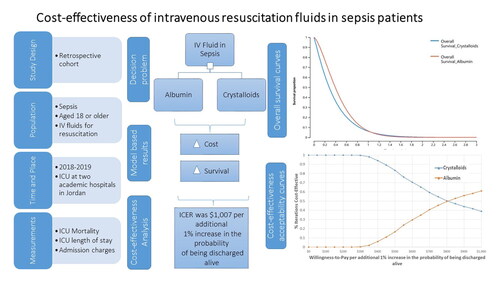
Abstract
Aim
Albumin role as fluid resuscitation in sepsis remains understudied in low- and middle-income countries. This study aimed to evaluate the cost-effectiveness of intravenous (IV) Albumin compared to Crystalloids in sepsis patients using patient-level data in Jordan.
Methods
This was a retrospective cohort study of sepsis patients aged 18 or older admitted to intensive care units (ICU) at two major tertiary hospitals during the period 2018–2019. Patients information, type of IV fluid, and clinical outcomes were retrieved from medical records, and charges were retrieved from the billing system. A 90-day partitioned survival model with two health states (alive and dead) was constructed to estimate the survival of sepsis patients receiving either Albumin or Crystalloids as IV fluids for resuscitation. Overall survival was predicted by fitting a Weibull model on the patient-level data from the current study. To further validate the results, and to support the assessment of uncertainty, time-dependent transition probabilities of death at each cycle were estimated and used to construct a state-transition patient-level simulation model with 10,000 microsimulation trials. Adopting the healthcare system perspective, incremental cost-effectiveness ratios(ICERs) of Albumin versus Crystalloids were calculated in terms of the probability to be discharged alive from the ICU. Uncertainty was explored using probabilistic sensitivity analysis.
Results
In the partitioned survival model, Albumin was associated with an incremental cost of $1,007 per incremental1% in the probability of being discharged alive from the ICU. In the state-transition patient-level simulation model, ICER was $1,268 per incremental 1% in the probability of being discharged alive. Probabilistic sensitivity analysis showed that Albumin was favored at thresholds >$800 per incremental 1%in the probability of being discharged alive from the ICU.
Conclusion
IV Albumin use in sepsis patients might not be cost-effective from the healthcare perspective of Jordan. This has important implications for policymakers to readdress Albumin prescribing practice in sepsis patients.
PLAIN LANGUAGE SUMMARY
Sepsis is a life-threatening complication of infection, which usually requires resuscitation with intravenous fluids. Still, no conclusive evidence is available about the best fluid resuscitation to be used in sepsis patients especially in low- and middle-income countries. This study compared the costs and effectiveness of intravenous Albumin versus Crystalloids in sepsis patients. Findings from this study showed that resuscitation with Albumin is much more expensive compared to resuscitation with Crystalloids with no significant difference in mortality but with prolonged length of stay in the hospital and the intensive care unit. Decision makers are advised to change Albumin prescribing practices in a way that mitigates the associated clinical and financial burdens without compromising quality of care or resuscitate with Crystalloids.
KEY POINTS
Albumin use is associated with significant prolongation of hospital and ICU lengths of stay, but no evidence indicates that Albumin is associated with better mortality rates.
Albumin is associated with remarkable medical expenditures in sepsis patients compared to those treated with Crystalloids.
Albumin is not cost-effective in sepsis patients treated in the ICU setting.
Introduction
Sepsis is a major global health threat and a leading cause of death and disability worldwide, with millions of cases occurring each yearCitation1. Sepsis is a medical emergency that frequently leads to single or multiple organ dysfunction as a result of dysregulated body response to infectionCitation2. The treatment of sepsis typically involves the use of antibiotics to control infection and the administration of intravenous (IV) fluids to support blood pressure and perfusion to vital organsCitation3.
Fluid resuscitation, through the administration of IV solutions, is a core part in the management of sepsis in the intensive care unit (ICU)Citation4. During the early stage of sepsis, about two-thirds of patients respond well to fluid therapyCitation5. Fluid administration should continue with continuous improvement in hemodynamic parameters like cardiac output and stroke volumeCitation6. IV solutions are classified into two categories depending on their specific electrolyte and molecular composition. Firstly, Crystalloids, such as normal saline and Ringer’s lactate, permeate freely through membranes given smaller electrolyte molecules. Secondly, colloids, like Albumin and hydroxyethyl starch, are solutions containing macromolecules that cannot cross freely through capillary membranesCitation7.
Surviving Sepsis Campaign 2021 guidelines recommend Crystalloids as the initial fluid for resuscitationCitation8. Noteworthy, Albumin is also used as a resuscitation fluid in sepsis due to its ability to maintain intravascular volume and support oncotic pressure. However, the use of Albumin as a resuscitation fluid in sepsis remains controversialCitation9. Indeed, results from published studies on the use of Albumin in sepsis patients showed notable differences among health systems, populations, and time framesCitation10–15. Economic evaluations conducted in developed countries showed that Albumin might have some economic plausibility compared to CrystalloidsCitation16–18. Populations from low- and middle-income countries (LMICs) are extremely underrepresented in the current literature: only one evaluation was conducted in Iran and showed that Albumin might not be the best option in this settingCitation19.
Jordan is a developing LMIC located in the Middle East region. The per capita health care expenditure was $299 in 2020 compared to $11,702 in the USA, $6,619 in Canada, $4,769 in France, and $5,901 in AustraliaCitation20. Evidence synthesized in relevant settings and using real patient data is crucial to guide policies that target efficient use of resources in LMICs. Considering the conflicting results related to Albumin use in sepsis, and the dearth of studies conducted in ICU settings and populations from LMICs such as Jordan, the present study was conducted to assess the cost-effectiveness of Albumin administration as an IV resuscitation fluid compared to IV Crystalloids in sepsis patients.
Method
Study design and population
This was a retrospective cohort study conducted at two leading academic hospitals in Jordan; King Abdullah University Hospital (KAUH) and Jordan University Hospital (JUH). KAUH is a tertiary referral center in Irbid that serves patients from the North of Jordan. The total number of ICU beds at KAUH is 120 distributed over 7 ICUs. JUH is a referral teaching hospital located in Amman, the capital of Jordan. It serves patients from various parts of the country and has 84 ICU beds distributed over 9 ICUs.
Medical records and pharmacy data of sepsis patients admitted to ICU units in KAUH and JUH from January 1, 2018, to December 31, 2019 were retrieved and reviewed to identify eligible patients and collect patients’ demographics and clinical outcomes. These two years were selected to avoid the impact of the COVID-19 pandemic, which had a profound impact on the healthcare system and on patients’ clinical variables especially in the ICU setting.
Patients who were diagnosed with sepsis during ICU admission and confirmed to receive either Albumin or Crystalloids as IV fluids for resuscitation were deemed eligible to be included in the study. Regarding sepsis diagnosis, adult patients who were admitted to the ICU were identified using the sepsis International Classification of Diseases (ICD)10 code (A41). Subsequently, the sepsis diagnosis was verified for all retrieved admissions using medical records. Lastly, the medical records for eligible patients diagnosed with sepsis thus identified were reviewed by research staff to identify all other comorbidities and diagnoses. Patients aged less than 18 years, pregnant females, and those with no documented data about IV fluid use inside the ICU were excluded. Eligible patients were classified according to the type of IV fluid administered during ICU admission into two groups: Albumin treated patients (with or without Crystalloids) and Crystalloids treated patients (did not receive Albumin during the whole admission). Both the pharmacy data and clinical notes for eligible admissions were carefully reviewed to verify the IV fluid used. Both groups were followed until discharge or death.
The billing systems at KAUH and JUH were used to retrieve all charges for the eligible admissions including both total and itemized charges. Per-day admission charge was calculated for each patient by dividing the total admission charge by the length of stay. Components of admission charges and their contribution to total admission charges were evaluated for both treatment groups. All charges were retrieved in Jordanian dinars (JOD) and converted to 2019 US dollars (USD; $) at a rate of $1.41 per 1 JODCitation21.
Cost-effectiveness analysis
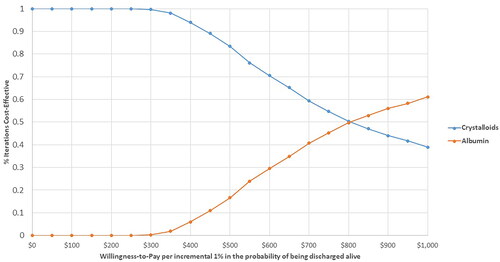
A 90-day partitioned survival model with two health states (alive and dead) was constructed to simulate the survival of a cohort of ICU adult patients diagnosed with sepsis who received either Albumin or Crystalloids as IV fluids for resuscitation. In the partitioned survival model, ICU sepsis patients were tracked for survival at daily intervals based on a Weibull survival model, which was fit on the study patient-level data and adjusted for Albumin level at admission, gender, Charlson Comorbidity Index (CCI), and Sequential Organ Failure Assessment (SOFA) score. The estimated scale (λ) and shape (γ) parameters of the Weibull distribution were used to estimate the survival function for both Albumin and crystalloid-treated patients. Costs and effectiveness were estimated from the healthcare system perspective and accumulated on a daily bases. Daily charges were estimated using admission charges and length of stays from the real patient data described above. The incremental cost-effectiveness ratio (ICER) of Albumin versus Crystalloids was calculated in terms of the cost per incremental 1% in the probability of being discharged alive from the ICUCitation22. To further validate the results of the partitioned survival model by applying time-dependent transition probabilities, and to support the assessment of uncertainty in the overall survival extrapolationCitation23, a state-transition patient-level simulation model was constructed, . Time-dependent transition probabilities were calculated over a 90-day time horizon (on daily-based cycles) using the estimated scale (λ) and shape (γ) parameters. In this model, patient survival was simulated in terms of transition to death status based on daily cycles (an ICU day) in a state transition simulation model with Markovian health states of death and alive, and 10,000 microsimulation trials. The incremental cost-effectiveness ratio (ICER) of Albumin versus Crystalloids was calculated in terms of the probability of being discharged alive from the ICU. Because a 90-day time horizon was considered, no discounting method was applied. A half-cycle correction was applied. Uncertainty was explored using probabilistic sensitivity analysis varying transition probabilities, survival probabilities, and per day admission charges for both treatment groups simultaneously over second order 1000 Monte-Carlo simulationsCitation22,Citation24. The cost-effectiveness acceptability curve was plotted to depict favored strategies over willingness to pay (WTP) values per incremental 1% in the probability of being discharged alive.
In order to validate our results in terms of hospital survival (rather than ICU survival), we conducted another partitioned survival analysis over a 6-months’ time horizon starting from ICU admission, in which the ICER was calculated in terms of the probability to be discharged alive from the hospital. Cost-effectiveness models were constructed using TreeAge Pro 2023 (TreeAge Software LLC, Williamstown MA).
Statistical analysis
Descriptive statistics such as arithmetic mean with standard deviation (SD), median with interquartile range (IQR), and frequency with percentage were applied to summarize patient characteristics and outcomes related to ICU admissions. Unadjusted comparisons between the two IV fluid groups were conducted using the independent samples t test, Mann Whitney test, or chi-square test, as appropriate. Multiple logistic regression was performed to assess potential predictors of 90-day mortality. Variables included in both logistic and survival models were selected using a backward stepwise process with a P value of 0.2 to stay. Data analyses were conducted using Stata version 17 software (StataCorp, 2021, “Stata: Release 17. Statistical Software,” College Station, TX: StataCorp LLC). The statistical significance level was set at 0.05 on both sides.
Ethics approval
This research was approved by the Institutional Review Board (IRB) committee at KAUH (Number: 17/141/2021) and the IRB committee at JUH (Ref number: 10/2021/25014).
Results
Patients’ demographic and clinical characteristics
During the study period, there were 250 eligible patients with sepsis during ICU admission, among whom, 29.20% were in surgical ICUs and the rest were in medical ICUs. Of eligible patients, 127 (50.8%) received Albumin and 123 (49.2%) received Crystalloids only. The average age of included patients was 72.38 (SD = 15.03) years, and 49.20% were males. ICU severity scores such as the average value of the Glasgow Coma Scale (GCS), SOFA, and Acute Physiology and Chronic Health Evaluation (APACHE II) (), and other clinical characteristics are described in . Details related to comorbid conditions and CCI are summarized in Supplemental Table S1.
Table 1. Characteristics of the study patients and admissions for the total sample and by IV fluid administered.
In terms of both hospital and ICU stays, the Albumin group had significantly longer stays compared to the Crystalloids group. Median stays (IQR) were 13 (7–22) vs. 6(3–12), and 10 (6–18) vs. 5(2–11) days for hospital and ICU stays, respectively (p values < .001). Patient clinical characteristics for the total sample and by IV fluid administered are summarized in Supplemental Table S2. After controlling for Albumin level at admission as well as for other potential confounders, there was no significant difference between IV fluid groups (Albumin and Crystalloids) in odds of mortality at 90 d (OR = 2.16, p value = .117). Detailed results are reported in Supplemental Table S3.
Average admission charges were $12,773.9 (SD= $17,070.9) and $4,404.4 (SD= $5,127.6) for Albumin treated patients and Crystalloids treated patients, respectively; p value < .001. The average per-day admission charge was also significantly higher for Albumin treated patients compared to Crystalloids treated patients ($631 (SD=$384) versus $530 (SD=$280), p value = .007). Components of admission charges and their contribution to total admission charges for both treatment groups are presented in Supplemental Figure S1.
Cost-effectiveness analysis
In the partitioned survival model, the estimated ICER for patients treated with Albumin compared to Crystalloids was $1,007 per incremental 1% in the probability of being discharged alive from the ICU, . Overall survival curves showed that patients treated with Albumin had a slightly longer survival during the first month of admission, however, this superiority faded after the first 30 days of admission, ending with almost identical survival curves, .
Figure 2. Overall survival curves for sepsis patients treated with Albumin and Crystalloids over 90-day horizon.
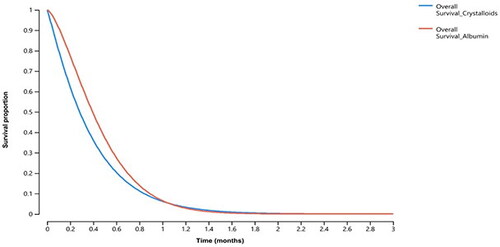
Table 2. The results of the cost-effectiveness analysis.
In the state-transition patient-level simulation model, base case ICER for patients treated with Albumin compared to Crystalloids was $1,268 per incremental 1% in the probability of being discharged alive from the ICU, .
Probabilistic sensitivity analysis showed that the probability of being cost-effective for IV Albumin was higher than the corresponding probability for IV Crystalloids over a range of cost-effectiveness thresholds larger than $800 per incremental 1% in the probability of being discharged alive, .
In the partitioned survival analysis conducted over 6 months’ time horizon, the calculated ICER was $1,991.25 per incremental 1% in the probability of being discharged alive from the hospital.
Discussion
The following are the principal findings of this retrospective cohort study conducted in the two major teaching hospitals in Jordan: First, Albumin use did not improve sepsis patients’ survival compared to Crystalloids over a 90-day time horizon. Second, Albumin use was associated with prolonged hospital and ICU lengths of stay. Third, IV Albumin use in sepsis patients is not cost-effective compared to Crystalloids.
The uncertain role of Albumin in sepsis was reported in previous literature. In a multicenter randomized clinical trial, Albumin did not improve the rate of survival at 28 and 90 dCitation10. Findings from a recent systematic review and meta-analysis showed that the in-hospital mortality for sepsis was not significantly different between patients receiving Albumin plus Crystalloid solution and those receiving Crystalloid solution aloneCitation25. In contrast, some studies showed a beneficial effect of Albumin in sepsisCitation26–28. The conflicting results might be related to the population studied and follow-up duration. The majority of these studies used data from developed countries, which might have different ICU settings and populations. Compared to Crystalloids-based resuscitation, Albumin-based resuscitation is very expensive across the world. A recent study conducted highlighted that normal saline (0.9%) had ∼27 times lower-cost compared to Albumin at equi-effective dosingCitation29.
No formal willingness to pay threshold has been established in Jordan, however, taking the cost equivalent of 3 times the gross domestic product (GDP) per capita ($4,204.5 in 2022Citation30) or $12,613.5, can be justified as an appropriate and relevant willingness to pay threshold. Further, considering also the average age in the current study (around 72 years old), the expected life expectancy of the Jordanian population (74 years oldCitation31) and the poor prognosis for older adults who survived sepsisCitation32, the estimated ICERs in both the base-case and sensitivity analyses in the current study markedly exceeded what might be perceived as cost-effective in Jordan.
Only a few studies have evaluated the cost-effectiveness of Albumin as fluid therapy in sepsis patients and a systematic review highlighted the dearth of evidence available to support current guidelines and practice regarding the use of IV resuscitation fluids particularly in sepsis patientsCitation33. Noteworthy, cognizant interpretation of the results of cost-effectiveness literature is of utmost importance as it implies the expert’s multidimensional judgment regarding cost and effectiveness outcomes used, as well as the population and setting studied. A study conducted in 2007 from a third-party payer perspective in France, showed that Albumin infusion might be cost-effective in severe sepsisCitation16. The favorable cost-effectiveness of Albumin compared to Crystalloids was also reported in a similar study conducted from the French National Health System viewpoint and was restricted to septic shock patientsCitation17. In one evaluation conducted from the US payer perspective, Albumin was associated with increased effectiveness (life years gained) compared to both hydroxyethyl starch and Crystalloid and decreased cost compared to hydroxyethyl starchCitation18. With the dearth of data published from developing countries, we found only one published study conducted in Iran, that was designed to evaluate the cost-effectiveness of using Albumin particularly in sepsis patientsCitation19. Tigabu et al. found in a retrospective cohort study that the addition of Albumin in the fluid therapy of patients with septic shock increased the total cost of treatment by $3,846.07 and had no significant increase in life-year gain from the healthcare payers’ perspectiveCitation19. The estimated ICER was $5,740.4 per life year gained, and accordingly Albumin was not cost-effective considering the GDP per capita of IranCitation19. A study conducted in Jordan found that Albumin use was not associated with significant improvement in clinical outcomes in ICU patients but with a substantial increase in admission chargesCitation34. However, no formal cost-effectiveness evaluation was conducted, and the study was not restricted to sepsis patients.
International variation in critical care services, practices, resources and staffing makes the delivery of critical care unique in each country and may affect outcomes markedly between countriesCitation35–37. Furthermore, legal and cultural issues can have a profound impact on practices and outcomes in different countries. For example, the “Do-not-resuscitate (DNR)” order is not allowed nor practiced in Jordan, which may impact ICU length of stay. Identifying the system and practice is important to identify problems and design policies to maximize the quality of care while minimizing costs.
Findings from the current study are important to inform decision makers about the importance of reevaluating policies for prescribing Albumin in the ICU for sepsis patients. The importance of enforcing restrictions to promote the judicious use of Albumin in ICU settings was repeatedly highlighted in previous literatureCitation38–40. Continuous medical education on clinical decision-making regarding Albumin administration in sepsis patients was shown to save an average of $593,890.77 per yearCitation41.
This study was multicenter and representative of the sector of teaching hospitals on the national level in Jordan. Actually, the current study was conducted with both demographics and settings related particularly to the Middle East region, which is underrepresented in the literature. However, some limitations should be acknowledged. Despite the use of a survival model adjusted for potential confounders and having the benefit of using real life patient data, these data were retrospective and may have induced selection bias. The Albumin treated group in the underlying retrospective cohort study received 20% Albumin strength only as this was the only concentration used in practice in both hospitals. Accordingly, the results may not be generalizable to other Albumin concentrations. Finally, the charges used in this study were expected to be higher than actual costs since charges include profits and are commonly used in negotiations with insurersCitation42. Further, charges were used consistently for both groups, hence this is not expected to bias the overall conclusions of the study. Charges are used widely in the literature as a metric of costs. Being readily available compared to actual cost data which are proprietary to the providers, they are considered a feasible alternative, provided that a clear cost-definition is declared explicitly in the economic evaluationCitation43.
Conclusion
In conclusion, Albumin use as an IV fluid in sepsis patients costs slightly over $1000 for an incremental 1% in the probability of being discharged alive from the ICU compared to Crystalloids. This finding suggests that Albumin might not be cost-effective from the healthcare perspective of LMICs such as Jordan. The extensive use of Albumin in the ICU setting without robust evidence to support is concerning. Policies to restrict using Albumin are warranted particularly in countries with limited resources such as most of middle east countries including Jordan.
Transparency
Author contributions
SA, EA: Conceptualization, Methodology, Data curation, Formal analysis, Supervision, Project administration, Funding acquisition, Writing- Original draft preparation, Writing- Reviewing and Editing. SM, RK: Conceptualization, Methodology, Writing- Original draft preparation, and Writing- Reviewing and Editing. MS, IA: Methodology, Writing- Original draft preparation, and Writing- Reviewing and Editing. KAH: Data curation, Writing- Original draft preparation, and Writing- Reviewing and Editing.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics approval
Ethical approval was obtained from the Institutional Review Board (IRB) Committee at KAUH (Number: 17/141/2021) as well as the Institutional Review Board (IRB) Committee at JUH (Number: 10/2021/25014).
Supplemental Material
Download MS Word (95 KB)Acknowledgements
None stated.
Declaration of funding
This work was supported by the Deanship of Scientific Research at Jordan University of Science and Technology [grant numbers 20210314, 2021]. The funding agency was not involved in the study design, conduct, writing or decision to submit the article for publication.
Declaration of financial/other relationships
The Authors declare that they have no conflicts of interest to disclose.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC.
- Napolitano LM. Sepsis 2018: definitions and guideline changes. Surg Infect. 2018;19(2):117–125. doi: 10.1089/sur.2017.278.
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):775–787. doi: 10.1001/jama.2016.0289.
- Dellinger RP, Carlet JM, Masur H, et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–873. doi: 10.1097/01.ccm.0000117317.18092.e4.
- Leisman DE, Doerfler ME, Schneider SM, et al. Predictors, prevalence, and outcomes of early crystalloid responsiveness among initially hypotensive patients with sepsis and septic shock. Crit Care Med. 2018;46(2):189–198. doi: 10.1097/CCM.0000000000002834.
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of intensive care medicine. Intensive Care Med. 2014;40(12):1795–1815. doi: 10.1007/s00134-014-3525-z.
- Casey JD, Brown RM, Semler MW. Resuscitation fluids. Curr Opin Crit Care. 2018;24(6):512–518. doi: 10.1097/MCC.0000000000000551.
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337.
- Melia D, Post B. Human albumin solutions in intensive care: a review. J Intensive Care Soc. 2021;22(3):248–254. doi: 10.1177/1751143720961245.
- Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–1421. doi: 10.1056/NEJMoa1305727.
- Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256.
- Finfer S, McEvoy S, Bellomo R, et al. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37(1):86–96.
- Patel A, Laffan MA, Waheed U, et al. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ. 2014;349:g4561. doi: 10.1136/bmj.g4561.
- Winters ME, Sherwin R, Vilke GM, et al. What is the preferred resuscitation fluid for patients with severe sepsis and septic shock? J Emerg Med. 2017;53(6):928–939. doi: 10.1016/j.jemermed.2017.08.093.
- Zou Y, Ma K, Xiong JB, et al. Comparison of the effects of albumin and crystalloid on mortality among patients with septic shock: systematic review with meta-analysis and trial sequential analysis. Sao Paulo Med J. 2018;136(5):421–432. doi: 10.1590/1516-3180.2017.0285281017.
- Guidet B, Mosqueda GJ, Priol G, et al. The COASST study: cost-effectiveness of albumin in severe sepsis and septic shock. J Crit Care. 2007;22(3):197–203. doi: 10.1016/j.jcrc.2006.11.005.
- Guidet B, Ghout I, Ropers J, et al. Economic model of albumin infusion in septic shock: the EMAISS study. Acta Anaesthesiol Scand. 2020;64(6):781–788. doi: 10.1111/aas.13559.
- Farrugia A, Bansal M, Balboni S, et al. Choice of fluids in severe septic patients – A cost-effectiveness analysis informed by recent clinical trials. Rev Recent Clin Trials. 2014;9(1):21–30. doi: 10.2174/1574887108666131213120816.
- Tigabu BM, Davari M, Kebriaeezadeh A, et al. A cost-effectiveness analysis of albumin in septic shock: a patient-level data analysis. Clin Ther. 2019;41(11):2297–2307.e2. doi: 10.1016/j.clinthera.2019.08.023.
- Global health expenditure database. 2023 [cited 2023 Oct 4]. http://apps.who.int/nha/database/
- XE. 1 USD to JOD – Convert US Dollars to Jordanian Dinars. 2023. https://www.xe.com/.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
- Woods BS, Sideris E, Palmer S, et al. Partitioned survival and state transition models for healthcare decision making in oncology: where are we now? Value Health. 2020;23(12):1613–1621. doi: 10.1016/j.jval.2020.08.2094.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. United Kingdom: Oxford University Press; 2006.
- Liu P, Zhi D, Wang Y, et al. Effects of albumin supplements on in-hospital mortality in patients with sepsis or septic shock: a systemic review and meta-analysis. Evid Based Complement Alternat Med. 2022;2022:2384730. doi: 10.1155/2022/2384730.
- Xu JY, Chen QH, Xie JF, et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: a meta-analysis of randomized clinical trials. Crit Care. 2014;18(6):702. doi: 10.1186/s13054-014-0702-y.
- Ge C, Peng Q, Chen W, et al. Association between albumin infusion and outcomes in patients with acute kidney injury and septic shock. Sci Rep. 2021;11(1):24083. doi: 10.1038/s41598-021-03122-0.
- Zhou S, Zeng Z, Wei H, et al. Early combination of albumin with crystalloids administration might be beneficial for the survival of septic patients: a retrospective analysis from MIMIC-IV database. Ann Intensive Care. 2021;11(1):42. doi: 10.1186/s13613-021-00830-8.
- Taylor C, Yang L, Finfer S, et al. An international comparison of the cost of fluid resuscitation therapies. Aust Crit Care. 2021;34(1):23–32. doi: 10.1016/j.aucc.2020.06.001.
- World Bank WDI. GDP per capita (current US$) – Jordan. 2022 [cited 2023 Oct 12]. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=JO.
- World Bank WDI. Life expectancy at birth, total (years) – Jordan 2021 [cited 2023 Oct 12]. https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=JO.
- Dong J, Chen R, Song X, et al. Quality of life and mortality in older adults with sepsis after one-year follow up: a prospective cohort study demonstrating the significant impact of frailty. Heart Lung. 2023;60:74–80. doi: 10.1016/j.hrtlng.2023.03.002.
- Higgins AM, Brooker JE, Mackie M, et al. Health economic evaluations of sepsis interventions in critically ill adult patients: a systematic review. J Intensive Care. 2020;8(1):5. doi: 10.1186/s40560-019-0412-2.
- Altawalbeh SM, Almestarihi EM, Khasawneh RA, et al. Clinical and economic outcomes associated with intravenous albumin fluid use in the intensive care unit: a retrospective cohort study. Expert Rev Pharmacoecon Outcomes Res. 2023;23(7):789–796. doi: 10.1080/14737167.2023.2215431.
- Murthy S, Wunsch H. Clinical review: international comparisons in critical care – lessons learned. Crit Care. 2012;16(2):218. doi: 10.1186/cc11140.
- Khanna AK, Labeau SO, McCartney K, et al. International variation in length of stay in intensive care units and the impact of patient-to-nurse ratios. Intensive Crit Care Nurs. 2022;72:103265. doi: 10.1016/j.iccn.2022.103265.
- Wunsch H, Angus DC, Harrison DA, et al. Variation in critical care services across North america and Western Europe. Crit Care Med. 2008;36(10):2787–2793, e1-9. doi: 10.1097/CCM.0b013e318186aec8.
- Fink RJ, Young A, Yanez ND, et al. Cohort study of albumin versus lactated ringer’s for postoperative cardiac surgery fluid resuscitation in the intensive care unit. Pharmacotherapy. 2018;38(12):1241–1249. doi: 10.1002/phar.2195.
- Torbic H, Bauer SR, Militello M, et al. Evaluation of albumin 25% use in critically ill patients at a tertiary care medical center. Hosp Pharm. 2020;55(2):90–95. doi: 10.1177/0018578718823727.
- Udeh CI, Wanek M, Udeh BL, et al. Application of unit-level cost transparency, education, enhanced audit, and feedback of anonymized peer ranking to promote judicious use of 25% albumin in critical care units. Hosp Pharm. 2020;55(3):154–162. doi: 10.1177/0018578719828341.
- Vukovic M, Gvozdenovic BS, Rankovic M, et al. Can didactic continuing education improve clinical decision making and reduce cost of quality? Evidence from a case study. J Contin Educ Health Prof. 2015;35(2):109–118.
- Arora V, Moriates C, Shah N. The challenge of understanding health care costs and charges. AMA J Ethics. 2015;17(11):1046–1052.
- Tunis SL. A cost-effectiveness analysis to illustrate the impact of cost definitions on results, interpretations and comparability of pharmacoeconomic studies in the US. Pharmacoeconomics. 2009;27(9):735–744. doi: 10.2165/10899600-000000000-00000.