Abstract
Objective
To assess the costs of treated recurrence and survival in elderly patients with early breast cancer (EBC) at high risk of recurrence using Surveillance Epidemiology and End Results (SEER) registry-Medicare linked claims data.
Methods
This retrospective study included patients aged ≥65 years with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 negative (HER2−), node-positive EBC at high risk of recurrence. Treated recurrences were defined based on treatment events/procedure codes from claims. Primary outcomes were monthly total extra costs and cumulative extra costs of treated recurrence relative to patients with non/untreated recurrence. Costs were calculated using a Kaplan-Meier sampling average estimator method and inflated to 2021 US$. Secondary outcomes included analysis by recurrence type and overall survival (OS) after recurrence. Subgroup analysis evaluated costs in patients with Medicare Part D coverage.
Results
Among 3,081 eligible patients [mean (SD) age at diagnosis was 74.5 (7.1) years], the majority were females (97.4%) and white (87.8%). Treated recurrence was observed in 964 patients (31.3%). The monthly extra cost of treated recurrence was highest at the beginning of the first treated recurrence episode, with 6-year cumulative cost of $117,926. Six-year cumulative extra costs were higher for patients with distant recurrences ($168,656) than for patients with locoregional recurrences ($96,465). Median OS was 4.34 years for all treated recurrences, 1.92 years for distant recurrence, and 6.78 years for locoregional recurrence. Similar cumulative extra cost trends were observed in the subgroup with Part D coverage as in the overall population.
Limitations
This study utilizes claims data to identify treated recurrence. Due to age constraints of the dataset, results may not extrapolate to a younger population where EBC is commonly diagnosed.
Conclusion
EBC recurrence in this elderly population has substantial costs, particularly in patients with distant recurrences. Therapies that delay or prevent recurrence may reduce long-term costs significantly.
Background
Breast cancer (BC) is the most common non-cutaneous cancer and the second leading cause of cancer deaths among women in the United States (US)Citation1. A US population-based registry reported hormone receptor-positive (HR+), human epidermal growth factor receptor 2 negative (HER2−) BC as the most common subtype of BC, accounting for 73% of all BC casesCitation2. More than 90% of patients with the HR+, HER2− subtype are diagnosed with non-metastatic (stage I–III) early breast cancer (EBC)Citation3. Most patients with HR+, HER2− EBC have a good prognosis with a survival rate of 92.5% at 4 years without experiencing invasive recurrenceCitation4,Citation5. However, 20–30% of EBC patients are estimated to develop locoregional or distant recurrence over 10 yearsCitation6,Citation7. Certain clinicopathologic features, such as ≥4 positive axillary lymph nodes (ALNs); grade 3; tumor size ≥5 cm; and Ki-67 ≥ 20%, confer a higher risk of recurrenceCitation8,Citation9. A recent US-based retrospective study quantified the risk of recurrence in patients with HR+, HER2− EBC associated with a combination of high-risk clinicopathological features (consistent with the monarchE trial) and reported that the risk of recurrence was three times greater in the patients with high-risk clinicopathological features, warranting the need for better therapies to prevent or delay BC recurrenceCitation5.
At this juncture, recent clinical trials have investigated the addition of new adjuvant treatments to current therapies in HR+, HER2− EBC and were designed to target patients at high risk of recurrenceCitation10,Citation11. As a result, several treatment options, including adjuvant endocrine therapy and targeted therapies are now available or in development, either to prevent or delay BC recurrenceCitation10–15. Consequently, the management of BC is associated with a substantial economic burden.
Previous studies have evaluated the economic burden associated with the recurrence of EBCCitation16,Citation17. A study using the US Surveillance Epidemiology and End Results (SEER) registry-Medicare data (1991–2002) showed that BC recurrence (distant, local, and contralateral recurrence) after treatment leads to substantial increases in costs over 10 years (∼$11,000–$19,000), where cost estimates were based on Medicare payments adjusted to 2004 US$Citation16. Another study examining the economic burden associated with disease recurrence in patients with EBC using Henry Ford Health System (HFHS) registry data (1996–2002) reported substantially greater quarterly charges for continuing post-recurrence care than pre-recurrence care ($4,934 vs. $1,825, p <.001); the charges were inflated to 2003 US$Citation17. However, these studies did not cover outpatient pharmacy claimsCitation16,Citation17, an important component of costs as it comprises 77% of total Medicare drug costsCitation18. Therefore, it is pivotal to consider outpatient pharmacy claims for a meaningful health economic analysis.
In addition, longitudinal data on the costs of treating BC recurrence are limitedCitation16,Citation17. Thus, more up-to-date estimates of the costs after treated recurrence of BC are warranted to facilitate informed value assessments of treatment. Furthermore, previous studies included patients of varying risk of recurrence and regardless of biomarker status. The treatment based on these considerations, and hence the cost, can differ substantially. The current study was designed to examine the costs of treated recurrence and survival in elderly patients (age ≥65 years) with HR+/ HER2−, node-positive EBC at high risk of recurrence (eligibility criteria for monarchE) from the Medicare sector perspective, which includes payments by Medicare and cost-sharing payments by patients.
Methods
Study design and data source
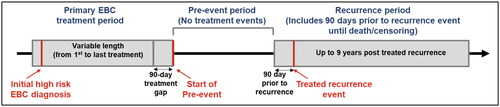
This retrospective study used linked patient data from the SEER registry (1 January 2010 to 31 December 2014) and Medicare claims (1 January 2009 to 31 December 2019), which included data from Part A (inpatient care), B (outpatient care, home health care, durable medical equipment, health care provider services), and optional Part D (outpatient prescription drugs) ()Citation19. The SEER registry is a cancer reporting system that collects and reports cancer incidence and survival data from population-based cancer registries, covering approximately 48% of the US populationCitation20. The SEER registry collects clinical, demographic, and cause of death information for persons diagnosed with incident cancerCitation21. The SEER registry-Medicare linked database combines the cancer registry data with administrative Medicare files. The Medicare files contain Medicare claims details for covered healthcare services from the time of a person’s Medicare eligibility (age ≥65 years, major disability, end-stage renal disease) until death.
Figure 1. Study design. Abbreviations: EBC, Early breast cancer; SEER, Surveillance Epidemiology and End Results; HCRU, Health-care resource utilization.
In this study, we only examined patient records related to BC that were obtained from the SEER registry-Medicare linked database. The retrospective data from SEER-Medicare used in this study did not require formal consent from patients, as the database follows Health Insurance Portability and Accountability Act (HIPAA) privacy standards with regards to deidentifying patient data. Data analyzed in this study were obtained through a Data Use Agreement (DUA) by the National Cancer Institute (NCI). The study was deemed exempt from review by the institutional review board.
Study population
Patients aged ≥65 years, with an initial diagnosis of HR+, HER2−, node-positive EBC (American Joint Commission on Cancer staging criteria stage I–IIIC) who are at high risk for recurrence, as identified in the SEER registry, were includedCitation22. Patients were considered to be at high risk of recurrence if they met the following criteria: pathological tumor involvement in four or more ipsilateral ALNs or one to three ALNs and at least one of the following: histologic/nuclear Grade 3 or pathological primary tumor size ≥5 cm. In addition, patients should have been continuously enrolled in Medicare Part A and Part B (for 3 years) from the time of their initial high-risk EBC diagnosis until either death or the cut-off date for the available Medicare claims data. Patients were excluded from the study if they were initially enrolled in Medicare due to disability or end-stage renal disease, had a history of previous malignancies, or had an unreported cancer stage at the time of diagnosis.
All patients who met the selection criteria were divided into two groups: patients with treated recurrence and patients with non/untreated recurrence. Patients in the non/untreated recurrence group include those for whom no BC recurrence was observed within the time constraints of the analysis, as well as patients who may have experienced BC recurrence but did not receive treatment. The SEER-Medicare linked data do not explicitly capture recurrenceCitation23. The National Cancer Institute explicitly cautions that recurrence is a limited measure in the SEER-Medicare linked data with many instances of recurrence likely to be untreated in this population, thus, potentially undetectable using claims dataCitation23–25. Non-recurrence and untreated recurrence were grouped due to this detectability limitation and assumed to have similar costs. The rationale for this assumption is that costs for these two groups would be similar with non-recurrence patients not requiring any treatment for recurrence and untreated recurrence patients not receiving treatment despite recurrence. Medicare data were reviewed to identify primary BC treatment events and associated claims data. The identification of primary BC events (including local, regional, distant, and contralateral) was in accordance with the methodology used by Stokes et al.Citation16 Second new (non-breast) primary cancers were identified by tumor location type codes from the SEER datafile. Relevant ICD-9/ICD-10 codes and Current Procedural Terminology (CPT) codes for each cancer treatment are presented in Supplementary Table S1. The type of recurrence was inferred by reviewing listed ICD-9-CM and ICD-10-CM diagnosis codes recorded in the medical claims data (Supplementary Table S2).
Treatment cost was defined as the sum of total payments for treatment (Medicare payments and patient out-of-pocket payments). Out-of-pocket payments include coinsurance and beneficiary deductibles where available (i.e. Medicare Provider Analysis and Review [MedPAR] Part B outpatient datasets) and were otherwise derived from total costs minus primary payer and Medicare costs. To identify and quantify the cost associated with treated recurrence, it was necessary to separate treatment events related to the treatment of primary EBC from subsequent treatment events associated with recurrence. The approach to defining the periods and identifying treated recurrence followed the general methodology used by Stokes et al.Citation16 The period of follow-up for each patient was divided into:
The primary EBC treatment period included the occurrence of any primary EBC treatment (mastectomy [including lumpectomy], chemotherapy, or radiotherapy) within 90 days of each other with the first in the chain falling between 30 days prior and 90 days after the SEER-documented BC diagnosis. It also included any similar chains of surgeries/mastectomies starting within 1 year after initial neoadjuvant endocrine therapy. When a gap in treatment of at least 90 days after primary treatment was encountered, it was assumed that the primary treatment had ended. The start and end dates of the period were identified by the dates of service associated with individual claims in the Medicare claims data.
The pre-event period began 90 days after the end of the last primary treatment event. For patients with a treated recurrence during the follow-up period, the pre-event period ended 90 days prior to the date of the treated recurrence. Contrastingly, for patients with non/untreated recurrence during the follow-up period, the pre-event period ended with death or censoring.
Treated recurrence period defined as any treatment event, (chemotherapy/immunotherapy, radiotherapy, or surgery signaling treated recurrence on or after the first day of the pre-event period) after the 90-day gap following the end of the primary EBC treatment period, and any subsequent treatment for BC was considered treated recurrent BC.
Outcomes and measures
The primary outcomes were monthly total extra costs and cumulative extra cost of treated BC recurrence. The cost associated with treated BC recurrence was determined in all the periods of follow-up. As the availability of sample size for analysis is small toward the end of the follow-up period, the standard errors of the associated estimates (monthly total extra cost and cumulative extra cost) are relatively large with wide confidence intervals. Accordingly, the presentation of results focuses on a follow-up period of 6 years (72 months). Patient-specific costs based on the healthcare resource utilization (HCRU) and pharmacy costs were collected for treated and non/untreated recurrence patients. Monthly extra costs were inferred by differencing the average costs of those without a treated recurrence (non/untreated recurrence) from the costs of those with a treated recurrence. Cumulative extra costs were the sum of survival-adjusted extra costs in each month over the follow-up period. Secondary outcome measures included overall survival (OS) after treated BC recurrence. Time to death was calculated from the date of recurrence to date of death sourced from the SEER data (provided as month and year). The death date was assumed to be the 15th of the month. For patients who had claimed after the 15th of the month, the death date was assumed to be the end of the month. The availability of outpatient pharmacy claims provided additional detail that could be used to detect recurrence treatment events. Therefore, subgroup analysis was performed involving EBC patients with Part D coverage with treated recurrence throughout the follow-up period. In addition, the extra cost of treated recurrence was assessed for subgroup of patients with Part D coverage.
The primary analysis included the overall population and characterized costs associated with any treated local, regional, distant, contralateral recurrence, and/or second primary (non-BC) cancer. These events were defined as per the original Standardized Definitions of Efficacy End Points criteria (2007), which were applied in the monarchE clinical studyCitation26. Secondary analysis involving patients with locoregional treated recurrence versus distant (metastatic) recurrence was conducted to analyze extra recurrence costs and the extent to which extra costs differ by type of recurrence.
Statistical analysis
Baseline patient characteristics and follow-up study measures were summarized descriptively. Mean per-patient cumulative post-recurrence costs of patients who experienced treated recurrence compared to those with non/untreated recurrence were estimated using Kaplan-Meier (KM) sampling average estimator by Lin et al.Citation27 Nonparametric bootstrap analysis was utilized to resample the study population and summary statistics (95% CI and SD) were obtained to draw the statistical inference on the point-estimator (per-patient cumulative post-recurrence costs). All cost values were inflated to December 2021 US$.
Patients were censored if they were alive and were continuously enrolled in traditional (fee-for-service) Medicare (Part C) through the date cut-off for the Medicare claims data or had discontinued enrolment in Medicare prior to death. OS was estimated using the KM method. Patients who survived the maximum feasible follow-up period were censored in KM analysis. All statistical analyses were performed using R version 4.1.0Citation28.
Results
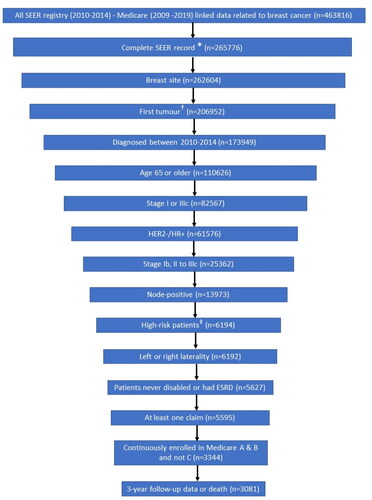
A total of 463,816 patients with BC were available in the SEER-Medicare database for the study period. After all inclusion and exclusion criteria were applied, 3,081 patients were found eligible for analysis (). Before considering the high-risk criteria, the criteria having the largest impact on attrition were completeness of SEER record and initial diagnosis at age ≥65 years.
Figure 2. Patient attrition and final sample size. Abbreviations: AJCC, American Joint Committee on Cancer; ESRD, end-stage renal disease; HR+/HER2−, hormone receptor positive/human epidermal growth factor receptor 2; ID, SEER, Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Notes: *Complete record defined as non-missing year of diagnosis, tumor site, AJCC stage, breast subtype, and AJCC N from SEER data. †Sequence number for first tumor is 0 when the breast cancer tumor is the patient’s first and only tumor and 1 when the breast cancer tumor is the patient’s first of multiple tumors. ‡High risk patients are defined as those who had 4–90 regional node-positive lymph nodes, or 1–3 regional node-positive lymph nodes (SEER variable: Regional_nodes_positive_1988) and either tumor grade 3 (SEER variable: Grade) or tumor size greater than or equal to 5 cm (SEER variable: CS_tumor_size_2004_2015).
Patient demographics and clinical characteristics
The mean (SD) age at diagnosis in the overall study sample was 74.5 (7.1) years, and the majority were female (97.4%) and white (87.8%). Similar baseline characteristics were observed in patients with Medicare Part D data. All other characteristics are presented in . In the overall population, treated and non/untreated recurrence were observed in 964 (31.3%) and 2,117 (68.7%) patients, respectively. Among patients with treated recurrence, distant recurrence (432; 44.8%) was the most common recurrence type identified, followed by locoregional recurrence (128; 13.3%) (). The type of recurrence was not identified in 395 (41.0%) patients due to the lack of a diagnosis code associated with a recognized treatment. Similar recurrence characteristics were observed in subgroup patients with Part D data, where recurrence was observed in 557 (31.3%) patients (Distant: 245 (44.0%); Locoregional: 78 (14.0%); recurrence type not identified: 232 (41.7%)). Patients in both the treated recurrence and non/untreated recurrence groups were predominately white and female with similar baseline characteristics. No initial EBC treatment was identified for 237 (11.2%) patients. Compared to patients in the treated recurrence group (964; 100%), a lower percentage (1,880; 88.8%) of patients in the non/untreated recurrence group received initial treatment as identified from claims. However, the receipt of initial treatment is conditional with surgery being the most common initial treatment in both groups (recurrence group: 81%; non/untreated group: 88%) followed by chemo/immunotherapy (recurrence group: 15.6%; non/untreated group: 8.7%).
Table 1. Patient demographics and clinical characteristics – overall population and Part D.
Table 2. Comparison of characteristics of treated recurrence in the primary analysis set and Part D subgroup.
Overall population
Pre-event cost
Treatment costs were similar throughout the pre-event period after the initial diagnosis. The 95% CI band of the monthly cost differences in pre-event period largely captures the x-axis (i.e. cost difference = $0), indicating no significant difference (Supplementary Figure S1). In the first year of the pre-event period, the mean (SE) total cost of the treated recurrence and non/untreated recurrent groups was $16,628 ($937) and $18,312 ($590), respectively. There was no significant difference in the total first-year costs between the two groups (bootstrap p = 0.137). The corresponding monthly cost for recurrent patients is $1,386, and for non/untreated recurrent patients is $1,526. This indicates that treatment costs for the patients who later experienced a treated recurrence and those who did not experience a treated recurrence are sufficiently similar, thereby making the non/untreated recurrence patients suitable as a comparison group for recurrence patients for estimating the extra cost of recurrence.
Extra cost of treated recurrence
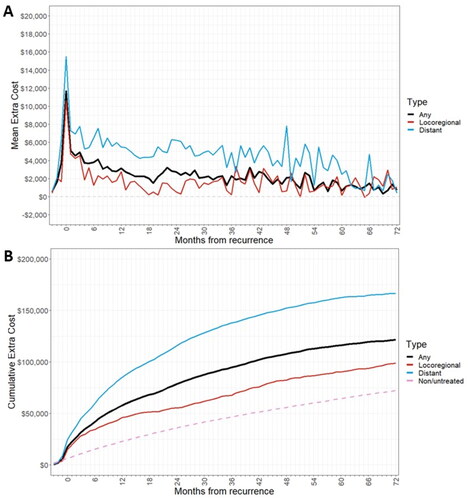
presents the estimated extra cost of treated recurrence by month for the first 6 years of follow-up. The extra cost of recurrence per treated patient was estimated as $5,627 over the 3 months immediately preceding treated recurrence (). The mean extra cost of treated recurrence was estimated as $21,582 over the first 3 months after treated recurrence (0–3 months cumulative), which declined to $11,693 (3–6 months), $9,946 (6–9 months), and $8,524 during the final months of the first-year post-recurrence (9–12 months). Furthermore, monthly extra costs were on average $2,457 during the second-year post-recurrence (12–24 months), and $2,142 in the third-year post-recurrence (34–36 months) and declined to $1,046 in the sixth year.
Figure 3. Extra cost of recurrence for all recurrence patients by month after recurrence over the first 6 years of follow-up period.
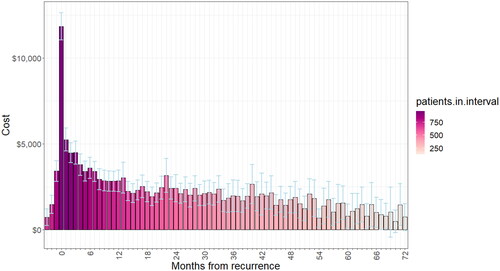
Table 3. Estimated mean extra cost of recurrence, by period after recurrence.
Cumulative extra costs of treated recurrence by type of recurrence
Patients with a distant recurrence had a higher extra cost of treated recurrence than those with a locoregional recurrence, especially after the initial recurrence treatment period (). In addition, estimated mean cumulative treatment costs over 6 years post-recurrence and adjusted for survival were greater for patients with a distant recurrence ($168,656) than those with locoregional recurrence ($96,465) ( and ).
Figure 4. (a) Extra cost of recurrence by month after recurrence, by recurrence type (b) cumulative treatment costs post-recurrence, by recurrence type.
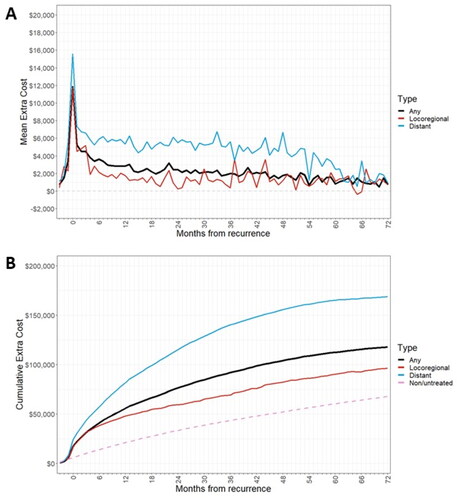
Table 4. Cumulative treated recurrence costs by recurrence type in primary analysis set and Part D subgroup.
Cumulative extra costs of treated recurrence by type of service
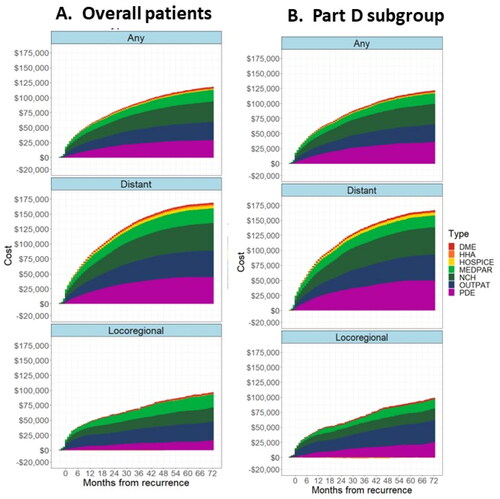
In the overall population and regardless of the type of recurrence, the largest contributor to overall cumulative costs (mean) was physician services [National Claims History (NCH)] cost ($34,278), followed by outpatient services costs ($30,261) and prescription drugs costs (PDE; $28,850). For patients with distant recurrence, the largest contributor to overall cumulative cost (mean) was NCH costs ($46,200), followed by PDE costs ($44,536) and outpatient services costs ($44,143). Whereas, for patients with locoregional recurrence, the largest contributor to overall cumulative cost was outpatient services ($31,410), followed by physician services ($22,898) and hospital services (MedPAR, $22,183) ().
Figure 5. Cumulative treatment costs by type of service (a) overall population and (b) Part D subgroup. Abbreviations: DME, Durable Medical Equipment; HHA, Home Health Agency; MEDPAR, Medicare Part A Inpatient; NCH, National Claims History (Medicare Part B Non-institutional); OUTPAT, Medicare Part B Institutional; PDE, Part D Drug Event. Notes: Primary population is the full primary analytical population. Part D subgroup is the analysis of the subset of patients who had continuous Part D enrollment from 1-month prior to their diagnosis of breast cancer until their exit date.
Subgroup analysis – patients with Part D coverage
Pre-event cost
In patients with Part D coverage data, pre-event costs for the non/untreated recurrence patients are similar to the pre-event costs for the treated recurrence patients, thereby making the non/untreated recurrence patients a suitable comparison group to be compared to recurrence patients for estimating the extra cost of recurrence. The monthly mean (SD) treatment costs for patients with recurrence and non/untreated recurrence during the pre-event period were $1,626 (206.70) and $2,036 (170.01) respectively, at baseline, and it was $1,050 (221.16) and $1,474 (224.50) for recurrence and non/untreated recurrence at 6 years (Supplementary Figures S2a and b).
Extra cost of treated recurrence
The estimated extra cost of treated recurrence by month after recurrence for the subset of patients who had continuous Part D coverage over the follow-up period (72 months) is shown in . Patients with a distant recurrence had a higher extra cost of treated recurrence than patients with a locoregional recurrence, especially after the initial recurrence treatment period (). In addition, estimated mean cumulative treatment costs over 6 years post-recurrence and adjusted for survival were greater for patients with a distant recurrence ($166,472) than those with locoregional recurrence ($98,741) ( and ).
Cumulative extra costs of treated recurrence by type of service
In patients with Part D coverage regardless of the type of recurrence, the largest contributor to overall cumulative cost (mean) was PDE cost ($35,653), followed by NCH cost ($34,134) and outpatient cost ($29,474). A similar trend was observed in patients with distant recurrence. However, for patients with locoregional recurrence, the largest contributor to overall cumulative cost was outpatient services ($37,121), followed by PDE ($24,993) and NCH ($19,018) ().
Survival
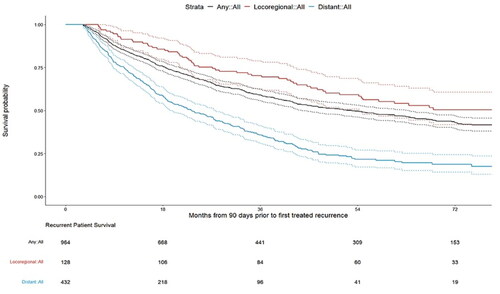
The median (95% CI) OS from recurrence for patients with any treated recurrence was 4.34 (3.66–5.20) years. Median OS was longer in patients with treated locoregional recurrence than with treated distant recurrence (6.78 years vs. 1.92 years) ().
Discussion
To the best of our knowledge, this is the first study to consider outpatient pharmacy claims, in addition to Medicare Part A and B claims, to evaluate and report the costs of treated recurrence in patients with HR+/HER2−, node-positive EBC at high risk of recurrence. The current study utilized the linked SEER registry-Medicare data to assess the extra cost (all-cause costs and not limited to drug cost) attributable to recurrence and survival in patients with HR+/HER2−, node-positive EBC at high risk of recurrence. Despite well-recognized clinicopathological risk factors for recurrence of BC such as nodal positivityCitation29 and higher stage of diseaseCitation30, there is no standard definition of high risk. Given the lack of a standardized definition, in the present study, we used the definition for the monarchE trial, which recently informed the FDA label for abemaciclib in high-risk EBC patientsCitation31. In the current study, identification of subset of patients with HR+/HER2−, node-positive EBC at high risk of recurrence was consistent with the monarchE trialCitation31 except for the Ki-67 expression inclusion criteria as this variable is not collected in the SEER registry over the time frame of the study.
In the present study, based on the diagnosis codes, treated recurrence was observed in 964 (31.3%) patients [distant (432; 44.8%), locoregional (128; 13.2%), contralateral (9; 0.9%), no type identified (395; 41.0%)]. These findings are consistent with a prior study that reported a recurrence rate of 36.8% after primary BC treatment using SEER-Medicare data (1991–1997) after 10 years of follow-upCitation30. The results of the current analysis showed that additional cost (all-cause costs, and not limited to drug cost) of treated BC recurrence, compared with non/untreated recurrence, was considerably higher at the beginning of a first treated BC recurrence episode, but overall cost remained substantial for several years after recurrence. These findings are on par with the recent report by Jan et al. which showed higher all-cause and BC-related HCRU among patients with recurrence after a diagnosis of triple-negative EBC, particularly metastatic recurrence ($8,575/month, adjusted to 2019 US$) compared to patients with non-recurrenceCitation32.
Additional cumulative extra costs over 6 years post-recurrence were lower for locoregional recurrence ($96,465) compared with distant recurrence ($168,656). In addition, similar patterns of extra cost of treated recurrence were observed in the overall population and patients with Part D drug coverage over the entire study period. These findings are consistent with a previous report by Lamerato et al. which showed higher mean total care charges in patients with contralateral ($43,803), locoregional ($66,927), and distant BC recurrences ($102,504) than those without BC recurrenceCitation17. However, the findings from Lamerato et al. are from the HFHS integrated healthcare system (1996–2002) and involved younger patients with EBC [mean (SD) age: 44 (23) years for patients in the recurrence group], regardless of recurrence risk, and measured costs based on charge list prices (rather than payments) inflated to 2003 US$Citation17.
Our analysis confirmed that survival following BC recurrence events varies widely depending on the type of event, where patients with locoregional recurrence experienced longer median OS than patients with distant recurrence. These findings follow the same trend found in a previous study, where Stokes et al. reported that median OS was longer for patients with local recurrence (36 months) than distant recurrence (8 months)Citation16; though the length of OS has increased substantially since that report, indicating the development and use of more efficacious treatments in the intervening timeframe.
Although our approach was based on the method used by Stokes et al., the current study includes several distinctions. Firstly, in the current analysis, we recognized mastectomies and other treatment-related surgeries up to 1 year following diagnosis of EBC as a part of the primary treatment for EBC if they were preceded by any neoadjuvant endocrine therapy. This allowed us to identify patients who might otherwise have appeared not to receive treatment in the first year and for whom we would not subsequently have been able to identify a recurrence event. However, Stokes et al.Citation16 did not recognize neoadjuvant therapies. Secondly, Stokes et al.Citation16 only used primary diagnosis codes to assign types of recurrence. However, Mues et al.Citation33 reported that diagnoses of interest may not always correspond to the primary diagnosis associated with a claim, it may instead be a condition present with a relatively high reimbursement rate. Therefore, we used all available diagnosis information that may lead to identification of more diagnosis codes associated with recurrence. Thirdly, the current analysis includes patients with and without Part D prescription drug coverage, therefore, we can include the costs of patients using endocrine therapies and other newer pharmaceutical treatment options including CDK4/6 and PI3K inhibitors to treat BC recurrence. Finally, in the current analysis, we estimated the cumulative extra costs as costs of each patient with treated recurrence minus the baseline costs estimated from the costs of non/untreated patients. Whereas Stokes et al.Citation16 estimated cumulative extra costs as the cost difference of treatments received by all patients with treated recurrence and non/untreated recurrence patients.
Strengths and limitations
A meaningful health economic analysis should include all relevant costs in the cost-effectiveness evaluation to guide decisions on the allocation of healthcare resourcesCitation17. Unlike previous studies, which estimated cost of recurrence without outpatient pharmacy claimsCitation16,Citation17, this study factored the Medicare Part D costs for a more inclusive assessment of costs. In addition, subgroup analysis of patients with continuous Medicare Part D enrollment confirmed that the overall population had similar proportions of patients amongst treated recurrent and non/untreated recurrent patients and similar costs amongst other claim types. Additionally, this analysis adjusts cumulative costs by recurrent patient survival, which helps to adjust the fact that patients with BC recurrence are less likely to survive than those with non/untreated recurrence, and therefore more likely to have lower cumulative costs.
As with all retrospective studies that rely on administrative data, the current study has potential limitations. The SEER database only provides detailed data about the initial cancer diagnosis and initial course of therapy and does not explicitly capture events of recurrence and subsequent details on treatment, claims, and costs. Due to this inherent limitation of the SEER database, untreated recurrences are likely to be present, and the sample of patients with no evidence of recurrence in the data may include patients with untreated recurrence. Therefore, claims data should be interpreted with cautionCitation23,Citation25.
In addition, our study specifically quantifies OS and extra costs of treated recurrence in elderly patients with HR+, HER2−, node-positive, high-risk EBC and does not address the cost in patients with other biomarker subtypes, lower-risk EBC, or a younger age where EBC is commonly diagnosed. Another limitation is that we did not use endocrine therapies (ET) to identify recurrence, as it is challenging to distinguish ET treatment occurring over time periods between initial treatment and recurrence (i.e. whether patients are taking ET for adjuvant treatment or to treat recurrent disease). Therefore, we would potentially fail to identify recurrence in patients receiving only ET to treat recurrence. Another limitation relates to the present analysis exclusion of patients who had Medicare Part C (Medicare Advantage) at any time during the study period. Thus, the extra cost estimates only applied to the subset of Medicare patients in the traditional fee-for-service Medicare plans. Moreover, the proportion of Medicare patients opting for Part C was steadily increasing during the study period, which could have affected the likelihood of exclusion for patients depending on their date of recurrence. Finally, the diagnosis and procedure codes entered into the claims data are used mainly for billing and may not always be accurate.
Conclusion
The cost of treated recurrence in elderly patients (≥65 years) with HR+/ HER2−, node-positive EBC at high risk of recurrence was considerably higher in patients with distant recurrences. The cost of treated recurrence was high, particularly in the first few months after diagnosis, and slowly decreased over time. However, costs remain elevated for at least 5 years following recurrence. This trend is consistent for all recurrence types and for the Part D subpopulation. However, the Part D subgroup showed slightly higher costs compared to the overall population. In addition, most patients with identified treated recurrence in the overall population experienced distant recurrence with shorter OS. Findings from the current analysis may offer insight into the magnitude of medical-care costs attributable to costs of treated recurrence in EBC patients over 10 years and may be useful in estimating the economic benefits of prevention strategies.
Transparency
Author contributions
Conception of work: RO
Design of the work: ASV, PM, AJ, SZ, RO, and SZ
Acquisition of data: RO
Data Analysis: ASV, PM, AJ, SZ, RO, and SZ
Data interpretation: AML, ASV, PM, AJ, SZ, RO, and SZ
Manuscript writing and critical revision: AML, ASV, PM, AJ, SZ, RO and SZ
Final approval: ASV, PM, SZ, AJ, RO, SZ and AML
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Consent to participate
Due to the nature of the study, consent to participate from patients was not required.
Consent to publication
The manuscript does not contain any individual person’s data in any form. Therefore, consent for publication was not required.
Previous presentations
A portion of these results was presented at the 2022 San Antonio Breast Cancer Symposium: December 6–10, 2022.
Supplemental Material
Download MS Word (176.8 KB)Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors. The authors would like to acknowledge the contributions of Jacqueline Brown (former employee of Eli Lilly and Company) and Mark Boye (Eli Lilly and Company) for the study concept/design. The authors would also like to thank Vengal Rao Pachava (Eli Lilly Services India Pvt. Ltd) for providing medical writing and editorial support.
Declaration of financial/other interests
ASV and AML are employees and stockholders of Eli Lilly and Company. PM, SZ, and AJ are employees of Medical Decision Modeling Inc. and performs contracted work for Eli Lilly and Company. RO is a consultant for Medical Decision Modelling Inc. and performs contracted work for Eli Lilly and Company. SZ is an employee of Tech Data Services Company and performs contracted work for Eli Lilly and Company.
Data availability statement
The data that support the findings of this study are available from the SEER-Medicare linked database (https://healthcaredelivery.cancer.gov/seermedicare/), but restrictions apply to the availability of these data, which were used under permission for the current study, and so are not publicly available. Data are available from SEER-Medicare upon reasonable request and with permission.
Additional information
Funding
References
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–541. doi: 10.3322/caac.21754.
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055.
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323.
- Howlader N, Cronin KA, Kurian AW, et al. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(6):619–626. doi: 10.1158/1055-9965.EPI-17-0627.
- Sheffield KM, Peachey JR, Method M, et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol. 2022;18(21):2667–2682. doi: 10.2217/fon-2022-0310.
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717.
- Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352.
- Mamounas EP, Tang G, Paik S, et al. 21-Gene Recurrence Score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: results from NSABP B-28/NRG Oncology. Breast Cancer Res Treat. 2018;168(1):69–77. doi: 10.1007/s10549-017-4550-8.
- Nitz U, Gluz O, Christgen M, et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat. 2017;165(3):573–583. doi: 10.1007/s10549-017-4358-6.
- Yamauchi H, Toi M, Takayama S, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. Breast Cancer. 2021;30(4):606–2405. doi: 10.1007/s12282-023-01468-z.
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571–1581. doi: 10.1016/j.annonc.2021.10.015.
- A study of imlunestrant versus standard endocrine therapy in participants with early breast cancer (EMBER-4). Available from: https://clinicaltrials.gov/ct2/show/NCT05514054.
- A study evaluating the efficacy and safety of adjuvant giredestrant compared with physician’s choice of adjuvant endocrine monotherapy in participants with estrogen receptor-positive, HER2-negative early breast cancer (lidERA Breast Cancer). Available from: https://clinicaltrials.gov/ct2/show/NCT04961996.
- A study to evaluate the efficacy, safety, and pharmacokinetics of giredestrant plus palbociclib compared with anastrozole plus palbociclib for postmenopausal women with estrogen receptor-positive and HER2-negative untreated early breast cancer (coopERA Breast Cancer). Available from: https://clinicaltrials.gov/ct2/show/NCT04436744.
- A trial to evaluate efficacy and safety of ribociclib with endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer (NATALEE). Available from: https://clinicaltrials.gov/ct2/show/NCT03701334.
- Stokes ME, Thompson D, Montoya EL, et al. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-medicare data. Value Health. 2008;11(2):213–220. doi: 10.1111/j.1524-4733.2007.00226.x.
- Lamerato L, Havstad S, Gandhi S, et al. Economic burden associated with breast cancer recurrence: findings from a retrospective analysis of health system data. Cancer. 2006;106(9):1875–1882. doi: 10.1002/cncr.21824.
- Tisdale RL, Ma I, Vail D, et al. Availability of cost-effectiveness studies for drugs with high medicare part D expenditures. JAMA Netw Open. 2021;4(6):e2113969. doi: 10.1001/jamanetworkopen.2021.13969.
- Parts of Medicare. Available from: https://www.ssa.gov/medicare/plan/medicare-parts.
- National Cancer Institute. Overview of the SEER program. Available from: https://seer.cancer.gov/about/overview.html.
- SEER-Medicaid: brief description of the SEER-medicaid database. Available from: https://healthcaredelivery.cancer.gov/seermedicaid/overview.
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388.
- Warren JL, Mariotto A, Melbert D, et al. Sensitivity of medicare claims to identify cancer recurrence in elderly colorectal and breast cancer patients. Med Care. 2016;54(8):e47–e54. doi: 10.1097/MLR.0000000000000058.
- Hassett MJ, Ritzwoller DP, Taback N, et al. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med Care. 2014;52(10):e65–e73. doi: 10.1097/MLR.0b013e318277eb6f.
- Measures that are limited or not available in the data. Available from: https://healthcaredelivery.cancer.gov/seermedicare/considerations/measures.html#8.
- Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523.
- Lin DY, Feuer EJ, Etzioni R, et al. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53(2):419–434. doi: 10.2307/2533947.
- R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. Available from: https://www.R-project.org/.
- Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34(9):927–935. doi: 10.1200/JCO.2015.62.3504.
- Cheng L, Swartz MD, Zhao H, et al. Hazard of recurrence among women after primary breast cancer treatment-a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev. 2012;21(5):800–809. doi: 10.1158/1055-9965.EPI-11-1089.
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–3998. doi: 10.1200/JCO.20.02514.
- Sieluk J, Song Y, Freimark J, et al. The economic burden of recurrence in triple-negative breast cancer among working age patients in the United States. Adv Ther. 2022;39(2):943–958. doi: 10.1007/s12325-021-01913-5.
- Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol. 2017;9:267–277. doi: 10.2147/CLEP.S105613.