range of etiologies. The proportion of patients with chronic (as opposed to non-chronic) etiology of IDA was estimated to be 63.95% based on an analysis of prescription data covering 146 hospitals in nine locations, including Guangzhou and ShanghaiCitation31. The proportion of patients receiving IV iron as outpatients (as opposed to inpatients) was taken to be 15.3% based on the same data source. Median times to symptom recurrence and retreatment in patients with chronic IDA were assumed to be 10 and 16 months, respectively (a repeat IV iron course was assumed to start if retreatment became necessary)Citation32. Background mortality was modeled using data from 2019 Chinese life tablesCitation33.
Table 1. Baseline population characteristics and disease parameters.
Clinical data
Data informing the clinical effectiveness and safety estimates used in the model were sourced preferentially from RCTs. Trials were identified from a 2019 systematic literature review that conducted an indirect treatment comparison of FDI and ferric carboxymaltose via iron sucroseCitation34 and a 2020 network meta-analysis on the risk of hypophosphatemia following IV ironCitation35, with incremental literature search updates performed in PubMed (including MEDLINE) in October 2021 to identify any newly published RCTs.
The effectiveness of FDI versus IS was modeled as the time to hematological response (Hb increase ≥2 g/dL from baseline), based on the PROVIDE RCT (NCT02130063), which was the only available RCT in patients with IDA of different etiologies that was powered to detect differences in time to hematological response between FDI and ISCitation24. For modeling, the proportion with a hematological response at different time points was preferred to achieving a fixed target hemoglobin level after a set period, as different target levels are used in the literature and in clinical practice in China for a different patient population with IDACitation7,Citation20,Citation21 and because response proportions over time are naturally suited to modelling efficacy as time in the model progresses. Data from PROVIDE showed a faster response with FDI than IS over the first 5 weeks (Supplementary Table 3). Beyond 5 weeks, response proportions were assumed to remain at their 5-week value for FDI and IS, respectively.
The incidence of CV events was sourced from two safety-focused RCTs comparing FDI and IS, namely the FERWON-IDACitation23 and FERWON-NEPHROCitation36 studies (Supplementary Table 4), as the PROVIDE trial did not report detailed CV events. The HSR incidence was taken from an indirect comparison study including FDI and ISCitation26 (Supplementary Table 4).
Resource use
The number of infusions per treatment course was calculated from baseline body weight and hemoglobin values as per the respective label insert. A simplified table approach could therefore be used for FDI (see Supplementary Table 5), while the more complex Ganzoni formula had to be used to determine the iron need for ISCitation24.
Assumptions around in- and outpatient resource utilization and laboratory tests were informed by the supporting physician survey. As most IV iron administrations are performed in inpatient settings, the mean number of days in the hospital per administration was elicited from the survey (Supplementary Table 6). The mean number of hospital days was calculated from physicians’ estimates as 1.27 for FDI and 1.36 for IS due to FDI’s improved safety profile and smaller risk of adverse events. Similarly, fewer outpatient visits and laboratory tests (routine blood, ferritin, and serum iron) were required per treatment course with FDI.
Productivity losses resulting from hospitalization, consultation, and travel were calculated for the patient and one caregiver accompanying the patient. An 8-hour working day was assumed. Surveyed physicians estimated that, on average, traveling to the treatment location was associated with a 1-hour one-way trip, while an outpatient visit was estimated to last 4 h. An inpatient stay was assumed to take up all working hours in a day for the patient, with no caregiver time required. Productivity losses were weighted by the proportion of patients and caregivers in employment in each age group, using labor force participation rates published by the National Bureau of Statistics (NBS).
Costs
Costs were expressed in 2021 Renminbi (RMB), with cost-related model results also presented as US dollars (USD) following conversion using the 2020 purchasing power parity for RMB relative to USD by the Organisation for Economic Co-operation and DevelopmentCitation37.
The cost of FDI was obtained from the national insurance tariff (1 mL containing 100 mg of FDI at a cost of RMB 192.5), for a unit cost of RMB 1.93 per mg. The cost of IS was obtained from public drug procurement platforms for the originator IS (5 mL containing 100 mg of IS at a cost of RMB 84.95). An alternative cost for use in a scenario analysis was calculated as the market share-weighted average cost of the originator and generic IS products, at RMB 42.1, with market shares obtained from the IQVIA Drug Sales database.
IV infusion administration costs consisted of service and equipment charges. The former was RMB 11.66 per administration, based on the median of public medical service fees in Beijing, Guangzhou, Shanghai, Shenzhen, and Xiamen; the latter was RMB 22.00 from a public hospital procurement platform, for a total per-administration fee of RMB 33.66 (). The daily cost of an inpatient stay, the cost per outpatient visit and laboratory test costs were obtained as the respective median public list price from the same five cities as above. Treatment costs of adverse events were obtained from the physician survey, the NBS, and the literatureCitation38–45.
Table 2. Unit costs.
These costs were used to model cost-utility outcomes from both a healthcare system perspective, which excluded transportation costs and productivity losses, and a societal perspective, which included all costs and productivity losses listed above.
Health state utilities
The baseline health state utility (HSU) value for patients with IDA was the general Chinese population mean of 0.985Citation46 to which an IDA-specific utility decrement of 0.15Citation47 was applied in line with a previous pharmacoeconomic evaluation of FDI conducted for the Canadian Agency for Drugs and Technologies in HealthCitation48. The annual per-infusion disutility was derived from a non-linear regression applied to the supporting TTO survey (see subsection on Supporting studies) and calculated to be 0.0161. Utility decrements associated with CV events and HSR were derived from the literature (Supplementary Table 4)Citation49–54.
Model parameters
The base case analysis was performed over a 5-year time horizon, which was considered sufficient to account for potential IDA relapse. The model was run with a monthly cycle length, which facilitated accurate modeling of multiple treatment courses and adverse events over the course of a year with a reasonable computational burden. Both benefits and costs were discounted at 5% per annumCitation55. As there is no formal willingness-to-pay (WTP) threshold in China, cost-utility results were assessed against the gross domestic product (GDP) per capita, which was RMB 72,447 in 2020Citation56.
Scenario and sensitivity analyses
The base case scenario was conducted from a healthcare system perspective, using the originator IS unit cost. A second scenario took the same perspective but used an IS unit cost calculated as the market share-weighted average of the originator and generic IS unit costs. A third scenario took a societal perspective while using the originator IS unit costs.
One-way sensitivity analyses were performed to assess the robustness of the model results in changes in input values, including patient baseline characteristics, costs, and utilities. In these analyses, baseline values were varied by ±20%. An additional analysis was conducted in which the difference in hematological response after 5 weeks was abolished, with values set to zero for both treatments for the remainder of the analysis.
Probabilistic sensitivity analysis (PSA) was performed using 1,000 simulated cohorts of 1,000 patients each. Baseline patient characteristics were sampled from lognormal distributions and the baseline utility from a beta distribution, while adverse event treatment costs and utility decrements were sampled from normal distributions (the remaining inputs were not sampled). An additional analysis of sampling costs from gamma distributions was conducted to assess the effect of parametric distribution selection on probabilistic model outcomesCitation57. Distributions were parametrized using mean and standard deviation (SD) values. Where SD values could not be obtained or calculated from the source, the SD was assumed to be 10% of the mean.
Results
Base case analyses
Survival and QoL
FDI was associated with an incremental gain in QALE relative to IS (). The difference of 0.078 QALYs in favor of FDI resulted from reduced anemia-related QALY loss (−0.572 QALYs with FDI versus −0.614 QALYs with IS) and lower incidence of adverse events and HSRs (−0.002 QALYs with FDI versus −0.003 QALYs with IS). In addition, treatment with FDI was associated with fewer iron infusions per patient (5.7 versus 19.6 infusions over five years), despite delivering more iron per treatment course (1,552.4 versus 1,235.4 mg) relative to IS; infusion-related reductions in QALE were also, therefore, smaller with FDI than IS (−0.057 QALYs versus −0.092 QALYs).
Table 3. Cost-utility outcomes, in RMB [and USD] per QALY, for different scenarios over a 5-year time horizon.
Cost and cost-utility
In the base case scenario, FDI was associated with incremental costs of RMB 1,934 (USD 462) relative to IS (). This difference was due to higher acquisitions costs of FDI (RMB 8,176 [USD 1,953] versus RMB 2,871 [USD 686]), which were partially offset by reduced costs for IV iron administration and treatment of adverse events and HSR (Supplementary Table 7). The increased QALE at increased costs for FDI relative to IS yielded an ICUR of RMB 24,901 (USD 5,949) per QALY gained in the base scenario.
In the alternative scenario using weighted IS unit costs, the total costs of IS were reduced to RMB 6,998 (USD 1,672), yielding a total cost difference of RMB 3,382 (USD 808) relative to FDI. The resulting ICUR was RMB 43,549 (USD 10,403) per QALY gained. In contrast, in the scenario using a societal perspective, FDI was associated with lower total costs relative to IS (RMB 12,396 [USD 2,961] versus RMB 14,258 [USD 3,406]) as direct nonmedical and transportation costs and productivity losses were reduced with FDI (Supplementary Table 7). FDI was therefore dominant as it was associated with improved QALE at lower societal costs relative to IS.
Sensitivity analyses
One-way sensitivity analyses
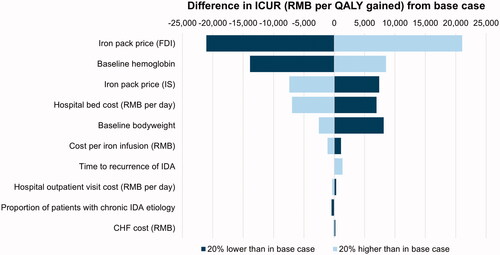
In deterministic, one-way sensitivity analyses, FDI acquisition costs and patients’ baseline Hb levels were identified as key drivers of ICURs across all three scenarios (). They were followed by IS acquisition costs and daily hospitalization costs in the base case scenario, hospitalization costs and patient’s baseline body weight in the alternative scenario using market share-weighted originator and generic IS costs, and by patients’ baseline body weight and IS acquisition costs in the scenario reporting a societal perspective. Additional drivers of ICURs included patient baseline body weight and age, the proportion of patients with chronic (as opposed to acute) IDA, the median time to retreatment for IDA, and costs of outpatient visits and infusion administrations.
Figure 2. Tornado diagram for the base case scenario Abbreviations: CHF; Congestive Heart Failure; FDI, Ferric Derisomaltose; ICUR, Incremental Cost-Utility Ratio; IDA, Iron Deficiency Anemia; IS, Iron Sucrose; QALY, Quality-Adjusted Life-Year; RMB, Renminbi.
When the hematological response was set to initial values (i.e. to zero) in both arms after 5 weeks, the ICURs were RMB 43,809 (USD 10,466) and RMB 76,618 (USD 18,303) per QALY gained for the base and first alternative scenario, while FDI was dominant over IS in the analysis from a societal perspective.
Probabilistic sensitivity analysis
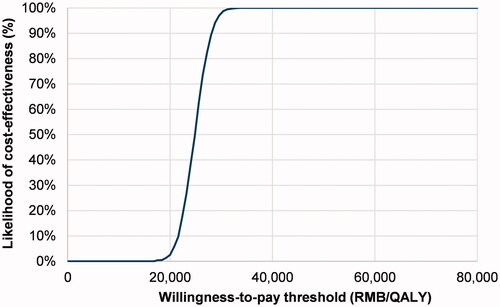
In the PSA around the base case scenario, results from all model iterations fell in the northeast quadrant of the scatterplot (Supplementary Figure 1), and there was a 100% likelihood that FDI would be cost-effective relative to IS at a WTP threshold equal to GDP per capita, i.e. RMB 72,477 (USD 17,307) per QALY gained (). In the alternative healthcare system scenario that used the market share-weighted originator and generic IS prices, there was also a 100% likelihood of FDI being cost-effective at a WTP threshold of RMB 72,477 (USD 17,307) per QALY gained and FDI was more likely to be cost-effective than not at WTP thresholds of RMB 44,000 (USD 10,501) per QALY gained and above (Supplementary Figures 3 and 4). Finally, from a societal perspective, FDI was cost-effective at all WTP thresholds as 99.9% of PSA iterations fell in the southeast quadrant of the scatterplot (Supplementary Figures 6 and 7), associated with reduced costs and QALY gains relative to IS. Using gamma distributions for costs did not materially affect health economic outcomes in any scenario (results not shown).
Discussion
This study used a newly developed patient-level cost-utility model to evaluate the costs and benefits of FDI relative to IS in the treatment of IDA in China. Accounting for both acute and chronic IDA as well as a range of adverse CV and HSR events, the analysis showed that, over 5 years, FDI was associated with ICURs between RMB 24,901 (USD 5,949) in the base case scenario and 41,939 (USD 10,019) per QALY gained in the alternative scenario relative to IS from a healthcare system perspective, and that FDI dominated IS from a societal perspective. At the 2020 GDP per capita value of RMB 72,447 (USD 17,307) in China, FDI would therefore likely provide good value for money relative to IS. These findings were aligned with previous analyses for other settingsCitation48 and reflected the improved effectiveness and safety of FDI relative to IS, which resulted in reduced costs and smaller QoL losses due to earlier hematological responseCitation24 as well as due to fewer adverse events and HSRCitation23,Citation26,Citation36, thereby partially offsetting higher FDI acquisition costs.
In addition, as a higher dose of iron can be delivered with FDI, iron deficits in the model could be corrected with fewer IV administrations than with IS, which further reduced administration costs and infusion-related reductions in QoL, while also saving patients and their caregivers’ time. Such economic benefits might be relatively limited in certain patient groups, such as those regularly undergoing dialysis; however, as current dialysis care in China is unable to meet IDA treatment needsCitation7,Citation20,Citation21, there are still likely to be economic benefits of using a more efficacious treatment that requires fewer administrations.
The physician survey and the TTO study conducted to inform model development confirmed the notion that an IV iron treatment option allowing for fewer consultations and infusions would be welcome in China, to increase patient compliance with treatment and reduce patient loss between infusions, while also meeting preferences for more convenient treatment. However, as no quantitative data on treatment compliance are currently available for China, treatment compliance could not be included in the cost-utility model, which represents a limitation of the present analysis.
A similar limitation resulted from basing differences in dosing in the model on those from an RCT (PROVIDE) which may overestimate iron doses delivered over a treatment course in clinical practice, where patient follow-up and management is less rigorous than in an RCT. No real-world data were available to correct for this limitation, so readers should bear in mind that iron doses might be lower in clinical reality than estimated here, particularly given the compliance concerns for formulations such as IS that require multiple administrations.
More broadly, the analysis was limited by a lack of country-specific data on IV iron infusions in general, similar to previous health economic analyses of anemia treatments in ChinaCitation58. Data on FDI and IS efficacy and safety as well as on some health state utilities, therefore, had to be sourced from non-Chinese populations, including several US-based RCTsCitation23,Citation24,Citation36, or, in the case of missing variation around some input values, had to be assumedCitation48. In contrast, patient baseline characteristics, costs and resource use could be sourced mostly from Chinese sources. While a more homogenous, China-specific set of input parameters would have been preferable, physicians’ and decision-makers’ need for timely information regarding the benefits and costs of a newly available IV iron formulation was considered to outweigh the limitations associated with the use of non-Chinese clinical data.
The analysis also had several strengths. The use of a patient-level simulation model, compared to a cohort-level approachCitation30,Citation58, allowed to better account for the non-linearity in the relationship between Hb and body weight as well as patient-level infusion timing and heterogeneity in Hb, body weight, and age, thereby yielding a more nuanced representation of patient trajectories in IDA. The present model also expanded on previous analysesCitation48,Citation58 by including a range of cardiovascular adverse events and HSR, each associated with a specific disutility and cost. An additional strength of this study is its contribution to resource use and costing data as well as to utility data associated with anemia, iron deficiency, and IDA in China, through a dedicated physician survey and TTO study.
Conclusions
The present cost-utility analysis was the first comparison of the costs and benefits of FDI relative to IS for the treatment of IDA in China. Relative to IS, FDI was projected to reduce costs associated with IV infusions and treatment of adverse events (at higher acquisition costs) and was associated with improved infusion-related QoL outcomes. Model results suggested that FDI was good value for money in China relative to IS and would likely reduce societal costs associated with IDA.
Transparency
Declaration of funding
Pharmacosmos A/S funded the development of the simulation model and analysis, preparation of the manuscript, and the article processing charge for the manuscript.
Declaration of financial/other relationships
SH, LL, DW, and YZ have received honoraria from Pharmacosmos China for participation in advisory board meetings conducted by Pharmacosmos China. RFP is a director and shareholder in, and JP is a full-time employee of, Covalence Research Ltd, which received consultancy fees from Pharmacosmos A/S to develop the simulation model and analysis and prepare the manuscript.
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
RFP was involved in the conception and design of the analysis. RFP developed the simulation model and conducted the statistical and health economic analyses. JP prepared the first draft of the manuscript, which was revised critically for intellectual content by all authors. All authors approved the final version to be published. All authors agree to be accountable for all aspects of the work.
Previous presentations
The supporting time trade-off study was previously presented at Virtual ISPOR Europe 2021 (November 30 to December 1, 2021) as poster POSA319.
Supplemental Material
Download PDF (372.6 KB)Supplemental Material
Download MS Word (1 MB)References
- Kassebaum NJ. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30(2):561–308.
- Tan J, He G, Qi Y, et al. Prevalence of anemia and Iron Deficiency Anemia in Chinese Pregnant Women (IRON WOMEN): a national cross-sectional survey. BMC Pregnancy Childbirth. 2020;20(1):670.
- Wu Y, Ye H, Liu J, et al. Prevalence of anemia and sociodemographic characteristics among pregnant and non-pregnant women in southwest China: a longitudinal observational study. BMC Pregnancy Childbirth. 2020;20(1):535.
- Yamamoto K Wang N Takita M, et al. Iron deficiency anaemia: its prevalence among women of reproductive age in Shanghai and Tokyo and links to body mass index. Cureus. 2020;12:e9436.
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822.
- Peng Z, Wang J, Yuan Q, et al. Clinical features and CKD-related quality of life in patients with CKD G3a and CKD G3b in China: results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). BMC Nephrol. 2017;18(1):311.
- Shen Y, Wang J, Yuan J, et al. Anemia among Chinese patients with chronic kidney disease and its association with quality of life – results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol. 2021;22(1):64.
- Haalen H v, Sloand J, Moon R, et al. Drug treatment patterns and work productivity in chronic kidney disease patients with anemia in China: cross sectional analysis of real-world data. Kidney Res Clin Pract. 2020;39(3):318–333.
- Huang Y, Su C, Jiang H, et al. The association between pretreatment anemia and overall survival in advanced non-small cell lung cancer: a retrospective cohort study using propensity score matching. J Cancer. 2022;13(1):51–61.
- Kalra SK, Thilagar B, Khambaty M, et al. Post-operative anemia after major surgery: a brief review. Curr Emerg Hosp Med Rep. 2021;9:89–95.
- Lin J, Wang C, Liu J, et al. Prevalence and intervention of preoperative anemia in Chinese adults: a retrospective cross-sectional study based on national preoperative anemia database. EClinicalMedicine. 2021;36:100894.
- DeLoughery TG. Safety of oral and intravenous iron. Acta Haematol. 2019;142(1):8–12.
- Auerbach M, Gafter-Gvili A, Macdougall IC. Intravenous iron: a framework for changing the management of iron deficiency. Lancet Haematol. 2020;7(4):e342–e350.
- Stein J, Haas JS, Ong SH, et al. Oral versus intravenous iron therapy in patients with inflammatory bowel disease and iron deficiency with and without anemia in Germany – a real-world evidence analysis. CEOR. 2018;10:93–103.
- Keeler BD, Simpson JA, Ng O, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104(3):214–221.
- Elhenawy AM, Meyer SR, Bagshaw SM, et al. Role of preoperative intravenous iron therapy to correct anemia before major surgery: a systematic review and meta-analysis. Syst Rev. 2021;10(1):36.
- Gafter-Gvili A, Rozen-Zvi B, Vidal L, et al. Intravenous iron supplementation for the treatment of chemotherapy-induced anaemia – systematic review and meta-analysis of randomised controlled trials. Acta Oncol. 2013;52(1):18–29.
- Hufnagel DH, Mehta ST, Ezekwe C, et al. Prevalence of anemia and compliance with NCCN guidelines for evaluation and treatment of anemia in patients with gynecologic cancer. J Natl Compr Canc Netw. 2021;19(5):513–520.
- Li LJ, Zhang LS. 缺铁性贫血规范化诊治的若干问题 [considerations on the standardized diagnosis and treatment of iron-deficiency anemia]. Zhonghua Yi Xue Za Zhi. 2021;101(40):3266–3270.
- Li Y, Shi H, Wang W-M, et al. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: first multicenter, cross-sectional study. Medicine. 2016;95(24):e3872.
- Zhou Q, Jiang J, Wu S, et al. Current pattern of Chinese dialysis units: a cohort study in a representative sample of units. Chin Med J. 2012;125(19):3434–3439.
- Hao C-M, Wittbrodt ET, Palaka E, et al. Understanding patient perspectives and awareness of the impact and treatment of anemia with chronic kidney disease: a patient survey in China. Int J Nephrol Renovasc Dis. 2021;14:53–64.
- Auerbach M, Henry D, Derman RJ, et al. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J Hematol. 2019;94(9):1007–1014.
- Derman R, Roman E, Modiano MR, et al. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol. 2017;92(3):286–291.
- Kassianides X, Bodington R, Bhandari S. An evaluation of ferric derisomaltose as a treatment for anemia. Expert Rev Hematol. 2021;14(1):7–29.
- Pollock RF, Biggar P. Indirect methods of comparison of the safety of ferric derisomaltose, iron sucrose and ferric carboxymaltose in the treatment of iron deficiency anemia. Expert Rev Hematol. 2020;13(2):187–195.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 2022;20(1):23.
- Wu D, Zhang Y, Boegelund M, et al. PR4 health utility assessment of different options for treatment of iron deficiency anemia: results from time-trade-off study in China. Value Health. 2021;24:S197.
- Pollock RF, Muduma G. A patient-level cost-effectiveness analysis of iron isomaltoside versus ferric carboxymaltose for the treatment of iron deficiency anemia in the United Kingdom. J Med Econ. 2020;23(7):751–759.
- Davis S, Stevenson M, Tappenden P, et al. NICE DSU Technical Support Document 15: Cost-effectiveness modelling using patient-level simulation [Internet]. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2014. [cited 2022 Jan 04]. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD15_Patient-level_simulation.pdf.
- IQVIA. Prescription database; 2021. Shanghai: IQVIA.
- Kulnigg S, Teischinger L, Dejaco C, et al. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol. 2009;104(6):1460–1467.
- Global Health Observatory. Life tables by country: China. Geneva: World Health Organization; 2020.
- Pollock RF, Muduma G. A systematic literature review and indirect comparison of iron isomaltoside and ferric carboxymaltose in iron deficiency anemia after failure or intolerance of oral iron treatment. Expert Rev Hematol. 2019;12(2):129–136.
- Bellos I, Frountzas M, Pergialiotis V. Comparative risk of hypophosphatemia following the administration of intravenous iron formulations: a network meta-analysis. Transfus Med Rev. 2020;34(3):188–194.
- Bhandari S, Kalra PA, Berkowitz M, et al. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol Dial Transplant. 2021;36(1):111–120.
- Organisation for Economic Co-operation and Development. Purchasing power parities. Paris: Organisation for Economic Co-operation and Development; 2021.
- Hu J, Fan X. Song S. 64例胺碘酮治疗老年冠心病心律不齐的临床疗效分析 [clinical efficacy analysis of 64 cases of amiodarone in the treatment of arrhythmia in elderly people with coronary heart disease]. China Med Guide. 2014;12:103–104.
- Xin Y. 高血压控制与治疗费用的观察 [observation of hypertension control and treatment costs]. Qinghai Med J. 2012;42:74–75.
- Gao D. 大剂量地塞米松治疗过敏性休克的疗效及安全性分析 [analysis of the efficacy and safety of high-dose dexamethasone in the treatment of anaphylaxis]. Chin Folk Rem. 2020;028:68–69.
- Zhang L, Liu B, Wang C. Pharmaceutical analysis of different antibiotic regimens in the treatment of lower respiratory tract infection. Exp Ther Med. 2018;16:2369–2374.
- Jackson JD, Cotton SE, Bruce Wirta S, et al. Burden of heart failure on patients from China: results from a cross-sectional survey. Drug Des Devel Ther. 2018;12:1659–1668.
- Yang L, Li G. IV3000透明敷贴联合水胶体敷料治疗PICC相关性皮肤过敏的效果观察 [observation on the effect of IV3000 transparent dressing combined with hydrocolloid dressing in the treatment of PICC-related skin allergy]. Contemporary Nursing. 2020;27:127–128.
- Wen X. 如何预防和治疗低血压 [How to prevent and treat hypotension] [Internet]. 大众健康报 [Popular Health News]; 2020. [cited 2022 Jan 04]. Available from: http://zkbctech.com/article/1852/2360.html.
- Lin S, Chen H. 舒血宁联合葛根素治疗不稳定型心绞痛的临床疗效评价 [evaluation of clinical efficacy of Shuxuening injection combined with puerarin in the treatment of coronary heart disease with unstable angina pectoris]. Chin J Prim Med Pharm. 2018;25:2628–2631.
- Wu C, Gong Y, Wu J, et al. Chinese version of the EQ-5D preference weights: applicability in a Chinese general population. PLOS One. 2016;11(10):e0164334.
- Strauss WE, Auerbach M. Health-related quality of life in patients with iron deficiency anemia: impact of treatment with intravenous iron. Patient Relat Outcome Meas. 2018;9:285–298.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Pharmacoeconomic review report: iron isomaltoside 1000 (monoferric) (pharmacosmos a/S). Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH); 2020.
- Davies EW, Matza LS, Worth G, et al. Health state utilities associated with major clinical events in the context of secondary hyperparathyroidism and chronic kidney disease requiring dialysis. Health Qual Life Outcomes. 2015;13:90.
- Li C, Zhou H, Wang P. Health utility of type 2 diabetes patients using basal insulin in China: results from the beyond II study. Acta Diabetol. 2021;58(3):329–339.
- Wehler E, Storm M, Kowal S, et al. PS1442 A health state utility model estimating the impact of ivosidenib on quality of life in patients with relapsed/refractory acute myeloid leukemia. Poster session presented at: 36. Quality of life, palliative care, ethics and health economics. 23rd Congress of the European Hematology Association; 2018. Jun 14–17; Stockholm.
- Greenhawt M, Shaker M. Determining levers of cost-effectiveness for screening infants at high risk for peanut sensitization before early peanut introduction. JAMA Netw Open. 2019;2(12):e1918041.
- Ronaldson S, Taylor M, Bech PG, et al. Economic evaluation of SQ-standardized grass allergy immunotherapy tablet (Grazax®) in children. Clinicoecon Outcomes Res. 2014;6:187–196.
- Shiroiwa T, Fukuda T, Tsutani K. Health utility scores of colorectal cancer based on societal preference in Japan. Qual Life Res. 2009;18(8):1095–1103.
- Chen Y, Guan H, Han S, et al. China guidelines for pharmacoeconomic evaluations: 2020. edition [Internet]. Chinese Pharmaceutical Association, China Society for Pharmacoeconomics and Outcomes Research and ISPOR Beijing Chapter; 2020 [cited 2022 Jan 04]. Available from: https://tools.ispor.org/PEguidelines/source/China-Guidelines-for-Pharmacoeconomic-Evaluations-2020.pdf.
- National Bureau of Statistics of China. Statistical communiqué of the People’s Republic of China on the 2020 national economic and social development. Beijing: National Bureau of Statistics of China; 2021.
- Thompson SG, Nixon RM. How sensitive are cost-effectiveness analyses to choice of parametric distributions? Med Decis Making. 2005;25(4):416–423.
- Hu Z, Tao H, Shi A, et al. The efficacy and economic evaluation of Roxadustat treatment for anemia in patients with kidney disease not receiving dialysis. Expert Rev Pharmacoecon Outcomes Res. 2020;20(4):411–418.