Abstract
Aims
This economic evaluation of axicabtagene ciloleucel (axi-cel) versus previous standard of care (SOC; salvage chemotherapy followed by high-dose therapy with autologous stem cell rescue) in the second line (2L) large B-cell lymphoma population is an update of previous economic models that contained immature survival data.
Methods
This analysis is based on primary overall survival (OS) ZUMA-7 clinical trial data (median follow-up of 47.2 months), from a United States (US) payer perspective, with a model time horizon of 50 years. Mixture cure models were used to extrapolate updated survival data; subsequent treatment data and costs were updated. Patients who remained in the event-free survival state by 5 years were assumed to have achieved long-term remission and not require subsequent treatment.
Results
Substantial survival and quality of life benefits were observed despite 57% of patients in the SOC arm receiving subsequent cellular therapy: median model-projected (ZUMA-7 trial Kaplan–Meier estimated) OS was 78 months (median not reached) for axi-cel versus 25 months (31 months) for SOC, resulting in incremental quality-adjusted life year (QALY) difference of 1.63 in favor of axi-cel. Incrementally higher subsequent treatment costs were observed in the SOC arm due to substantial crossover to cellular therapies, thus, when considering the generally accepted willingness to pay threshold of $150,000 per QALY in the US, axi-cel was cost-effective with an incremental cost-effectiveness ratio of $98,040 per QALY.
Conclusions
Results remained consistent across a wide range of sensitivity and scenario analysis, including a crossover adjusted analysis, suggesting that the mature OS data has significantly reduced the uncertainty of axi-cel’s cost-effectiveness in the 2L setting in the US. Deferring treatment with CAR T therapies after attempting a path to transplant may result in excess mortality, lower quality of life and would be an inefficient use of resources relative to 2L axi-cel.
Introduction
Axicabtagene ciloleucel (axi-cel) was approved in the United States (US) by the Food and Drug Administration for second-line (2L) large B-cell lymphoma (LBCL) based on the pivotal ZUMA-7 trial where it was compared against previous standard of care (SOC; salvage chemoimmunotherapy followed by high-dose therapy with autologous stem cell rescue for responders)Citation1. The mature primary OS analysis of ZUMA-7 recently demonstrated superior OS with a 27.4% reduction in the risk of death with axi-cel versus SOC after nearly 4 years of follow-up (median: 47.2 months)Citation2. This significant benefit was observed despite 57% of patients in the SOC arm crossing over to receive subsequent cellular therapy, off-protocol, following disease progression.
Previous economic analyses using ZUMA-7 primary event-free survival (EFS) data, with a median trial follow-up of 24.9 months, showed that axi-cel was highly cost-effective versus SOCCitation3,Citation4, but were limited by the duration of survival follow-up data at the time. Given the observed treatment benefit with axi-cel, uncertainty of the earlier results was acceptable in the context of the value assessment and unmet need in this population. Increased certainty in estimates regarding long-term outcomes improves the assessment of whether the cost of a new intervention provides “good value”. Longer-term follow-up also reduces the time period required for extrapolation of survival in economic evaluations. Thus, reassessing the cost-effectiveness of therapies with more mature survival data can provide greater certainty in long-term efficacy estimatesCitation5 and subsequently, the cost-effectiveness estimates themselves.
CAR T therapies represent a paradigm shift in the treatment of previously incurable hematological malignancies. While uptake of CAR T has been steadily increasingCitation6, there is a need to fully understand the value they bring to the healthcare system given their upfront costs. In light of the recently available mature ZUMA-7 data (primary OS analysis), the aim of this study was to reassess the cost-effectiveness of axi-cel versus SOC in 2L LBCL.
Methods
Model updates
Updates were made to the inputs of the previously developed partitioned survival model in 2L LBCL patients, with three mutually exclusive health states (event-free, post-event or death)Citation3. Time-to-event outcomes were updated based on mature ZUMA-7 data (primary OS analysis). EFS, time to next treatment (TTNT) and OS were fit independently and extrapolated using mixture cure models (MCMs); MCMs have previously been shown to be the most accurate approach when predicting outcomes in LBCL patients treated with axi-celCitation7. Functional forms for the extrapolation of time-to-event data were selected based on best statistical fit (using Akaike’s and Bayesian Information Criteria [AIC and BIC, respectively]), as well as expert validation for clinical plausibility. Details on MCM methods are described in the Supplemental Data.
Updated costs included treatment and long-term care costs to reflect 2023 US prices (). Subsequent treatment patterns were also updated using the ZUMA-7 primary OS analysis (Table S4); in line with the previous economic evaluation, the cost of experimental treatments was not included. Patients who remained in the EFS state by 5 years were assumed to have achieved long-term remission and not require subsequent treatment.
Table 1. Model inputs.
Analysis
Consistent with the previous analysis, the model time horizon was 50 years (lifetime) and costs and utilities were discounted 3.0% annually; a US third-party payer perspective was considered. Detailed incremental costs, incremental life years (LYs), incremental quality-adjusted life years (QALYs), along with the incremental cost-effectiveness ratio (ICER) are reported. Deterministic (costs and utilities are varied by their SE/95% confidence intervals, or when not available, ± 20%) and probabilistic sensitivity analyses (where all parameters are simultaneously varied) were performed.
Scenario analyses
Though CAR T has increased in its coverage across the US, some jurisdictions do not have access to axi-cel in third-line (3L) or higher settings. Therefore, in alignment with guidelinesCitation8, a scenario analysis removing the confounding effects of subsequent CAR T in the SOC arm was performed. Briefly, as 57% of SOC patients received off-protocol cell therapy, if axi-cel has a beneficial effect on OS, the standard ITT analysis would underestimate the OS benefit in the absence of crossing over. Using the guideline-recommended methodology of rank preserving structure failure time (RPSFT) modelsCitation9, the SOC OS curve was adjusted with the hazard ratio (HR) from first dose of cell therapy until death/censoring using full recensoring. Full details of this approach and methods are available in the Supplemental Data). Additional scenario analyses of alternate curve distributions of OS data with lower estimated cure fractions were also explored, given that extrapolations are one of the key drivers of uncertainty in any economic evaluation.
Budget impact model
Using the previously built modelCitation3, the budget impact model within the cost-effectiveness model engine of axi-cel in the 2L was updated using updated costs of 2L and subsequent treatments. The epidemiological and projected treatment pattern inputs to determine the population flow in the budget impact model are presented in . The budget impact was calculated as the difference of a practice where axi-cel is included as a treatment option for 2L LBCL patients versus practice where no CAR T cell therapy is included in the 2L, over a cumulative 5-year period (lower and upper range).
Results
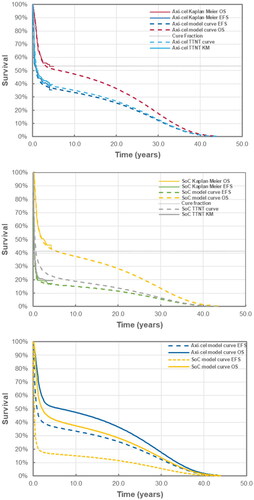
All selected base case curves were selected based on the best statistical fit (i.e. the curves had the lowest combined AIC and BIC; Supplemental Data Table S1–S3) and aligned with expert opinion. In the base case analysis, the model estimated 5-year EFS to be 36.6% and 16.4% for axi-cel and SOC, respectively. The difference in 4-year projected OS was 11.7% (52.0% vs. 40.3% for axi-cel and SOC, respectively) and the difference in estimated cure fractions was 13% (54% vs 41% for axi-cel and SOC, respectively). The cure fraction difference for OS between arms ranged from 5% to 14% in favor of axi-cel. Despite substantial crossover to 3L or later cellular therapy in the SOC arm, 10-year model OS was projected to be 10% higher with axi-cel in the base case (). The median model-projected OS of 78 months for axi-cel and 25 months for SOC was longer than previous model estimates of 59 and 24 months for axi-cel and SOC, respectivelyCitation3.
Figure 1. Time to event plots for axi-cel (a), SOC (B) and modelled extrapolated survival (C). Abbreviations. EFS, event-free survival; KM, Kaplan Meier; OS, overall survival; SOC, standard of care; TTNT, time to next treatment.
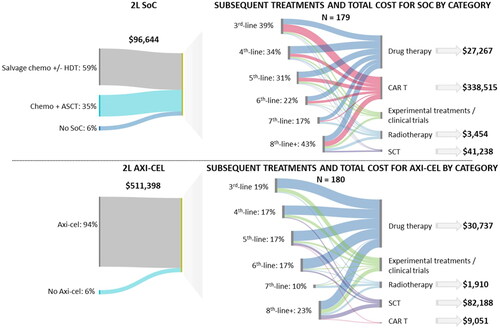
Base case cost-effectiveness results are presented in . Patients treated with axi-cel, as compared to SOC, had incrementally higher LYs and QALYs (1.74 LYs and 1.63 QALYs), with higher gains observed in the event-free state (3.54 event-free LYs and 2.88 event-free QALYs). Incrementally higher subsequent treatment costs were observed in the SOC arm due to substantial crossover to cellular therapies, with the highest proportion of subsequent treatment costs attributable to CAR T therapies in subsequent lines ($309,359) (). Among patients receiving 2L axi-cel, the highest proportion of subsequent treatment costs was stem cell therapy (). When considering the generally accepted willingness to pay (WTP) threshold of $150,000 per QALY In the USCitation10, axi-cel was cost-effective with an ICER of $98,040 per QALY versus SOC. Deterministic sensitivity analyses found that the ICER was most sensitive to the acquisition cost of axi-cel, the proportion of SOC patients receiving CAR T in 3L + and the proportion of axi-cel patients receiving allo-SCT (Figure S6). In probabilistic sensitivity analyses, axi-cel was cost-effective compared to SOC in 54% and 82% of simulations at WTP thresholds of $100,000 and $150,000 per QALY, respectively (Figures S7 and S8).
Table 2. Base case cost-effectiveness results (discounted, 2023 USD).
Axi-cel remained cost-effective with an ICER of $112,327 per QALY in the scenario analysis where crossover to subsequent cellular therapy in the SOC arm was removed (see Table S8 Supplemental Data for detailed results). In this analysis, the incremental QALYs were higher (3.59 QALY) due to the increased treatment effect of axi-cel, however, given that there was less use of subsequent treatments in the SOC arm, incremental costs were also higher ($402,988). When the most pessimistic curves by AIC/BIC were selected for OS, the resulting ICERs was $114,860, per QALY and a one-way analysis using a time horizon of 20 years (versus the baseline of 50 years) results in an ICER of $121,114 per QALY (data not shown).
In the budget impact analysis, an estimated 9 LBCL patients in a million member plan would be eligible for axi-cel in the 2L. Based on the updated costs, providing access to axi-cel for patients with 2L LBCL leads to a total 5-year cumulative budget impact of $0.06–$0.08 USD per member per month.
Discussion
This updated economic evaluation includes mature primary analysis OS data from ZUMA-7, thus greatly reducing the uncertainty regarding the cost-effectiveness of axi-cel versus previous SOC in 2L LBCL patients in the US. The more mature data reveals a significant and greater treatment effect on OS with axi-cel: the model found that for every ten 2L LBCL patients if all patients were treated with axi-cel versus SOC, one additional patient would be alive at 10 years. Further, axi-cel remains cost-effective at generally accepted WTP thresholdsCitation10, with an ICER of $98,040 per QALY. The incremental clinical benefit of axi-cel was found to be driven by the increased survival time and quality of life of patients treated with axi-cel, notably in the EFS state, contributing to a longer and higher quality of life event-freeCitation11.
This analysis also confirmed that MCM extrapolations are an appropriate method for extrapolating survival of curative therapies such as CAR T, as the survival extrapolations this analysis were over 95% consistent with the previous model’s extrapolations based on the primary EFS analysisCitation3. Furthermore, both arms appear to have robust plateau in the OS data, lasting several years, suggesting that while patients in either arm could be functionally cured, as noted above, 10% more patients would be alive at the 10-year mark if they were treated with axi-cel as compared to SOC in 2L, as some patients may die in 2L while waiting for a stem cell transplant or from disease progression. The estimated cure fraction for axi-cel for OS was above 50% regardless of the functional form used to extrapolate the data versus 41–45% for SOC. Thus, a higher proportion of patients may experience a long-term response with treatment with axi-cel compared to SOC.
Use of CAR T in 2L also highlights the increased cost offsets from reduced use of cell therapy as a subsequent treatment for those who do not respond to previous 2L SOC. These results reinforce the importance of early use of 2L cell therapy to reduce the need for cycling through lines of non-CAR T standard therapy followed by CAR T as a last resort. A previous study noted that CAR T eligible patients may not receive CAR T treatment for reasons including death and dropouts, and that delays in access to CAR T impacted treatment effectivenessCitation12. A second study among CAR T treatment centers found that despite a median time on the waiting list of 6 months, only 25% of patients were reported to receive CAR T therapyCitation13. Thus, increasing access to CAR T should be prioritized. Although the uptake of CAR T is still growing, delays in access to CAR T need to be addressed, including systemic challenges such as awareness, coverage challenges by some insurance plans, and a complete referral systemCitation14. Of note, access to CAR T therapies in the real-world may vary due to logistical, socioeconomic or provider considerations and use of CAR T therapies as a subsequent treatment in this model may therefore not reflect current treatment patterns. However, as supported by our study, delaying access to CAR T not only increases the risk of poor clinical outcomes, but results in increased costs in subsequent treatments to the health system.
This analysis is also significant in the context of assessing both the value of CAR T therapies and evaluating treatments in the context of available data. While this study highlights the cost-effectiveness of a CAR T therapy, qualifying innovative therapies as cost-effective, despite large incremental gains in QALYs remains a challenge in some jurisdictionsCitation15. Compounding this assessment of value is the uncertainty in long-term effectiveness that can arise from the use of surrogate endpoints such as EFS in oncology trials. Assessing a therapy’s clinical value when the results in terms of meaningful outcomes (such as OS) are not yet available is a challenging balancing act between addressing unmet need in treatments and confirming the value of said treatments. Economic evaluations are dependent on the data feeding the analysis, as such, the use of long-term follow-up patient-level data in this analysis reduces the uncertainty in long-term survival extrapolations. Previous studies have relied on alternative trials, historical controls, or curve digitizationCitation4,Citation16,Citation17, and/or the use of ZUMA-7 primary EFS dataCitation3,Citation4,Citation16. Providing an updated cost-effectiveness analysis using primary OS data allows decision makers to evaluate the cost-effectiveness of a therapy without the use of a surrogate endpoint and based on mature data, thereby reducing uncertainty. In this current analysis, the mature OS data further confirmed the previous cost-effectiveness analysis of axi-cel with the interim survival analysisCitation3.
This economic analysis should be interpreted in the context of its limitations. As with the previous economic evaluation, the cost of managing adverse events and related disutilities for subsequent treatments were not included as not all subsequent treatments had marketing approval in DLBCL, thus, specific adverse event profiles for these were not available. Our results are, however, still a reasonable approximation, given that neither adverse events nor disutilities in the second-line were key model drivers. While all available data was used to inform the model, experimental treatments that were used in a subset of patients (such as bi-specific antibody agents), or treatments not commercially available during the course of the ZUMA-7 trial, were not accounted for due to a lack of publicly available pricing and approval. Of note, bi-specific antibody agents have since been approved in some jurisdictionsCitation18. Further, drug wastage was not included; while axi-cel is a one-time infusion, other therapies included in this analysis could potentially be impacted by drug wastage. Given that therapies potentially impacted by drug wastage are present in the SOC arm, the results in this analysis could be considered more conservative than should drug wastage have been included.
Treatment with axi-cel in 2L LBCL remains cost-effective, well below the generally accepted WTP threshold of $150,000 per QALY in the US. This analysis using primary OS data provides increased certainty in the clinical and economic value of axi-cel in the 2L in the US. Earlier 2L treatment with axi-cel provides substantial clinical benefits in combination with an efficient use of resources. The significant projected lifetime survival benefits observed, despite 57% of patients in the SOC arm receiving subsequent cellular therapy, suggests that a strategy of deferring treatment with CAR T therapies after attempting a path to transplant may result in excess mortality. Reducing delays and barriers to access, while increasing patient awareness to CAR T therapies are remaining challenges to address.
Transparency
Author contributions
AP, CF, NJS conceived and designed the analysis. AP, SV, and CF collected the data. RB and NJS performed the analysis. OO, MAP, MD and JP contributed to the interpretation of the analysis. AP, CF and NJS wrote the first draft of the manuscript. All authors provided feedback on the manuscript and all authors approved the final version of the manuscript.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they received consultant fees from Gilead srl. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose. All peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download PDF (475.2 KB)Acknowledgements
The authors would like to thank Lianne Barnieh, of the Maple Health Group, for her contributions to the preparation of this manuscript.
Declaration of financial/other interests
OO reports consultancy and advisory board work with Pfizer, Kite, Gilead, AbbVie, Janssen, TGR therapeutics, ADC, Novartis, Epizyme, Curio science, Nektar, Cargo, Caribou and has received institution funding from Kite, Pfizer, Daichi Sankyo, Allogene and honoraria from Pfizer and Gilead. ARP, SV, and CF are employees of Kite, a Gilead company and have stock ownership. NJS and RB are employees of Maple Health Group, who received fees from Gilead Consulting for the conduct of this work. MD reports honoraria from Roche, Amgen, MSD, Janssen, Bristol-Myers Squibb, Novartis, Gilead Sciences and Abbie; he has received consulting fee or has played an advisory role for Novartis, Bristol-Myers Squibb, Gilead Sciences, Janssen, Abbvie and Genmab. MD has received institutional research support from Novartis, Roche, Takeda, Celgene, MSD, Abbvie, and Lilly. PJ has nothing to disclose. MAP reports honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board of NexImmune. MAP has ownership interests in NexImmune, Omeros and OrcaBio; he has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis.
Additional information
Funding
References
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2021;386(7):640–654. doi: 10.1056/NEJMoa2116133.
- Westin JR, Oluwole OO, Kersten MJ, et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. 2023;389(2):148–157. doi: 10.1056/NEJMoa2301665.
- Perales MA, Kuruvilla J, Snider JT, et al. The cost-effectiveness of axicabtagene ciloleucel as second-line therapy in patients with large B-cell lymphoma in the United States: an economic evaluation of the ZUMA-7 trial. Transplant Cell Ther. 2022;28(11):750.e1-750–e6. doi: 10.1016/j.jtct.2022.08.010.
- Kambhampati S, Saumoy M, Schneider Y, et al. Cost-effectiveness of second-line axicabtagene ciloleucel in relapsed refractory diffuse large B-cell lymphoma. Blood. 2022;140(19):2024–2036. doi: 10.1182/blood.2022016747.
- Tai T-A, Latimer NR, Benedict Á, et al. Prevalence of immature survival data for anti-cancer drugs presented to the national institute for health and care excellence and impact on decision making. Value Health. 2021;24(4):505–512. doi: 10.1016/j.jval.2020.10.016.
- Borgert R. Improving outcomes and mitigating costs associated with CAR T-cell therapy. Am J Manag Care. 2021;27(13 Suppl):S253–s261. doi: 10.37765/ajmc.2021.88737.
- Vadgama S, Mann J, Bashir Z, et al. Predicting survival for chimeric antigen receptor T-cell therapy: a validation of survival models using follow-up data from ZUMA-1. Value Health. 2022;25(6):1010–1017. doi: 10.1016/j.jval.2021.10.015.
- NICE. Final appraisal document: axicabtagene ciloleucel for treating relapsed or refractor diffuse large B-cell lymphoma after first-line chemoimmunotherapy. 2023.
- Latimer NR, Abrams KR. NICE DSU Technical Support Document 16: adjusting survival time estimates in the presence of treatment switching. 2014.
- Gold MR. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 1996.
- Kersten MJ, Qiao Y, Shah R, et al. Quality-adjusted time without symptoms or toxicity: analysis of axicabtagene ciloleucel versus standard of care in patients with relapsed/refractory large B cell lymphoma. Transplant Cell Ther. 2023;29(5):335.e1–335-e8. doi: 10.1016/j.jtct.2023.01.008.
- Chen AJ, Zhang J, Agarwal A, et al. Value of reducing wait times for chimeric antigen receptor T-cell treatment: evidence from randomized controlled trial data on tisagenlecleucel for diffuse large B-cell lymphoma. Value Health. 2022;25(8):1344–1351. doi: 10.1016/j.jval.2022.02.007.
- Kourelis T, Bansal R, Patel KK, et al. Ethical challenges with CAR T slot allocation with idecabtagene vicleucel manufacturing access. J Clin Oncol. 2022;40(16_suppl):e20021–e20021. doi: 10.1200/JCO.2022.40.16_suppl.e20021.
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. J Clin Oncol Oncol Pract. 2022;18(12):800–807. doi: 10.1200/OP.22.00315.
- Shafrin J, Quddus S, Marin M, et al. A decade of health innovation: the impact of new medicines on patient health and the implications for NICE’s size of benefit multiplier. Value Health. 2023;26(10):1435–1439. doi: 10.1016/j.jval.2023.06.009.
- Choe JH, Abdel-Azim H, Padula WV, et al. Cost-effectiveness of axicabtagene ciloleucel and tisagenlecleucel as second-line or later therapy in relapsed or refractory diffuse large B-cell lymphoma. JAMA Netw Open. 2022;5(12):e2245956. doi: 10.1001/jamanetworkopen.2022.45956.
- Vijenthira A, Kuruvilla J, Crump M, et al. Cost-effectiveness analysis of frontline polatuzumab-rituximab, cyclophosphamide, doxorubicin, and prednisone and/or second-line chimeric antigen receptor T-cell therapy versus standard of care for treatment of patients with intermediate- to high-risk diffuse large B-cell lymphoma. J Clin Oncol. 2023;41(8):1577–1589. doi: 10.1200/JCO.22.00478.
- Tavakkoli M, Barta SK. 2024 Update: advances in the risk stratification and management of large B-cell lymphoma. Am J Hematol. 2023;98(11):1791–1805. doi: 10.1002/ajh.27075.
- Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21(12):1238–1245. doi: 10.1080/13696998.2018.1529674.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–420. doi: 10.1177/0272989X06290495.
- NICE. Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies [TA567]. Technology appraisal guidance. London: National Institute for Health and Care Excellence; 2019.
- RED BOOK. 2023. [cited 20021 Nov 1]. Available from: https://www.ibm.com/products/micromedex-red-book.
- Liu R, Oluwole OO, Diakite I, et al. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J Med Econ. 2021;24(1):458–468. doi: 10.1080/13696998.2021.1901721.
- Centers for Medicare and Medicaid Services. Physician fee schedule 2023 [cited 2023 Mar 31]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched.
- Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00.
- Pelletier EM, Smith PJ, Dembek CJ. Payer costs of autologous stem cell transplant: results from a U.S. claims data analysis. Blood. 2008;112(11):2373–2373. doi: 10.1182/blood.V112.11.2373.2373.
- Broder MS, Quock TP, Chang E, et al. The cost of hematopoietic stem-cell transplantation in the United States. Am Health Drug Benefits. 2017;10(7):366–374.
- Kutikova L, Bowman L, Chang S, et al. Medical costs associated with non-Hodgkin’s lymphoma in the United States during the first two years of treatment. Leuk Lymphoma. 2006;47(8):1535–1544. doi: 10.1080/10428190600573325.
- Surveillance EaERP. Cancer stat facts: NHL - diffuse large B-cell lymphoma (DLBCL). 2021. [cited 2021 Nov 1]. Available from: https://seer.cancer.gov/statfacts/html/dlbcl.html.
- Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842–858. doi: 10.1056/NEJMra2027612.
- Maurer MJ, Ghesquières H, Jais J-P, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–1073. doi: 10.1200/JCO.2013.51.5866.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Program. 2011;2011(1):498–505. doi: 10.1182/asheducation-2011.1.498.