Abstract
Aims
Focal therapy treats individual areas of tumour in non-metastatic prostate cancer in patients unsuitable for active surveillance. The aim of this work was to evaluate the cost-effectiveness of focal therapy versus prostatectomy and external beam radiotherapy (EBRT).
Materials and methods
A Markov cohort health state transition model with four health states (stable disease, local recurrence, metastatic disease and death) was created, evaluating costs and utilities over a 10-year time horizon for patients diagnosed with non-metastatic prostate cancer. National Health Service (NHS) for England perspective was used, based on direct healthcare costs. Clinical transition probabilities were derived from prostate cancer registries in patients undergoing radical prostatectomy, EBRT and focal therapy using cryotherapy (Boston Scientific) or high-intensity focused ultrasound (HIFU) (Sonablate). Propensity score matching was used to ensure that at-risk populations were comparable. Variables included age, prostate-specific antigen (PSA), International Society of Urological Pathology (ISUP) grade group, maximum cancer core length (mm), T-stage and year of treatment.
Results
Focal therapy was associated with a lower overall cost and higher quality-adjusted life year (QALY) gains than either prostatectomy or EBRT, dominating both treatment strategies. Positive incremental net monetary benefit (NMB) values confirm focal therapy as cost-effective versus the alternatives at a willingness to pay (WTP) threshold of £30,000/QALY. One-way deterministic sensitivity analyses revealed consistent results.
Limitations
Data used to calculate the transition probabilities were derived from a limited number of hospitals meaning that other potential treatment options were excluded. Limited data were available on later outcomes and none on quality of life data, therefore, literature-based estimates were used.
Conclusions
Cost-effectiveness modelling demonstrates use of focal therapy (cryotherapy or HIFU) is associated with greater QALY gains at a lower overall cost than either radical prostatectomy or EBRT, representing good value for money in the NHS.
Plain Language Summary
Focal therapy can be used for the primary treatment of individual areas of cancer in those patients with prostate cancer whose disease has not spread (localized or non-metastatic prostate cancer) and whose disease is unsuitable for active monitoring. Focal therapy in these patients results in similar control of the cancer to more invasive therapies, such as surgical removal of the prostate and radiotherapy, with the benefit of fewer sexual, urinary and rectal side effects. This work considered whether using focal therapy (either freezing the cancer cells using cryotherapy or using high-intensity focused ultrasound [HIFU] to destroy cancer cells) was good value for money in the National Health Service (NHS) compared with surgery or radiotherapy. An economic model was developed which considered the relative impact of treatment with focal therapies, surgery or radiotherapy within the NHS in England. Previously collected information from people undergoing treatment for their prostate cancer, together with published literature and clinical opinion, was used within the model to predict the treatment pathway, costs incurred and the results of treatment in terms of patient benefits (effectiveness and quality of life). The model showed that focal therapy using either cryotherapy or HIFU was associated with a lower overall cost and higher patient benefit than either surgery or radiotherapy, indicating that focal therapy represents good value for money in the NHS.
1. Introduction
The treatment of non-metastatic prostate cancer in patients unsuitable for active surveillance has centred on treating the whole gland with extirpative surgery or radiation therapy. Such treatments confer a small survival benefit in patients with intermediate and high-risk diseases but have deleterious genito-urinary and rectal side effectsCitation1,Citation2. The use of focal therapy in select patients to target individual areas of cancer has been shown to have a 5 to 10-fold reduction in such side effectsCitation2–5, with observational studies and propensity score matched analyses comparing outcomes to radical therapies resulting in comparable medium-term rates of cancer control in terms of rates of salvage local therapy, metastases and survivalCitation3,Citation5–8.
Current European Association of Urology (EAU) treatment guidelines for prostate cancer recommend radical prostatectomy and external beam radiotherapy (EBRT) for the treatment of non-metastatic prostate cancer, offered as equal in terms of cancer control although with different side-effects and treatment deliveryCitation9. In the UK, where this work was carried out, the National Institute for Health and Care Excellence (NICE) guidelines stipulate that radical prostatectomy or radical radiotherapy can be offered as treatment options for patients with localized prostate cancer of all risks and a hierarchy of prostatectomy for different groups is not stipulatedCitation10. Both the European guidelines and NICE guidelines recommend the use of focal therapies (including cryosurgery and high-intensity focused ultrasound [HIFU]) in patients with non-metastatic prostate cancer within a clinical trial setting, or in a well-designed prospective cohort study or registryCitation9,Citation10. Indeed, there is considerable interest in the use of focal therapy in the UK, with the registries HIFU Evaluation and Assessment of Treatment (HEAT) and International Cryotherapy Evaluation (ICE) collecting patient data. This registry data provides insight into the benefits of focal therapies within appropriately selected patientsCitation11.
There is currently limited evidence supporting the cost-effectiveness of radical treatments for localized prostate cancerCitation12,Citation13. Such analyses are vital in the application of treatments in healthcare settings that consider treatment efficacy along with cost efficiencies, as seen in the UK and other middle- and high-income countries.
Given changing guidelines that allow for focal therapy to be provided in a wider healthcare setting, we believe it is important to consider not just the clinical, safety and side effect profile, but also the potential cost implications in providing this treatment. We, therefore, evaluated the cost-effectiveness of focal therapy compared to radical prostatectomy and external beam radiotherapy (EBRT) in patients newly diagnosed with localized intermediate and high-risk prostate cancer unsuitable for active surveillance, treated in the UK.
2. Methods
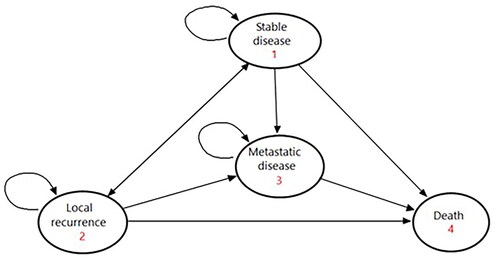
A Markov cohort health state transition model was created, evaluating costs and utilities over a 10-year time horizon for patients diagnosed with non-metastatic prostate cancer (prostate-specific antigen [PSA] < 20 ng/ml; Gleason score ≤7; MRI stage ≤2c). Four health states were defined: stable disease, local recurrence, metastatic disease and death (). The perspective was the National Health Service (NHS) for England, based on direct healthcare costs only. The cycle length was 1 month and a discount rate of 3.5% pa was applied to both costs and utilities. All modelling was carried out using TreeAge Pro Healthcare v22.1.2 (TreeAge Software LLC, Williamstown MA, USA).
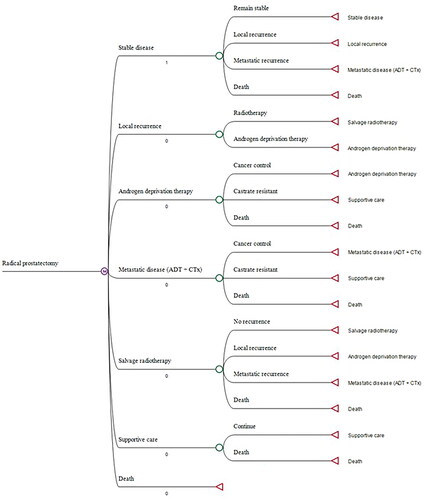
Three treatment options were explored for the index procedure: radical prostatectomy, EBRT and focal therapy (assuming a 50:50 case mix of cryosurgery and HIFU). The initiation of treatment was within 1 month of diagnosis, in cases of radical radiotherapy the start of treatment was defined as the start of neoadjuvant androgen deprivation therapy (ADT). Active surveillance was not considered as a treatment option since patients suitable for the explored treatment options would not be suitable for active surveillance and would be advised at a multidisciplinary team meeting to have active treatment which included radical and focal options. Subsequent treatment options included: re-treatment with the index procedure (for focal therapy only), salvage treatment with one of the comparator procedures, ADT (±chemotherapy [CTx]) and best supportive care (commonly known as palliation). Progression through the treatment pathway for each index treatment option was informed by clinical opinion and validated from published literature. demonstrates the pathway followed for patients receiving radical prostatectomy – equivalent pathways were explored for focal therapy and EBRT (see Figures 1 and 2, in the Supplementary Material).
Figure 2. Model schematic – example for radical prostatectomy. Note that all patients start in “stable disease” state.
2.1. Clinical input parameters
Early-stage clinical transition probabilities were derived from a series of prostate cancer disease registries documenting short- and long-term clinical outcomes for patients undergoing radical prostatectomy, EBRT and focal therapy (using cryotherapy [Visual ICE, Boston Scientific, Marlborough, MA, USA] or HIFU [Sonablate, Sonablate Corp, Charlotte, NC, USA]). Patient and disease demographics and clinical outcomes from these series have previously been publishedCitation3,Citation5–7. A propensity score matching process was undertaken to ensure that the at-risk populations were clinically and demographically comparable. Variables used in the matching process were age, PSA, International Society of Urological Pathology (ISUP) grade group, maximum cancer core length (mm), T-stage and year of treatment. The mean (standard deviation) age of patients following propensity score matching was 66 years (7.3), and 66 years (7.4) for the radical and focal group respectively. Patients in the data set used for propensity score matching predominantly had intermediate or high-risk localized diseases. Overall, of the patients in whom risk was specified 88% had intermediate or high-risk disease (23% intermediate favorable risk, 32% intermediate unfavorable risk and 33% high risk). Clinical analyses from this dataset have previously been published, with a full description of the statistical approach adopted for the weighting processCitation6. Time-to-event transition probabilities were then estimated by parameterizing the weighted Kaplan-Meier survival curves, extrapolating as required to 10 years, and extracting the data as a series of tables documenting the monthly probability of the outcome in question, from months 1–120.
Later-stage transitions (beyond the first local recurrence) were too infrequent to allow meaningful analysis to be undertaken. Consequently, transition probabilities for second and subsequent recurrences are derived from literature-based survival analyses. A summary of the inputs used is shown in .
Table 1. Summary of clinical parameters used in the model.
2.1.1. Utility valuation
Utilities were based on the state-specific results reported by Torvinen et al. based on EQ-5D questionnaires completed by 630 men with prostate cancerCitation14. Utility values used were:
Localized disease (first 6 months post diagnosis): 0.90
Localized disease (6–18 months post diagnosis): 0.89
Localized disease (>18 months post diagnosis): 0.87
Metastatic disease: 0.74
Supportive care: 0.59
2.1.2. Cost inputs
Costings were applied from the perspective of NHS England and are detailed in Table 1 in the Supplementary Material. Hospital service costs are principally drawn from NHS Reference Costs for 2019-20, on the basis that this best reflects NHS practice before the COVID-19 pandemic. Drug costs are drawn from the NHS Drug Tariff for March 2022. Estimates of complication rates are based on literature reviews with costings applied subsequently ()Citation15–20. For the purposes of this model, supportive care was assigned a zero cost. The literature did not reveal any usable estimates and carrying out a detailed costing exercise was beyond the scope of this analysis. Given that it is not expected that there will be any systematic differences between treatment groups in time to end-stage disease, it was felt that the omission of this element should not influence the validity of the overall cost estimates.
Table 2. Estimation of complication costs.
2.2. Analyses
Primary deterministic analyses were carried out to assess net monetary benefit (NMB) for each treatment option and incremental cost-effectiveness ratio (ICER) ranking for focal therapy versus prostatectomy and EBRT.
Results were based on a 10-year time horizon, to avoid extrapolation beyond the limits of the source data. Assessment of cost-effectiveness was based on a willingness to pay (WTP) threshold of £30,000/quality-adjusted life year (QALY) which is in the range of ICER thresholds used by the UK National Institute of Health and Care Excellence (NICE)Citation21. For the base case analyses, both costs and benefits were discounted at 3.5% per year. A scenario analysis was carried out to present undiscounted results for the base case.
2.2.1. Sensitivity analyses
One-way sensitivity analyses were carried out for all fixed-value variables. In the absence of information regarding variance for most parameters, a standard range of ±20% was applied to the central estimate. Transition estimates were constrained to the range 0–1, with symmetrical limitation being applied to the tested range, where necessary. For the time-sensitive transition probabilities, two scenario analyses were carried out, exploring the impact of simultaneously increasing and decreasing all per-cycle estimates by 20%.
3. Results
presents the results for the base-case analysis and for the undiscounted scenario. Focal therapy was associated with a lower overall cost and higher QALY gains than either prostatectomy or EBRT. Consequently, focal therapy dominates the other two strategies. The positive incremental NMB values confirm that focal therapy is expected to be cost-effective versus the alternatives at a WTP threshold of £30,000/QALY.
Table 3. Monte Carlo cost-effectiveness ranking using WTP = £30,000/QALY.
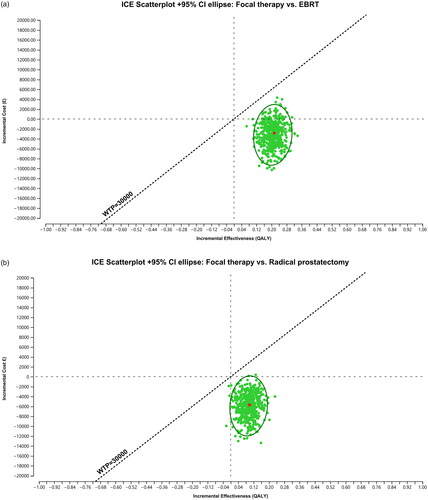
illustrates the incremental cost-effectiveness plots, based on a probabilistic sensitivity analysis of the 10 most influential parameters identified in the deterministic sensitivity analyses. The results suggest that focal therapy almost always dominates both alternative treatments, being both more effective and incurring lower costs.
Figure 3. Monte Carlo cost-effectiveness ranking using WTP = £30,000/QALY, (a) for comparison of focal therapy vs EBRT and b) for comparison of focal therapy vs prostatectomy.
3.1. Sensitivity analyses
Figure 3 in the Supplementary material presents the results of the one-way deterministic sensitivity analyses for focal therapy versus EBRT and for focal therapy versus prostatectomy. All parameters tested yielded results suggesting that focal therapy dominates both alternative treatments.
In each case, the primary drivers of the results are (a) the cost of the index procedures, (b) the distribution of secondary procedures undertaken following first local recurrence, (c) utilities associated with metastatic disease and supportive care states and (d) the cost of managing complications for the index procedure. All other variables have a minimal effect on the results. Two additional scenario analyses were carried out, exploring the consequence of a 20% increase and decrease in the cycle-specific risk of treatment failure, results remained comparable to the base case. Table 2 in the Supplementary material shows the results of this analysis and reveals that increasing or decreasing the time-sensitive transition probabilities does not have an impact on the results.
4. Discussion
This analysis demonstrated that in patients with non-metastatic prostate cancer (PSA <20 ng/ml; Gleason score ≤7; MRI stage ≤2c), the use of focal treatments (cryotherapy and HIFU) is associated with a lower overall cost and greater QALY gain over 10 years than either radical prostatectomy or EBRT. The calculation of a positive incremental NMB for focal therapy versus either comparator confirms that the incremental savings associated with focal therapy exceed the incremental costs and that this is consequently expected to be a cost-effective intervention for the NHS in England.
These results appear to be robust: exploration using multiple one-way deterministic sensitivity analyses yielded consistent results, with all estimates of ICER outcomes demonstrating dominance for focal therapies. Scenario analyses investigating variation in the time to treatment failure similarly showed qualitatively consistent results.
These results are principally driven by the lower cost per procedure for focal therapies, coupled with an ability to repeat the primary procedure in the event of localized recurrence in the first 2 years following the index intervention. Longer term outcomes are generally similar between the three treatment options and in consequence, these contribute little to the overall result. Indeed, the intention of this analysis was to model the cost-effectiveness of three different primary treatment strategies from a real-world perspective, based on actual data accrued within a UK multicenter prospective registry. Given that the median follow-up duration was 59 months, with a maximum follow-up duration of 143 months and 96.5% of the cohort were still alive on study completion, longer-term outcomes are not available within the real-world evidence. It would have been possible to extrapolate clinical outcome and survival curves beyond the chosen 10-year time horizon to a lifetime horizon, however, this would have required making a substantial set of assumptions to arrive at a point where >50% of the cohort could be assumed to have died. If this approach had been performed the majority of the costs and QALYs accrued would have been attributable to these estimated outcomes, rather than an assessment of the UK source data, which was the primary target of the analysis.
For individuals with non-metastatic prostate cancer, there are a range of treatment options available. Although patient factors may make one or other strategy the preferred option, for those with no factors that are strongly directed toward one or other treatment, the decision may be made based on both clinical and cost-effectiveness grounds. Whilst the clinical effectiveness of focal therapy has been published elsewhere, the cost-utility analysis model presented here addresses the economic impact on the NHS.
There is a limited economic evidence base for focal therapy in prostate cancer but what exists is broadly consistent with the conclusions of our analysis. Ramsay et al. carried out a literature-based economic assessment of ablative therapy in localized prostate cancer in 2015, although this was predominantly based on whole-gland ablation rather than focal therapyCitation22. This used a network meta-analysis to generate estimates of treatment effect for three ablative therapies (cryotherapy, HIFU, brachytherapy) and three comparators (active surveillance, radical prostatectomy and EBRT). The results were analyzed in an individual simulation Markov model, which explored nine different potential treatment pathways. In common with our data, the authors concluded that both HIFU and cryotherapy achieved higher utility gains than either prostatectomy or EBRT. Compared to prostatectomy, whole-gland ablative therapies were dominant, as the overall costs were also lower, whereas compared to EBRT they were associated with an additional cost. Concern was expressed by the authors that the evidence base for all ablative therapies was largely limited to non-randomised data.
Boyd et al. modelled the use of either cryotherapy or ADT in a population with radiation-recurrent prostate cancerCitation23. Cryotherapy was shown to dominate ADT in this population, although clearly, the population is not comparable to the population evaluated in our analysis, being significantly further advanced along the disease pathway. Finally, Hu et al. carried out a simplistic cost comparison in the form of a commentary, only stating upfront treatment costs, but not evaluating costs of further treatment in the radical therapies, downstream costs of managing complications, side-effects and failures nor utilities associated with each treatment strategyCitation24.
The principal strength of this analysis is that it is based on real-world evidence from UK patients treated within an NHS context. All data were prospectively gathered and included sufficient information to allow the risk status of the individuals to be accurately defined. In the UK, it is compulsory for all focal therapy cases to be inputted into a national registry, so the likelihood of case selection bias is low. Moreover, patients treated in the centers contributing to this study were all discussed in multidisciplinary meetings regarding the suitability of treatment options and then offered all appropriate treatments. We believe this reduces selection bias still further. The collection of registry data allowed a propensity score matching process to be applied, thereby minimizing the risk that between-group differences in time-to-event analysis reflected selection bias at the time of index treatment allocation.
Our study has some limitations. The data used to calculate the transition probabilities were derived from a limited range of units delivering focal therapy, and radical treatment provided by an individual high-volume tertiary cancer center, meaning that the patient pool is relatively restricted. Indeed, patients in focal therapy units received either cryotherapy or HIFU, therefore we were unable to explore other available strategies such as irreversible electroporation (Nanoknife). Radical therapy could not include brachytherapy as the cancer volume metrics from biopsy, which are key inclusion criteria for brachytherapy, were not available for the patients in the EBRT and prostatectomy groups. There were very small numbers of patients with outcomes documented beyond the second local recurrence. For this reason, the later stages of the model relied on literature-based estimates. Whilst every effort was made to ensure that appropriate studies were used to match the modelled population, it is not possible to guarantee absolute equivalence. As discussed earlier, we chose to limit the time horizon of the model to 10 years, in order not to rely on uncertain extrapolation to yield a “lifetime” result. However, this meant that we were unable to capture all deaths from prostate cancer in the cohorts. Given that radical prostatectomy and EBRT confer a lower risk of long-term mortality than focal therapies this may bias the results towards focal therapies, although, of course, patients may undergo radical prostatectomy or EBRT post-focal therapies. The dataset that we used did not include contemporaneously collected quality-of-life data. For this reason, we used literature-based estimates of the three health state utilities used in the model. Although individual studies yielded information on single health states, we were only able to identify a single study that used the same approach to estimate utilities for all three simultaneouslyCitation14. This approach means that we were unable to capture potential differences in treatment-specific health-state utilities: i.e. it assumes that localized disease carries the same utility, regardless of whether the patient has been treated with surgery, focal therapy or EBRT. This approach may have overestimated the utility of a treatment such as EBRT, which has long-term sequelae, while underestimating the utility of focal treatment, which would have been expected to have only a transient impact on quality of life. However, given that there are no studies in the prostate cancer literature that assess utilities across a full range of treatment-specific health states, this would have required sourcing the estimates for each of the treatment groups from separate studies, which would have introduced substantial potential selection bias and undermined the validity of the results. Although the face validity of the estimates used seemed plausible, we were unable to validate the results externally. Given that utility estimates were identified in the sensitivity analyses as a potential source of variability in the results, this may be considered a weakness in the analysis.
5. Conclusion
The primary purpose of this analysis was to consider the cost-effectiveness of a range of primary management strategies in localized prostate cancer, based on a real-world prospective registry of 1360 patients who received their initial intervention in five UK hospitals over the period 2006–2018. A propensity score weighting was applied to the dataset prior to the assessment of the outcomes used in the analysis, allowing dissimilar patient groups to be legitimately compared in a meaningful way. Using this approach, this cost-effectiveness model demonstrates that the use of focal therapy, cryotherapy or HIFU, in patients with localized prostate cancer represents good value for money for the UK NHS, as it is associated with greater QALY gains at a lower overall cost than either radical prostatectomy or EBRT.
Transparency
Author contributions
The economic model was devised and produced by JB, with input from HUA and DR to ensure that the model reflected UK clinical practice. The first draft of the paper was written by JB and reviewed by HUA and DR. All authors reviewed the final manuscript prior to submission.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (417.6 KB)Acknowledgements
We would like to acknowledge Tricia Dixon of JB Medical Ltd, who provided editorial support, which was funded by Boston Scientific. We would also like to acknowledge clinicians and fellows who have contributed to the diagnosis and management of patients treated with focal therapy and radical prostatectomy. This includes Philip M Huber, Derek Lomas, Arnas Rakauskas, Saiful Miah, David Eldred-Evans, Stephanie Guillaumier, Neil McCartan, Massimo Valerio, Naveed Afzal, Henry Lewi, Damian Greene, Chris Ogden and Raj Persad.
Declaration of financial/other relationships
DR has received funding from Prostate Cancer UK for research and travel grants from Sonablate Corp and Imperial College Healthcare Charity.
MvS has no relationships to declare.
MP has no relationships to declare.
MBT has no relationships to declare.
TD is a paid proctor for HIFU and is paid for training other surgeons in this procedure.
RGH is a paid proctor for HIFU and is paid for training other surgeons in this procedure. He is also a paid proctor for Rezum for the treatment of benign prostate hyperplasia.
AE has no relationships to declare.
SM has no relationships to declare.
CO has no relationships to declare.
IS has no relationships to declare.
SM has no relationships to declare.
RN has no relationships to declare.
JV has no relationships to declare.
CMM receives funding from the National Institute for Health Research, The European Association of Urology Research Foundation, MRC, Cancer Research UK, Prostate Cancer UK, Movember and the Cancer Vaccine Institute, for clinical prostate cancer research. She has received advisory board fees for Genomic Health. She is also a proctor for HIFU and is paid for training other surgeons in this procedure.
MA is a paid proctor for HIFU and cryotherapy and is paid for training other surgeons in these procedures.
TTS has received funding from Prostate Cancer UK and the St Peters Trust for clinical research and has received funding for the conference attendance from Astellis, Ferring and Galil Medical.
MW receives a travel grant and previously a loan of the device from Zicom Biobot
ME has research support from UK NIHR UCLH/UCL Biomedical Research Centre. He was awarded NIHR Senior Investigator in 2015. He is a consultant to Sonacare Inc. and Angiodynamics Inc. He has received reimbursement for travel, training, teaching and for providing professional advice from both entities.
AF has no relationships to declare.
JB is a Director of JB Medical which received a grant from Boston Scientific to fund this work and the preparation of the manuscript.
HUA is supported by core funding from the UK NIHR Imperial Biomedical Research Centre. He currently receives funding from the Wellcome Trust, Medical Research Council (UK), Cancer Research UK, Prostate Cancer UK, National Institute for Health Research (UK), The Urology Foundation, BMA Foundation, Imperial Health Charity, NIHR Imperial BRC, Sonacare Inc., Trod Medical and Sophiris Biocorp for trials in prostate cancer. He was a paid medical consultant for Sophiris Biocorp in the previous 3 years and is a proctor for HIFU, cryotherapy and Rezum and is paid for training other surgeons in these procedures.
EC has no relationships to declare.
CH has no relationships to declare.
Additional information
Funding
References
- Hamdy FC, Donovan JL, Lane JA, et al. Active monitoring, radical prostatectomy and radical radiotherapy in PSA-detected clinically localised prostate cancer: the ProtecT three-arm RCT. Health Technol Assess. 2020;24(37):1–176. doi: 10.3310/hta24370.
- Lane JA, Donovan JL, Young GJ, et al. Functional and quality of life outcomes of localised prostate cancer treatments (prostate testing for cancer and treatment [ProtecT] study). BJU Int. 2022;130 (3):370–380. doi: 10.1111/bju.15739.
- Reddy D, Peters M, Shah TT, et al. Cancer control outcomes following focal therapy using high-intensity focused ultrasound in 1379 men with nonmetastatic prostate cancer: a multi-institute 15-year experience. Eur Urol. 2022;82(3):e74–e75. doi: 10.1016/j.eururo.2022.01.005.
- Lovegrove CE, Peters M, Guillaumier S, et al. Evaluation of functional outcomes after a second focal high-intensity focused ultrasonography (HIFU) procedure in men with primary localized, non-metastatic prostate cancer: results from the HIFU evaluation and assessment of treatment (HEAT) registry. BJU Int. 2020;125(6):853–860. doi: 10.1111/bju.15004.
- Shah TT, Peters M, Miah S, et al. Assessment of return to baseline urinary and sexual function following primary focal cryotherapy for nonmetastatic prostate cancer. Eur Urol Focus. 2021;7(2):301–308. doi: 10.1016/j.euf.2019.09.004.
- van Son MJ, Peters M, Reddy D, et al. Conventional radical versus focal treatment for localised prostate cancer: a propensity score weighted comparison of 6-year tumour control. Prostate Cancer Prostatic Dis. 2021;24(4):1120–1128. doi: 10.1038/s41391-021-00369-6.
- Shah TT, Peters M, Eldred-Evans D, et al. Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol. 2019;76(1):98–105. doi: 10.1016/j.eururo.2018.12.030.
- Shah TT, Ahmed H, Kanthabalan A, et al. Focal cryotherapy of localized prostate cancer: a systematic review of the literature. Expert Rev Anticancer Ther. 2014;14(11):1337–1347. doi: 10.1586/14737140.2014.965687.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. doi: 10.1016/j.eururo.2020.09.042.
- National institute for health and care excellence. Prostate cancer: diagnosis and management, London 2021.
- Habashy D, Reddy D, Peters M, et al. Evaluation of outcomes following focal ablative therapy for treatment of localized clinically significant prostate cancer in patients >70 years: a multi-institute, multi-energy 15-year experience. J Urol. 2023;210(1):108–116. doi: 10.1097/ju.0000000000003443.
- Noble SM, Garfield K, Lane JA, et al. The ProtecT randomised trial cost-effectiveness analysis comparing active monitoring, surgery, or radiotherapy for prostate cancer. Br J Cancer. 2020;123(7):1063–1070. doi: 10.1038/s41416-020-0978-4.
- Andersson SO, Andren O, Lyth J, et al. Managing localized prostate cancer by radical prostatectomy or watchful waiting: cost analysis of a randomized trial (SPCG-4). Scand J Urol Nephrol. 2011;45(3):177–183. doi: 10.3109/00365599.2010.545075.
- Torvinen S, Farkkila N, Sintonen H, et al. Health-related quality of life in prostate cancer. Acta Oncol. 2013;52(6):1094–1101. doi: 10.3109/0284186X.2012.760848.
- Protopapa E, Smith SC, Brew-Graves C, et al. TrueNth UK post surgery – urinary function in the 1st post-operative year in a 1000 man contemporary radical prostatectomy cohort. J Urol. 2018;199(4S):E739. doi: 10.1016/j.juro.2018.02.1755.
- Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138–1144. doi: 10.1056/NEJMsa011788.
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311.
- Prabhu V, Sivarajan G, Taksler GB, et al. Long-term continence outcomes in men undergoing radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65(1):52–57. doi: 10.1016/j.eururo.2013.08.006.
- Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol. 2015;68(2):216–225. doi: 10.1016/j.eururo.2015.02.029.
- National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013 [Internet]. London: National Institute for Health and Care Excellence (NICE); 2013 Apr 4. Process and Methods Guides No. 9.
- Ramsay CR, Adewuyi TE, Gray J, et al. Ablative therapy for people with localised prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2015;19(49):1–490. doi: 10.3310/hta19490.
- Boyd KA, Jones RJ, Paul J, et al. Decision analytic cost-effectiveness model to compare prostate cryotherapy to androgen deprivation therapy for treatment of radiation recurrent prostate cancer. BMJ Open. 2015;5(10):e007925. doi: 10.1136/bmjopen-2015-007925.
- Hu JC, Laviana A, Sedrakyan A. High-Intensity focused ultrasound for prostate cancer: novelty or innovation? JAMA. 2016;315(24):2659–2660. doi: 10.1001/jama.2016.5002.