 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Leveraging the safety profile of the synthetic hygroscopic cervical dilator (SHCD), one potential way to reduce the burden-of-care provision in the labor-and-delivery unit without compromising safety is to introduce a low-acuity care room (ripening room) for patients undergoing cervical ripening as a part of labor induction at term.
Methods
Implementing a ripening room using SHCDs was compared to scenarios using prostaglandins including a dinoprostone insert (PGE2 insert) or gel (PGE2 gel) and misoprostol given orally (oral PGE1) or vaginally (vaginal PGE1). A theoretical, cost-consequence model was developed to assess costs, staff time, and selected clinical outcomes related to cervical ripening. The model assessed a hypothetical cohort where patients remained in hospital from admission for induction of labor (IOL) to delivery, taking the US labor-and-delivery unit perspective. Model inputs were taken from structured searches of PubMed and ClinicalTrials.gov, published US guidance, and clinical practice. Results are presented as mean (95% credible interval [CrI]).
Results
The ripening room using SHCDs cost US$3,210 and required 10.22 hours (h) of nurse time on average per patient. The cost difference to prostaglandins ranged from −$894 (−$2,269 to $398) for PGE2 gel to +$460 (−$1,467 to $1,539) for vaginal PGE1. Mean nurse time was shorter than all prostaglandins, with time savings ranging from −7.05 (−24.55 to 5.73) h for PGE2 insert to −0.97 (−14.69 to 9.59) h for vaginal PGE1. When outcomes of the probabilistic sensitivity analysis were ranked from 1 (best) to 5 (worst), the ripening room using SHCDs ranked 1.94 for costs and 1.97 for nurse time. In a nulliparous population, results improved for the ripening room using SHCDs relative to all prostaglandins.
Conclusion
In this theoretical study, implementing a ripening room using SHCDs resulted in the lowest time burden and the second lowest costs. The cheapest option for preinduction cervical ripening was vaginal misoprostol.
PLAIN LANGUAGE SUMMARY
It is estimated that approximately 20% of deliveries in the USA are submitted to cervical ripening prior to induction of labor to facilitate a vaginal delivery. Cervical ripening can be achieved either by administering a synthetic hormone, called a prostaglandin (e.g. misoprostol or dinoprostone), or by using mechanical means of stretching the cervix (e.g. using the synthetic hygroscopic cervical dilator – SHCD). Prostaglandins have been associated with an increased risk of overstimulating uterus contractions such that the person undergoing cervical ripening with prostaglandins requires close monitoring. Each method for cervical ripening has advantages and disadvantages and there is no high-quality evidence to recommend one from the others based on clinical outcomes. In this theoretical study, we estimated the hospital costs and staff time for induction of labor care when using the SHCD in a lower acuity setting within the hospital, without monitoring facilities, in comparison to the patient remaining in the labor-and-delivery room using misoprotol or dinoprostone preparations. Our results suggest that misoprostol resulted in the least expensive option closely followed by the SHCD in a lower acuity setting, both with the potential for notable cost savings when compared to using dinoprostone preparations for cervical ripening. In addition, we associated up to several hours less staff time with the use of the SHCD in a lower acuity setting in comparison to misoprostol and dinoprostone. Patients that were delivering for the first time benefitted more from using the SHCD in a lower acuity setting in comparison to those who had delivered previously.
1. Introduction
Optimal use of healthcare resources is of increasing concern. In particular, the ongoing COVID-19 pandemic has placed exceptional strain on the healthcare system, and especially on physician and nursing staff. Given that childbirth is the most common reason for an inpatient stay in the United States (US) and can result in intense resource useCitation1–3, optimizing the care pathway for childbirth may have a positive impact on hospital resources. More than one in four deliveries in the US are inducedCitation4. Over 80% of inductions require cervical ripening, which is the physical softening, thinning, and dilation of the cervix in preparation for labor and birthCitation5,Citation6. With this work, we modeled how moving patients to a lower acuity setting in the hospital during the cervical-ripening phase of induction of labor (IOL) might affect the care pathway and resource use including nurse time and costs. Patients eligible for the lower acuity “ripening room” would be at low risk of complications during the cervical-ripening phase. Reducing resource use for low-risk patients has the potential to free capacity in labor and delivery (L&D) for more critical case.
The need for a ripening room may be even greater given that IOL after 39 weeks is expected to rise in light of evidence from the ARRIVE trialCitation7. This multicenter, randomized, controlled trial enrolled low-risk nulliparous patients from 41 US hospitals and assigned them to either IOL at 39 weeks 0–4 d (3,062 patients) or to expectant management (3,044 patients). Results showed that IOL patients required a significantly lower frequency of cesarean deliveries when inducedCitation7. Routine adoption of IOL at 39 weeks raises the concern of overburdening existing and already stretched resourcesCitation8–10. Although some resource burden may be offset by reduced antepartum hospitalization, outpatient visits, treatments, and tests, studies showed that IOL patients spent a median of six hours longer in L&D, and their intrapartum and delivery care costs were 16.9% higherCitation7,Citation9,Citation10. Outpatient cervical ripening is one option for reducing patient time in L&DCitation11,Citation12. But not all patients can, or want to, leave the hospital for medical, personal, or even logistical reasons. Therefore, safe and practical solutions for reducing hospital resource use for patients remaining in hospital for cervical ripening would be beneficial.
Moving cervical ripening to a lower acuity ripening room would require use of a cervical ripening agent (CRA) that requires minimal to no monitoring and can remain in place for an extended time without risk to the patient or fetus. Current standard protocols for cervical ripening mainly involve synthetic prostaglandins, the most common being dinoprostone, delivered as an insert or gel, and low-dose misoprostol, delivered orally or vaginallyCitation5. US protocols for administering synthetic prostaglandins involve continuous or frequent fetal monitoring during cervical ripeningCitation5,Citation13, making them unsuitable for use in a ripening room. Mechanical methods for cervical ripening, on the other hand, may be used with limited or no monitoring after a non-stress test ensures fetal wellbeingCitation5,Citation13. Mechanical methods, such as the synthetic hygroscopic cervical dilator (SHCD), support the natural production of endogenous prostaglandins by exerting pressure on the cervical tissue causing collagen degradation, which leads to a softening of the cervixCitation13. The SHCD is safe and effective for cervical ripening as part of IOLCitation13, can remain in-situ for up to 24 h, and requires minimal to no fetal monitoring after initial placementCitation5,Citation13,Citation14. A key concern when changing care pathway is the patient impact. In this regard, recent systematic reviews, meta-analyses, reviews, and guidelines have failed to identify a superior CRA regarding clinical outcomesCitation5,Citation14–16. Mechanical methods could have advantages over synthetic prostaglandins in that they may have fewer side effects such as excessive contractions of the uterus (uterine hyperstimulation)Citation14. If contractions are too long or very close together, the baby may not receive sufficient oxygenCitation14.
The ripening room could be a dedicated, multi-occupancy room without the specialty resources of an L&D room. In settings without dedicated space, further options could be an available antepartum or postpartum room – or any free hospital room within a reasonable distance. Advantages of the proposed ripening room would be the close proximity to professional care and equipment while increasing the capacity of L&D units. To realize the potential for the ripening room, cervical ripening would switch from using a prostaglandin as the standard of care to using the SHCD. To assess the impact of such a ripening room using SHCDs, we developed a cost-consequence model to estimate the CRA-dependent cost and staff time for IOL, alongside selected clinical outcomes, when comparing to any of the common prostaglandins.
2. Materials and methods
2.1. Patient population
Included in this assessment was a theoretical cohort of patients requiring IOL with an unfavorable cervix that was indicated to receive prostaglandins as the standard of care. The model uses population averages as inputs. Patients were modeled to remain in the hospital for the entire duration from admission for cervical ripening until birth. Patients given prostaglandins remain in the L&D unit with extensive fetal monitoringCitation5. Patients considered for the ripening room should have no medical requirement for continuous fetal monitoring during preinduction cervical ripening.
2.2. Standard-of-care induction of labor with an unfavorable cervix
To model the standard of care for preinduction cervical ripening and IOL for patients with an unfavorable cervix, we assumed that all patients received a synthetic prostaglandin for ripening the cervix. Please note that the characteristics below describe a hypothetical patient cohort for a theoretical, health-economic assessment; no individual patients were processed or simulated. All patients were admitted to a fully-equipped room within the L&D unit immediately or transferred from an obstetrics triage unit. As illustrated in , patients were admitted and prepared to receive a CRA, the CRA was administered, and fetal monitoring occurred as per protocol for each prostaglandinCitation5. If further cervical ripening was required, and the maximum number of administrations of the CRA had not yet been givenCitation5, the patient underwent another round of preparation, CRA administration, and fetal monitoring. After cervical ripening was complete or unsuccessful, and the patient was not in spontaneous labor, then they were assumed to be given an infusion of oxytocin to augment or induce contractions. Each of the steps above was assumed to take place within the average time to delivery, which was dependent on the CRA administered (Supplementary Material). Cesarean sections required due to fetal or maternal distress before spontaneous or induced labor were not modeled specifically because no CRA-specific differences were identified in the literature.
Figure 1. Illustration of the cost-consequence model. (A) The decision tree for assigning a CRA. In the standard-of-care arm a prostaglandin is assigned and in the comparison arm the SHCD is assigned (grey box). A single patient receives only one type of CRA. Patients are modeled separately depending on their parity. (B) The process from admission to delivery is divided into a ripening, labor, and delivery phase. The ripening room (grey box) is a lower acuity room in comparison to an L&D room without facilities for fetal monitoring or for labor or delivery, and is only used in the model for patients undergoing cervical ripening with the SHCD. A cesarean section required prior to attempted labor was not modeled because it is expected to be rare in a patient population with low to moderate risk pregnancies and it would not change model outcomes. All other events (white boxes) occur in a standard L&D room. Key cost and time parameters are listed in the right-hand box. Abbreviations. CRA, cervical ripening agent; IV, intravenous; L&D, labor and delivery; PGE1, misoprostol; PGE2, dinoprostone; prep., patient preparation before CRA administration; SHCD, synthetic hygroscopic cervical dilator.
For this work, we assessed the most commonly administered synthetic prostaglandins including: a dinoprostone vaginal insert referred to as PGE2 insert (Cervidil, Ferring Pharmaceuticals Inc, Parsippany-Troy Hills, NJ); a dinoprostone intracervical gel referred to as PGE2 gel (Prepidil, Pharmacia and Upjohn company LLC, New York, NY); and misoprostol (Cytotec, G.D. Searle LLC, Skokie, IL) with 25 mcg per dose given as a quartered 100 mcg tablet orally (referred to as oral PGE1) or vaginally (referred to as vaginal PGE1). Oral or vaginal administration of misoprostol was considered as separate CRAs with individual IOL protocols and clinical and cost inputs. The PGE2 gel is rarely used in the US but was included in a recent Cochrane reviewCitation14.
2.3. Comparator setting
In the comparison, patients received the SHCD (Dilapan-S, MEDICEM Technology, Czechia). Assessing all possible mechanical methods went beyond the scope of this work. The SHCD was considered to be more suitable than the Foley balloon for a lower acuity setting because (1) patients receiving the SHCD reported increased patient satisfaction outcomes, (2) the SHCD is FDA cleared for preinduction cervical ripening and the Foley balloon is not, (3) for the SHCD there is no protrusion from the introitus or need to keep it under tension, and (4) clinical safety and efficacy outcomes were reported to be non-inferiorCitation13. Other hygroscopic/osmotic dilators such as laminaria tents are primarily used during pregnancy terminationCitation5, whereas there is increasing evidence that the SHCD is safe for preinduction cervical ripeningCitation13,Citation17,Citation18. Please note that the predecessor of Dilapan-S was called Dilapan and was originally manufactured by Gynotech Inc., NJ until 1997. Early concerns of device fragmentation of Dilapan have been corrected and are not reported for Dilapan-SCitation19.
After the theoretical patient was admitted to the L&D or obstetrics triage unit and preparation, SHCD administration, and initial fetal monitoring was performed. The patient transferred to and stayed in the ripening room until cervical ripening was completed, the CRA fell out, re-administration of CRA was required, or complications occurred. Depending on local hospital processes and setup, the ripening room could be the room where the initial assessment and SHCD administration was performed and a fetal monitor could be shared between multiple assessment rooms, or a dedicated room or lounge located within reasonable distance from the L&D unit. We assumed the ripening room is at least within walking distance of the L&D unit and that the patient can walk between locations at no cost.
2.4. Model setup
The costs and clinical consequences of using each CRA and its associated care protocol were estimated using a cost-consequence model, commonly used in health economics to assess medical devices. This type of analysis allows the reader to weigh estimated costs against predicted consequences of the modeled scenarios. The cost-consequence model adheres to general ISPOR guidelines for economic modelingCitation20,Citation21, and is reported here following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist, provided in the Supplementary Material. The perspective of an L&D unit at a US hospital was taken, using US-specific costs, protocols, and clinical outcomes wherever possible. The time horizon was modeled from admission for cervical ripening until delivery. No discount rate was applicable because only a few days were assessed.
The overall model design is illustrated in . A decision tree was used to assign patients to a CRA, and to model oxytocin augmentation and delivery events. The hospital care pathway from admission for IOL to delivery is shown in alongside the cost and time parameters we considered to be affected by the type of CRA administered. Cost inputs that were not taken from current data (year 2020 or 2021) were inflated to 2020 US dollars.
The cost-consequence analysis assessed an average US protocol for IOL taken from the Association of Women’s Health, Obstetrics, and Neonatal Nurses (AWHONN) guidelinesCitation5,Citation22. Incidences of clinical outcomes and times for phases of delivery were taken from available scientific literature and normalized when required. Selected model inputs with their sources are given in (for clinical consequences compiled from the literature). The full list of input parameters including calculations performed to transform original source data into model-input data are given in the Supplementary Material.
Table 1. Base-case cost parameters.
Table 2. Selected base-case parameters for population and IOL protocols.
Table 3. Overview of calculated CRA-related costs and consequences.
2.5. Estimating costs related to each CRA
We estimated costs directly related to the use of a specific CRA ( over the three following aspects: (1) time in the L&D and the ripening room when applicable, (2) purchase and administration cost for the CRA, and (3) purchase and administration cost for the incidence of patients requiring oxytocin augmentation. All parameters for computing the costs are given in .
The cost for the time spent in hospital () depends on the time spent in a ripening room (
) and in a fully-equipped L&D room (
). The times, given in hours, are multiplied by the cost per hour for the ripening (
) or the L&D (
) rooms, respectively:
The cost of the L&D room is considered to cover the room, all facilities and equipment needed for a standard L&D, and standard staffing requirements for oxytocin augmentation, labor, and delivery. The cost of the ripening room is estimated as a percentage of the cost of the L&D room, which can be updated based on hospital requirements and in the base case is assumed to be 30%.
Next, the purchase and administration cost directly related to the CRA was considered:
where
is the purchasing cost for one administration of the CRA,
is the cost for administering the CRA by the hospital staff for the first time,
is the staff administration cost for any subsequent administrations, and
is the average number of administrations required for a patient receiving the CRA. Finally, the oxytocin purchase (
) and administration cost (
) is considered for the proportion of patients requiring oxytocin augmentation, depending on the CRA used (
):
The parameter per CRA is given in as a clinical consequence and is included in the cost calculation because it influences the cost of care depending on the CRA used. Included in the
was 30 units of oxytocin (Pitocin), 500 mL of saline with 5% dextrose, and 1000 mL normal saline bag for the mainline IV, non-sterile gloves, the IV catheter, and infusion pump tubing required per patient. Reusable equipment was assumed to be included in the cost of the L&D room. For
the cost for initiating the IV was considered, however, the staff cost for monitoring and adjusting the oxytocin infusion was assumed to be included in the L&D room cost.
Other costs that may accumulate during the time in L&D – such as instrumental birth, use of various analgesics, fetal and maternal monitoring, catheterization other than for oxytocin, and episiotomy, equipment use for labor and/or birth – were not considered because these added complexity and there was insufficient data to assess their impact accurately. Differences in cesarean section and vaginal delivery rates have been reported between CRAs, however, these may only be significant for PGE1 preparations (low- to moderate-quality evidence as defined by the referenced work)Citation13,Citation14.
2.6. Cost inputs
The cost of pharmaceuticals was taken from the IBM Micromedex RED BOOK Online databaseCitation23. Costs for items for oxytocin augmentation were taken from the current list price provided to the sponsor by Henry Schein. The list price for the SHCD was provided by the study sponsor and multiplied by the average number of rods required per administration taken from the DILAFOL trialCitation13. Other costs were sourced from recent literature as referenced in and inflated to 2020 USD as required.
2.7. Estimating staff time related to each CRA
The time to delivery for each CRA was divided into three phases: cervical ripening, labor, and delivery (). We assessed staff time dependent on the CRA being administered for nurses, residents, and physicians individually. The impact on staff time was calculated separately for each phase. Note that the focus was on times that were considered to be directly related to the type of CRA being administered. All parameters for calculating staff time are given in the Supplementary Material.
During the cervical-ripening phase, we included time parameters for total procedure, nurse, resident, and physician for each of the following steps: (1) patient preparation before the first administration, (2) patient preparation before subsequent administrations, and (3) administering the CRA including time for waiting for the availability of staff or resources. Patient preparation includes a fetal non-stress test. Guidance published by AWHONN was used to estimate time spent on fetal monitoring after each administration of the CRA using the recommended monitoring times and nurse-to-patient ratios suggested for fetal monitoringCitation5,Citation22. Times were then multiplied by the number of average administrations expected, taking the time difference in first and subsequent administrations into account. An upper bound for fetal monitoring time is given by the total time parameter for the cervical-ripening phase. For example, if constant fetal monitoring was required, then the time spent on patient preparation and administration was subtracted from the total ripening time given for that type of CRA, and the remaining time was considered time spent on fetal monitoring. Patients who were not currently involved in preparation, administration, or monitoring were assumed to not take up any additional staff time that was dependent on the CRA being administered. In the model base case, we assumed that the time spent in the ripening room by patients receiving the SHCD could be estimated by a US trial using the time saved when comparing outpatient with inpatient cervical ripeningCitation11. Because the trial assessed only a nulliparous population, multiplication factors were used to adjust these times to a multiparous population, with these taken from published trials (see Supplementary material)Citation13,Citation31. We considered the time saved comparing outpatient with inpatient cervical ripening to offer a lower bound to the average possible time a patient could spend in the ripening room. Many patients could spend up to at least 10 h per administration in the ripening room, given the protocol of 12 h of insertion time for SHCD but up to 24 h of insertion time is considered safeCitation13. Some patients may, though, start labor spontaneously or go to an L&D room due to concerns or complications lowering the overall average time saved in L&D. Increasing the total time spent in the ripening room was assessed in a scenario analysis.
Time to active labor and time to delivery are commonly reported in clinical trials comparing the efficacy of different CRAs. In addition, for this model, we required the time until oxytocin (if used) would begin. This allowed the period of ripening only and the period of ripening plus oxytocin augmentation before the onset of active labor to be estimated, because these periods require differing nurse-to-patient ratios and time. For fetal monitoring during the ripening-only time, a 1:2 nurse-to-patient ratio was usedCitation5,Citation25. A 1:1 nurse-to-patient ratio for patients requiring oxytocin augmentation was used for the time between the ripening-only phase until active laborCitation5,Citation22. This was only applied to the incidence of patients requiring oxytocin augmentation, which varied per CRA. Patients not needing oxytocin augmentation were assumed to not require additional nursing interventions until active labor. For the time from active labor to delivery, a 1:1 nurse-to-patient ratio was assumed, which was taken as an average of applicable ratios given by AWHONN guidelines for L&DCitation22.
2.8. Literature search strategy
Comparative clinical outcomes dependent on the CRA received during IOL were identified through a structured search of the PubMed database of peer-reviewed scientific literature. Search specifications are given in the Supplementary Material. In addition, a structured search of the ClinicalTrials.gov database was performed to identify any IOL trials based in the US (see the Supplementary Material). To supplement identified literature, manual searches were performed to identify further costs, IOL guidelines, and protocols that are relevant to US practice. When possible, we relied on data derived from US studies and prioritized randomized over observational studies.
2.9. Clinical efficacy and safety outcomes as consequences
The primary consequence assessed by the model was staff, in particular nurse, time that was dependent on the CRA being administered. In addition, the impact of each CRA on key clinical efficacy and safety outcomes were compared for: primary cesarean section rate (patients with a previous cesarean section were excluded from the model), vaginal delivery not within 24 h, and fetal hyperstimulation with fetal heart-rate changes. Time to delivery and oxytocin augmentation events were also reported but note that these items were included in the cost estimation of the CRAs. Clinical events were taken from recent US-based clinical trials when available – selected from all studies identified using the described structured literature search (see also the Supplementary Material). Otherwise, incidences were estimated by using a recent Cochrane reviewCitation14. Clinical studies directly comparing all four prostaglandins to the SHCD were not available, however, all of the CRA agents have been compared to the balloon catheterCitation13,Citation14. For this reason, the balloon catheter was used to normalize clinical and safety outcomes to improve comparability between studies. Full calculations are provided in the Supplementary Material. Neonatal outcomes were not included in this analysis because the focus of this analysis is on the L&D unit, and the care costs for neonates are accrued in a different setting. Furthermore, data comparing neonatal outcomes is mostly of low-quality evidenceCitation14.
2.10. Primary outcome (base case)
The estimated CRA-dependent cost impact on IOL to delivery is presented for each CRA separately. The estimated cost is presented alongside the expected clinical outcomes and resource use, specifically staff time, to allow the reader to assess for themselves whether the implementation of a ripening room using the SHCD would be beneficial.
2.11. Scenario analyses
To assess the stability of predicted costs and nurse times to variations in inputs, the following scenarios were evaluated:
Analysis of the cost of the ripening room in proportion to the L&D room. Proportional costs were iterated in intervals of ten percentage points from 10% to 100%. This gives the reader an indication of outcomes depending on the proportional cost of the lower acuity ripening room because it is impossible to generalize solutions for all hospitals for a situation that does not yet exist.
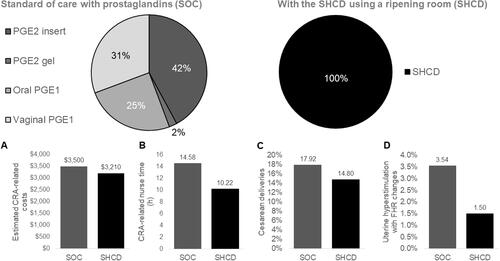
Due to staff and patient preferences, contraindications, and coverage, it is rare for only a single CRA to be used in a hospital. As such, in addition to assessing populations that all receive the same prostaglandin, we evaluated a mixed-case scenario where a hospital allows the use of different prostaglandins in different patients, dependent on the provider’s discretion or hospital guidelines. We compared the mixed-case-prostaglandin use to a population using only the ripening room with SHCDs. The relative proportions of each of the four prostaglandins were estimated from US literature: 42.1% patients received the PGE2 insertCitation5,Citation32, 2.2% received the PGE2 gelCitation5,Citation32, 25.0% received oral PGE1Citation32,Citation33, and 30.7% received vaginal PGE1Citation32,Citation33. Further details on how these proportions were determined are given in the Supplementary Material.
In the base case, the time spent in the ripening room was estimated by the difference in hospital time between inpatient and outpatient ripening. This is likely a conservative estimate of time spent in the ripening room; therefore, we performed a scenario analysis where patients receiving the SHCD could spend the entire cervical ripening time in the ripening room minus the time required for patient preparation, administrations, and fetal monitoring (the non-stress test after SHCD administration). This scenario gives an upper estimate of potential cost and time savings.
2.12. Sensitivity analyses
We performed probabilistic multivariate sensitivity analyses to assess the robustness of model outcomes. In all simulations, the results of the ripening room using SHCDs were compared to each of the prostaglandins individually and to the case-mix of prostaglandins used in scenario 2. The values for all input parameters were selected at random within the given range of uncertainty by using a seeded random number and a distribution depending on the type of input parameter. The uncertainty for model inputs used the following: the binomial proportion 95% confidence interval for all incidences of clinical parameters; a standard deviation of 10% of the base-case value for cervical-ripening protocol and cost parameters; and minimum and maximum times were calculated from the literature for the ripening-only times, times to active labor, and times to delivery. Because costs vary considerably between settings, we also tested standard deviations of 25% and 50% for cost parameters to determine whether this had an effect on the ranking of CRAs. For selecting possible model inputs, the beta distribution was applied to proportions, the lognormal distribution was applied to time parameters, and finally the gamma distribution was used for cost parameters. Sampling a unique set of model inputs was repeated 2,000 times and outcomes were summarized using the 95% credible interval (CrI) and by the percentage of simulations where the ripening room using SHCDs outperformed the comparator. For the mixed-case scenario, the mean rank of the ripening room and SHCD over all simulations is given from 1 (best in all simulations) to 5 (worst in all simulations). The ranking for each CRA given one set of parameters was also computed for each of the 2,000 simulations and plotted for CRA-related costs and nurse time.
There exists a lot of uncertainty in the time parameters; therefore, we also performed a univariate deterministic sensitivity analysis for all time parameters, assessing their impact on estimated costs and nurse time using the calculated minimum and maximum times as model inputs, which covered a wide range of possible times. See the Supplementary Material for further details. In addition, for each comparison between the ripening room and one of the prostaglandins, we assessed the minimum time-to-delivery difference that was required to achieve cost savings for the ripening room using SHCDs by keeping the prostaglandin times fixed and iterating over possible times for the SHCD. The same cost-minimization strategy was performed for the purchase cost of one round of SHCD administration.
3. Results
We report results from the cost-consequence model comparing the use of a ripening room in combination with SHCDs as the primary CRA to each of the commonly used prostaglandins. Whenever just the SHCD is mentioned in results, this implies that a ripening room was used during the cervical-ripening phase unless explicitly mentioned otherwise. Costs and consequences are given as an average over a hypothetical patient cohort.
3.1. CRA-dependent costs comparing the ripening room using SHCDs with each prostaglandin
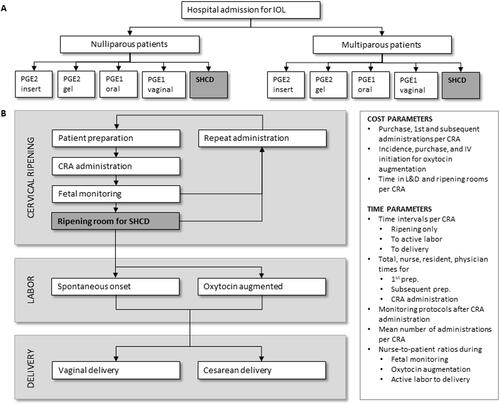
The estimated costs related to each type of CRA ranged between $2,750 for vaginal PGE1 to $4,104 for the PGE2 gel (). The relative per-patient cost for the ripening room using SHCDs in comparison to each prostaglandin was: −$894 (PGE2 gel), −$818 (PGE2 insert), −$271 (oral PGE1), and +$460 (vaginal PGE1).
Figure 2. Estimated costs associated with the administration of each type of CRA based on an average US clinical practice for IOL. (A) Total CRA-related cost per CRA in the base case. (B) Cost difference when comparing the SHCD to each of the prostaglandins stratified by parity. The total estimated cost for the ripening room using SHCDs was $2,936 for multiparous and $3,647 for nulliparous patients using the base case for all other parameters. Abbreviations. CRA, cervical ripening agent; PGE1, misoprostol; PGE2, dinoprostone; SHCD, ripening room using the synthetic hygroscopic cervical dilator. Costs are given in 2020 USD.
When splitting the total CRA-related cost into subparts (), we observed that the PGE2 gel was considered to have the highest purchase and administration cost; PGE1 was cheapest in this area due to a cost of only $1 for the drug acquisition per round. Oxytocin augmentation did not impact the overall costs substantially, with the greatest driver of costs being the time in hospital ().
When stratifying estimated costs by parity, both nulliparous and multiparous populations performed similarly to the base case in the overall trend (; ). The cost differences, however, were more pronounced for a nulliparous in comparison to a multiparous population with potential cost savings of over $1,000 per patient for the ripening room using SHCDs in comparison to the standard protocol for a PGE2 insert (). In general, the ripening room using SHCDs outperformed any route of PGE2 administration. The ripening room using SHCDs cost more than for multiparous populations receiving either oral (+$67) or vaginal PGE1 (+$199) but was cost saving for all other comparisons by parity ().
3.2. Staff-time requirements per CRA
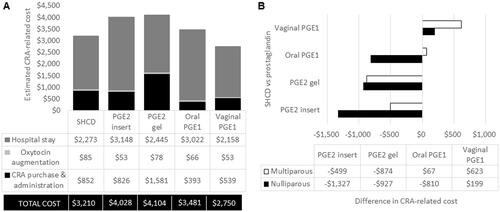
The mean CRA-dependent nurse time from admission for IOL to delivery was estimated to range from 10.22 h with the ripening room using SHCDs to 17.27 h with the PGE2 insert (). Relative differences in nurse time for the ripening room using SHCDs in comparison to the prostaglandins were: −7.05 h (PGE2 insert), −3.54 h (PGE2 gel), −4.03 h (oral PGE1), and −0.97 h (vaginal PGE1). As with costs, the benefit of the ripening room using SHCDs with respect to nurse time was greater with a nulliparous population than a multiparous population (; ). In all but one instance, the exception being a multiparous patient receiving the vaginal PGE1 (+6.6 min), the ripening room using SHCDs resulted in reduced nurse time compared to other prostaglandins ().
Figure 3. Estimated nurse time associated with the administration of each type of CRA based on an average US clinical practice for IOL from admission to delivery. (A) Total CRA-related nurse time for each CRA in the base case. (B) Difference in nurse time when comparing the SHCD to each of the prostaglandins stratified by parity. The total nurse time for the SHCD was 9.13 h for multiparous and 11.98 h for nulliparous patients. Abbreviations. h, hours; PGE1, misoprostol; PGE2, dinoprostone; SHCD, ripening room using the synthetic hygroscopic cervical dilator.
In addition to nurse time, we also assessed demands on resident and the attending physician time during cervical ripening only. In the model, the resident was assumed to administer the CRA, and only minor differences in their estimated time burden were identified: 12.6 minutes (min) (ripening room using SHCDs), 12.0 min (PGE2 insert), 15.6 min (PGE2 gel), 22.2 min (oral PGE1), and 16.8 min (vaginal PGE1). No differences between the CRAs were identified for the attending physician.
3.3. Comparison of clinical efficacy and safety outcomes
In most cases, the clinical efficacy and safety outcomes combined from multiple sources of published clinical trials and meta-analyses (Supplementary Material) were comparable, see . According to the calculations performed in this analysis, the ripening room using SHCDs is effective in that it had a lower rate of cesarean sections but it resulted in slightly fewer vaginal deliveries within 24 h and required substantially more oxytocin augmentations. The patients experiencing events for uterine hyperstimulation with fetal heart-rate changes were calculated to be slightly lower for SHCDs (1.5%) than for all prostaglandin CRAs, which ranged from 1.9% for oral PGE1 to 4.3% for the PGE2 insert (). Please note that comparative clinical studies for all prostaglandins were not identified for the SHCD but these clinical consequences were estimated using balloon catheters. Results for clinical consequences are to be considered as theoretical estimates and need to still be confirmed in comparative clinical trials.
3.4. Scenario analyses
Scenarios 1–3 test the robustness of model outcomes and give insights into clinical practices that vary from the base case.
3.4.1. Scenario 1
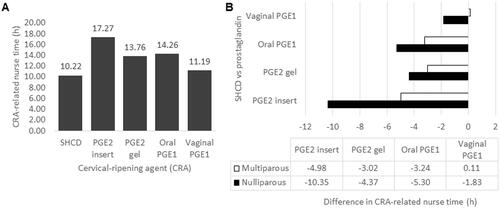
In scenario 1, we tested the impact of changing the cost of the ripening room proportionally to the L&D room. illustrates that the per-patient cost for the ripening room using SHCDs ranged between $3,107 to $3,570, with $3,570 representing the cost when SHCD is used without a ripening room. In this instance, SHCD is $89 more expensive than oral PGE1 () but is still less costly than both PGE2 preparations.
Figure 4. Illustrating the impact of changing the cost of the ripening room proportionally to the L&D room cost when using the SHCD. The per-hour cost for the ripening room was tested from 10% to 100% of the L&D room (shown as a black line). The grey rectangle between 20% and 40% represents a reasonable cost range for the ripening room in comparison to the L&D room. For comparison, the prostaglandin costs are given as grey dots along the 100% line. If the patient remains in the L&D room and does not transfer to a ripening room, the maximum possible costs were $3,570 (at the 100% line in light grey), which $89 higher than the cost for oral PGE1. Costs are given in 2020 USD. Abbreviations. CRA, cervical ripening agent; L&D, labor and delivery; PGE1, misoprostol; PGE2, dinosprostone; SHCD, synthetic hygroscopic cervical dilator.
3.4.2. Scenario 2
In scenario 2, illustrated in , we compared a population using a mix of prostaglandin CRAs with a population only using the ripening room and SHCD. Here, estimates for both CRA-related costs and nurse time were decreased with the ripening room using SHCDs. In addition, cesarean deliveries and uterine hyperstimulation with fetal-heart-rate changes were estimated to be lower in the ripening room using SHCDs scenario.
Figure 5. In scenario 2, we compared using a mix of prostaglandins in the standard of care (SOC) with switching to protocol including a ripening room and SHCDs. Comparisons are made in terms of (A) estimated CRA-related costs, (B) CRA-related nurse time, (C) cesarean deliveries, and (D) uterine deliveries with fetal-heart-rate (FHR) changes. The proportions used for the prostaglandins were taken from studies based on US patient dataCitation5,Citation32,Citation33. Costs are given in 2020 USD. Abbreviations. CRA, cervical ripening agent; PGE1, misoprostol; PGE2, dinosprostone; SHCD, synthetic hygroscopic cervical dilator.
3.4.3. Scenario 3
For scenario 3, we increased the time spent in the ripening room to the entire remaining ripening time (upper estimate); compared to using a time in the ripening room estimated by outpatient versus inpatient cervical ripening in the base case (lower estimate)Citation11. Here the potential CRA-related cost decreased from $3,210 to $2,685 on average for the ripening room using SHCDs. In this instance, the ripening room using SHCDs was the lowest cost protocol, being -$65 compared to the cheapest prostaglandin, which is vaginal PGE1 at $2,750 (see or for the costs of the prostaglandins in the base case).
3.5. Performance of CRAs in sensitivity analyses
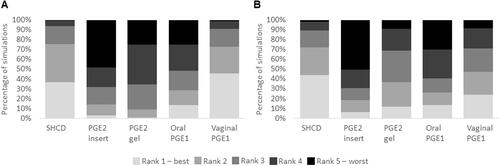
Results from the probabilistic multivariate sensitivity analysis are given in . In the 2,000 tested model simulations, costs with the ripening room using SHCDs were reduced in 92% of simulations compared with the PGE2 gel (upper end) and in 47% of simulations for vaginal PGE1 (lower end). For nurse time, a general trend was that the ripening room using SHCDs would likely result in reduced nurse-time requirements across all prostaglandin-based protocols: At the higher end, nurse time was reduced in 86% of simulations (PGE2 insert) and at the lower end, in 66% of simulations (vaginal PGE1).
Table 4. Sensitivity analyses for the base case and scenario 2 for costs and nurse time.
A further visualization of the sensitivity analysis using the rank of each CRA for each of the 2,000 simulations is shown in . Here you can see that the ripening room using the SHCD has a similar ranking profile in comparison to vaginal PGE1 for CRA-related costs () but is has a better ranking profile for nurse time (). The other three prostaglandins more often rank fourth or fifth for both costs and nurse time.
Figure 6. Ranking for each CRA separately when comparing (A) CRA-related costs and (B) nurse time in the sensitivity analysis. 2,000 parameter settings were tested and the output for each parameter set was the rank for each CRA from 1 (best) to 5 (worst). Abbreviations. CRA, cervical ripening agent; L&D, labor and delivery; PGE1, misoprostol; PGE2, dinosprostone; SHCD, synthetic hygroscopic cervical dilator.
In the univariate deterministic sensitivity analysis, general trends in cost and nurse time were confirmed (Supplementary material). As expected, the greatest impact was in the time to delivery as this heavily influences both overall costs and nurse time. The advantage of the ripening room using SHCDs was most apparent in comparison to the PGE2 insert where there was very little overlap in the uncertainty of these two CRAs.
Using a cost-minimization strategy, we identified minimum time-to-delivery thresholds for the ripening room using SHCDs for it to be considered cost saving in comparison to each prostaglandin by parity. For a nulliparous population, average time to delivery for the ripening room using SHCDs needed to be <33.47 h (at most 2.79 h longer) than for the PGE2 insert, <30.47 h (at most 8.63 h longer) than for the PGE2 gel, <29.60 h (at least 0.36 h shorter) than for oral PGE1, and <22.10 h (at least 0.70 h shorter) than vaginal PGE1 to achieve cost savings. For a multiparous population, average time to delivery for the ripening room using SHCDs needed to be <21.10 h (at most 1.95 h longer) than for the PGE2 insert, <23.95 h (at most 7.83 h longer) than for the PGE2 gel, <16.86 h (at least 1.2 h shorter) than for oral PGE1, and <12.70 h (at least 0.2 h shorter) than for vaginal PGE1 to achieve cost savings.
In a cost-minimization comparison for the purchase cost per round of CRA administration, the model calculated cost savings for the ripening room using SHCDs if it cost up to $667 more to purchase the SHCD than the PGE2 insert, up to $623 more for the PGE2 gel, and up to $639 more for oral PGE1. For vaginal PGE1, the ripening room using SHCDs scenario cost $7.55 more, even when the SHCD had zero purchase costs because the time to delivery is comparatively short for vaginal PGE1 ().
Increasing the standard deviation for cost parameters from 10% to 25% and also 50% resulted in no changes to overall CRA ranking: it made less than one percent difference in the percentages of scenarios where the ripening room using SHCDs was better for all cost and nurse-time comparisons.
4. Discussion
The focus of our study was on the cost and consequences associated with cervical ripening and IOL, as well as on analyzing how implementing a lower acuity room using the SHCD for preinduction cervical ripening may impact L&D units and their IOL care pathway. We assessed the administration of a single type of CRA followed, if required, by oxytocin augmentation. It was out of the scope of this work to include a comprehensive analysis of all other available CRAs or interventions for IOL, such as combinations of CRAs, balloon catheters, or amniotomy.
Our theoretical analysis indicates that switching to a strategy of a ripening room using SHCDs as the primary method of preinduction cervical ripening may save costs and nurse time in comparison to most prostaglandins except for vaginal PGE1 (misoprostol). Using vaginal PGE1 would likely save costs but increase nurse time. Key factors contributing to the decreased cost of vaginal PGE1 in comparison to the SHCD are (1) the lower acquisition costs and most importantly (2) fewer hours until delivery and thus less time in hospital. The lower cost of the ripening room could not counteract the reduced time in hospital. Value in healthcare comes from balancing costs and patient outcomes and one should consider that use of synthetic prostaglandins is associated with higher rates of uterine hyperstimulation with and without fetal heart-rate changes in comparison to mechanical methodsCitation14. In the IMPROVE trial, 23% and 31% of all patients had to stop the administration of PGE1 due to safety concerns for oral and vaginal PGE1, respectivelyCitation30. Healthcare professionals are encouraged to use their clinical judgement as well as consider costs and resource use when determining the best CRA for each patient. All provided options must consider patient preference and satisfaction and also whether they are comfortable with a lower acuity setting during cervical ripening.
From our literature review, this is the first economic assessment of the cost and staff-time requirements during inpatient IOL comparing different CRAs. Assessing staff-time requirements for IOL, though, is a likely area of interest given that the American College of Obstetricians and Gynecologists previously published an analysis of costs and staff time related to the use of misoprostol in the outpatient and inpatient settingsCitation25.
4.1. Limitations
As with all publications, there are limitations in our analysis. Foremost, this is a theoretical analysis and results should be validated by clinical studies. As patients with prior cesarean sections are contraindicated to receive prostaglandins they were omitted from our assessment because they could not receive the modeled standard of careCitation5. These patients may be able to benefit from the ripening room using SHCDs but their eligibility will depend on local clinical practice and guidelines. There is little direct comparative data for the SHCD versus prostaglandins, and as such, data for the balloon catheter was used as a link or proxy for many calculations. Using balloon catheter outcomes in this way is supported by a US-based study from Saad et al. showing that the SHCD is non-inferior to the Foley balloon when comparing several key clinical efficacy and safety outcomesCitation13.
Our analysis follows the time from admission to delivery, however, the costs and resource use that are directly related to the type of delivery (vaginal or cesarean section) and adverse events were not included; these are listed as clinical consequences of the CRA in this analysis. In the literature, delivery costs generally include the facility and staff costs for the total time in hospital for a delivery including postnatal careCitation34. In our model, we considered the cost for time in hospital for each CRA separately due to substantial differences in times to delivery depending on the CRA administered. Therefore, delivery costs were not included in cost estimates because they would result in double counting costs for hospitalization, which is the major cost factor for inpatient childbirth. Differences in neonatal outcomes were also not considered. Reasons were (1) associated costs are not a focus for L&D units and (2) they could have overinflated the benefit of the SHCD without the support of moderate-quality evidence such that we made the decision to err on the side of caution. In most comparisons, lower relative risks were reported for the balloon catheter versus prostaglandins for neonatal outcomesCitation14.
Another aspect that could be added to this analysis if data were available is analgesic use during cervical ripening, as differences in analgesics may impact overall costs and staff time. The average number of administrations for oral and vaginal PGE1 may be underestimated in the base case. These numbers were taken from the IMPROVE trial where after the first 25 mcg administration, 50 mcg of PGE1 was administered. However, we identified that 25 mcg is frequently given for all administrations in the US (Supplementary material)Citation30. Uterine hyperstimulation with and without fetal heart-rate changes have been associated with both 25 and 50 mcg doses, with higher doses associated with an increased rate of uterine hyperstimulationCitation5. From an economic viewpoint, changing the dosage could influence the number of required administrations, which is associated with additional fetal monitoring and administration costs. A further consideration is that although fetal monitoring is standard practice in the US during cervical ripeningCitation5, and we include 30 min of continuous fetal monitoring after each administration of the SHCD in the model, this may not be required for the SHCD when L&D units become more familiar with this CRA.
Where possible, parameter values were sourced from peer-reviewed published literature or official US national sources. Every model, though, requires assumptions for simplification and when data cannot be identified. Here, the patient population was limited to those patients not contraindicated to receive prostaglandins; however, this patient group may include some patients contraindicated to receive the SHCD, for example, those with a current vaginal infection. Another assumption was that staff time not considered by our clinical authors to be dependent on the type of CRA used was not included in overall time calculations. The CRAs were considered to impact times in the pathway from admission to delivery, but in the literature, these times are subject to large fluctuations, and the ripening-only time before oxytocin augmentation or spontaneous labor is not an outcome commonly reported in clinical trials. To account for the uncertainty in parameter values in this area, we performed a univariate deterministic sensitivity analysis with wide uncertainty ranges (Supplementary Material).
This work is based on clinical studies and public databases from the US and the model needs to be adapted to other countries before assuming that results are applicable to these settings. The model developed simulates the average pathway for a CRA in the average patient population. It is likely that most hospitals will deviate from the parameter values sourced from the literature review, and the results presented should be taken as an indication of potential outcomes. The analysis is not a costing study based on real-world data from a single institution and should thus be treated as an estimation of possible but not observed outcomes.
Given the potential limitations of this analysis, we consider this a first exploration of the potential for a ripening room and how switching to a SHCD-led pathway could impact hospital resource use. We encourage other researchers to explore the potential for ripening rooms and to consider undertaking studies to add additional evidence in this area. This could serve as the premise for quality-improvement initiatives.
5. Conclusion
Implementing the ripening room using SHCDs could save nurse time in L&D units. Compared to other CRA protocols, the ripening room using SHCDs was less expensive for all but vaginal misoprostol in our analyses. The benefits for a ripening room using SHCDs are most apparent in a nulliparous population, where greater cost and nurse-time savings were predicted than in a multiparous population.
Transparency
Declaration of funding
This work was funded by Medicem Inc., the US distributor/importer of Dilapan-S.
Declaration of financial/other interests
SJS and RTT are employees and RS is the owner of Coreva Scientific GmbH & Co KG, which received consultancy fees for performing, analyzing, and communicating the work presented here. TW is an employee of Medicem Inc, which paid the consultancy fees to Coreva Scientific GmbH & Co KG. JLG and BE were paid scientific consultants for Medicem Inc.
Author contributions
Conceptualization, SJS, TW, and RS; methodology and data collection, SJS, JLG, TW, RS, and BE; software, SJS.; validation, RTT and RS; writing – manuscript preparation, SJS; writing – review and editing, RTT, TW, JLG, RS, and BE; visualization, SJS; supervision, RS; project administration, SJS. All authors have read and agreed to the published version of the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (291 KB)Acknowledgements
The authors acknowledge Claudia Gersten, CNM MS FNP, for her insights into US-specific obstetrics and IOL practice, and on the current literature landscape.
References
- Sudhof L, Shah NT. In pursuit of value-based maternity care. Obstet Gynecol. 2019;133(3):541–551.
- National Partnership for Women & Families. Maternity care in the United States: we can – and must – do better. 2020. Available from: https://www.nationalpartnership.org/our-work/resources/health-care/maternity-care-in-the-united.pdf
- McDermott K, Elixhauser A, Sun R. Trends in hospital inpatient stays in the United States, 2005–2014 - statistical brief #225. US department of health and human services, agency for health care research and quality, healthcare cost and utilization project. 2017. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.pdf.
- Martin JA, Hamilton BE, Osterman MLK, et al. National vital statistics reports births: final data for 2018. Natl Vital Statis Rep. 2019;68(13):1–47.
- Simpson KR. Cervical ripening and labor induction and augmentation, 5th edition. J Obstet Gynecol Neonatal Nurs. 2020;49(5):S1–S41.
- McDonagh M, Skelly AC, Hermesch A, et al. Cervical ripening in the outpatient setting: comparative effectiveness review no. 238 (prepared by the pacific northwest evidence-based practice center under contract no. 290-2015-00009-I for the agency for healthcare research and quality and the patient). Agency Healthc. Res Qual AHRQ Public. 2021;193–195.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379(6):513–523.
- Clinical guidance for integration of the findings of the ARRIVE trial: labor induction versus expectant management in low-risk nulliparous women. ACOG. Available from: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2018/08/clinical-guidance-for-integration-of-the-findings-of-the-arrive-trial
- Grobman WA, Sandoval G, Reddy UM, et al. Health resource utilization of labor induction versus expectant management. Am J Obstet Gynecol. 2020;222(4):369.e1–369.e11.
- Einerson BD, Nelson RE, Sandoval G, et al. Cost of elective labor induction compared with expectant management in nulliparous women. Obstet Gynecol. 2020;136(1):19–25.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient foley catheter for induction of labor in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2020;136(3):597–606.
- Saunders SJ, Saunders R, Wong T, et al. Out-of-hospital cervical ripening with a synthetic hygroscopic cervical dilator may reduce hospital costs and cesarean sections in the United States-a cost-consequence analysis. Front Public Health. 2021;9:750.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220(3):275.e1-275–e9.
- de Vaan MD, Ten Eikelder ML, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;10:CD001233.
- Alfirevic Z, Keeney E, Dowswell T, et al. Which method is best for the induction of labour? A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess. 2016;20(65):1–584.
- Pierce S, Bakker R, Myers DA, et al. Clinical insights for cervical ripening and labor induction using prostaglandins. AJP Rep. 2018;8(4):e307–e314.
- Gupta JK, Maher A, Stubbs C, et al. A randomized trial of synthetic osmotic cervical dilator for induction of labor vs dinoprostone vaginal insert. Am J Obstet Gynecol MFM. 2022;4(4):100628.
- Gavara R, Saad AF, Wapner RJ, et al. Cervical ripening efficacy of synthetic osmotic cervical dilator compared with oral misoprostol at term: a randomized controlled trial. Obstet Gynecol. 2022;139(6):1083–1091.
- Saad AF, Gupta J, Hruban L, et al. Predictors of vaginal delivery after cervical ripening using a synthetic osmotic dilator. Eur J Obstet Gynecol Reprod Biol. 2020;246:160–164.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices - overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(6):796–803.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis - Principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Heal. 2014;17(1):5–14.
- Simpson KR, Lyndon A, Spetz J, et al. Adherence to the AWHONN staffing guidelines as perceived by labor nurses. Nurs Womens Health. 2019;23(3):217–223.
- Micromedex IBM. (R) [database online]. RED book online. Truven health analytics/IBM Watson gealth. 2021. https://www.micromedexsolutions.com
- Son SL, Benson AE, Hart Hayes E, et al. Outpatient cervical ripening: a cost-minimization and threshold analysis. Am J Perinatol. 2020;37(3):245–251.
- ACOG Education & Events. Inpatient versus outpatient induction of labor. The American College of Obstetricians and Gynecologists (ACOG). 2021. https://www.acog.org/education-and-events/creog/curriculum-resources/cases-in-high-value-care/inpatient-versus-outpatient-induction-of-labor
- Palmer PP, Lemus B, DiDonato K, et al. Cost of delivering intraveneous opioid analgesia in emergency departments in the United States. Value Heal. 2016;19(3):A246–A247.
- Hehir MP, Ananth CV, Siddiq Z, et al. Cesarean delivery in the United States 2005 through 2014: a population-based analysis using the robson ten group classification system. Am J Obstet Gynecol. 2018;219(1):105.e1–105.e11.
- Miller H, Goetzl L, Wing DA, et al. Optimising daytime deliveries when inducing labour using prostaglandin vaginal inserts. J Matern Fetal Neonatal Med. 2016;29(4):517–522.
- Ogbonna BN, Cabais F, Shabban M. Dinoprostone intracervical gel for cervical ripening. Int J Gynaecol Obstet. 2004;87(1):40–41.
- Haas DM, Daggy J, Flannery KM, et al. A comparison of vaginal versus buccal misoprostol for cervical ripening in women for labor induction at term (the IMPROVE trial): a triple-masked randomized controlled trial. Am J Obstet Gynecol. 2019;221(3):259.e1-259–259.e16.
- Levine LD, Downes KL, Elovitz MA, et al. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial HHS public access author manuscript conclusion-after censoring for cesarean and adjusting for parity, misoprostol-cervical foley resulted in twice the chance of deliver. Obs. Gynecol. 2016;128(6):1357–1364.
- Mendez-Figueroa H, Bicocca MJ, Gupta M, et al. Labor induction with prostaglandin E1 versus E2: a comparison of outcomes. J Perinatol. 2021;41(4):726–735.
- Dorr ML, Pierson RC, Daggy J, et al. Buccal versus vaginal misoprostol for term induction of labor: a retrospective cohort study. Am J Perinatol. 2019;36(7):765–772.
- Vesco KK, Ferrante S, Chen Y, et al. Costs of severe maternal morbidity during pregnancy in US commercially insured and medicaid populations: an observational study. Matern Child Health J. 2020;24(1):30–38.

