Abstract
Background
Limited real-world evidence exists on the economic burden of adverse events (AEs) to the healthcare system among patients with non-metastatic castration-resistant prostate cancer (nmCRPC) treated with second-generation androgen receptor antagonists (ARAs). Current data is needed to understand real-world clinical event rates among ARAs and the cost of these events.
Objectives
Describe the incidence of non-central nervous system (CNS)-related AEs and CNS-related AEs among nmCRPC patients treated in the United States with second-generation ARAs (apalutamide and enzalutamide) and evaluate healthcare resource utilization (HCRU) and costs for these patients.
Methods and study design
This was a retrospective observational cohort study using claims data from Optum Clinformatics Data Mart to identify adult males with prostate cancer, castration, no metastases, and >1 claim for apalutamide or enzalutamide. The study was conducted from January 2017 to March 2020, with a patient index identification period from January 2018 to December 2019. AEs were classified as CNS-related or non-CNS-related.
Results
Of 605 patients (156 apalutamide and 449 enzalutamide), most were ≥65 years (94%) and had ≥1 non-CNS-related AE (55%). Many had ≥1 CNS-related AE (32%). Pain (12%) and arthralgia (11%) were the most frequently reported non-CNS-related AEs. Fatigue/asthenia (14%) and dizziness (7%) were the most frequently reported CNS-related AEs. Among patients with versus without non-CNS-related AEs, 34% versus 8% had emergency room (ER) events, and 25% versus 2% had inpatient events. Among patients with versus without CNS-related AEs, 41% versus 14% had ER events, and 38% versus 4% had inpatient events. Adjusted per-patient per-year cost (in 2020 USD) differences were significant between patients with and without non-CNS-related AEs ($30,765, p = 0.0018) and between patients with and without CNS-related AEs ($40,689, p = 0.0017).
Conclusion
There is significant HCRU and cost burden among nmCRPC patients treated with ARAs developing AEs, highlighting the need for treatments with improved tolerability. Additional studies are warranted to include recently approved agents.
Introduction
Prostate cancer (PC) affects 1 in 8 men during their lifetime, making it the most common type of cancer, other than skin cancer, in men in the United States (US)Citation1. There were 268,490 new diagnoses in 2022, an increase from 248,530 in 2021Citation2,Citation3. Within 5 years of diagnosis, 10%–20% of patients with localized PC develop castration-resistant prostate cancer (CRPC) (non-metastatic or metastatic) – a biological or radiological progression occurring while receiving androgen deprivation therapy (ADT) and despite castrate levels of testosterone (<50 ng/dL following surgical or medical castration)Citation4,Citation5. Non-metastatic castration-resistant prostate cancer (nmCRPC) is the progression of PC despite ADT treatment, with no radiographic evidence of metastatic disease. Without treatment, patients with nmCRPC have a median overall survival (OS) of 4 years, with median bone metastasis-free survival between 25 and 30 monthsCitation6–8.
Clinical practice guidelines recommend treating patients with nmCRPC and a prostate-specific antigen doubling time of ≤10 months who are at increased risk of disease progression with second-generation androgen receptor antagonists (ARAs) in addition to continuing ADT to delay metastasesCitation9–10. Three approved second-generation ARAs, apalutamide, enzalutamide, and darolutamide, significantly delayed metastases and prolonged OS in clinical trialsCitation11–15. However, apalutamide and enzalutamide, presumably due to their ability to easily cross the blood–brain barrier, had an increased risk of central nervous system (CNS)-related events and other adverse events (AEs) in phase 3 studiesCitation11–14. A real-world retrospective analysis found that nmCRPC patients, treated second-line with enzalutamide, bicalutamide, or abiraterone for ≥1 year, with non-CNS-related AEs or CNS-related AEs experienced more annual inpatient, emergency room (ER), and outpatient visits than those without non-CNS-related AEs or CNS-related AEsCitation16. A matching-adjusted indirect comparison showed that darolutamide, apalutamide, and enzalutamide exhibited similar efficacy regarding metastasis-free survival and OS, though darolutamide demonstrated a lower AE riskCitation17,Citation18. We hypothesize that treatment with apalutamide and enzalutamide may cause undesirable CNS-related AEs and increased healthcare resource utilization (HCRU) and costs from AEs due to their high permeability across the blood-brain barrier.
There is limited real-world evidence on the economic burden of AEs experienced by nmCRPC patients treated with second-generation ARAs. This study sought to understand the burden of AEs and associated costs among nmCRPC patients treated with second-generation ARAs (apalutamide and enzalutamide). Darolutamide was not included since there were limited data, as it was introduced on the market in late July 2019 near the end of the study period. The primary objective was to describe the incidence of a diagnosis of non-CNS-related AEs and CNS-related AEs among nmCRPC patients treated with second-generation ARAs. The secondary objectives were to evaluate HCRU and direct medical costs for nmCRPC patients with and without non-CNS-related AEs and CNS-related AEs.
Methods
Study design and patient population
This retrospective, observational cohort study utilized Optum Clinformatics Data Mart, a large, single-payer claims database of administrative healthcare claims for members of a national managed care company affiliated with Optum. The database is composed of commercial health plan data and Medicare Advantage members. It includes administrative medical claims, pharmacy claims, and demographic characteristics of approximately 15 to 18 million annual covered lives across 50 US states for a total of 57 million unique covered lives from January 2007 to December 2017.
The study time frame was between January 1, 2017, and March 30, 2020. The patient index identification period was January 1, 2018 to December 31, 2019, to allow for at least a 3-month follow-up period. The index date was defined as the first claim for nmCRPC ARA treatment (apalutamide or enzalutamide). The baseline period was defined as the 12-month period prior to the index date and was used to identify the presence of a nmCRPC diagnosis and castration events and to assess patient demographics. The treatment time consisted of the index date when patients initiated treatment with apalutamide or enzalutamide, after meeting the nmCRPC inclusion criteria until metastatic diagnosis, discontinuation of ARA treatment, health plan disenrollment, death, or end of the study period, whichever occurred first. Discontinuation of ARA treatment was defined as any gap of ≥60 days from the end of a prescription for the index ARA, accounting for days supplied for the last fill. A minimum 3-month follow-up period was required, except for patients who died during this time to prevent survivor bias. The incidence of AEs (non-CNS-related AEs and CNS-related AEs) was evaluated during the treatment time period. New AEs were defined as those occurring in the treatment period, but not in the pre-index period, to avoid counting pre-existing AEs as AEs associated with these drugs.
Patients were included if they were aged ≥18 years and had a diagnosis of PC, evidence of surgical or pharmacological castration (or claim with an International Classification of Diseases, 10th Revision diagnosis of hormone-resistant malignancy), no evidence of metastases prior to initiation of second-generation ARA treatment, and ≥1 pharmacy claim for ARA (defined as the index date). Patients were excluded if they had a diagnosis of other non-PC/CRPC primary cancers during the 12-month baseline period or follow-up period, including the index date. See inclusion criteria in Supplemental Table 1.
Study variables and outcomes
The baseline variables included demographics (e.g. age, race, US region, health plan type) and clinical characteristics (e.g. comorbidities, type of castration procedure). The baseline variables were recorded on the index date or during the 12-month baseline period prior to index and were used in the multivariable analysis. Primary outcomes were new incidences of non-CNS-related AEs and CNS-related AEs, and the following measures were captured: mean time from index date to first occurrence of AE, predictors for AEs, and per-patient per-year (PPPY) AE count estimates. Patients were classified as having either a non-CNS-related AE or a new CNS-related AE. Patients were classified as having non-CNS-related AE if there was a new claim between the index date and the end of the treatment period with a diagnosis code for any of the following conditions (Supplemental File 1): arthralgia/joint pain, cardiovascular events, diarrhoea, oedema (peripheral), fractures, haematuria, hypertension, nausea, skin rash, upper respiratory infection, or urinary retention. These AEs were selected based on the common AEs reported in the interventional arms from the pivotal phase 3 trials leading to the approval of apalutamide (SPARTAN) and enzalutamide (PROSPER) for nmCRPCCitation11,Citation13. Patients were classified as having a CNS-related AE if there was a new claim between the index date and the end of treatment period, with a diagnosis code for any of the following conditions: amnesia/memory impairment, anxiety, ataxia, cognitive disorders, convulsions, dizziness, falls, fatigue/asthenia, hallucinations, headaches, insomnia, pain, paresthesia, seizures, weakness, or other CNS disorders. These CNS-related AEs were selected based on clinical events observed in patients taking enzalutamide per a large observational studyCitation19. AEs were selected in consultation of clinical experts. Secondary outcomes included HCRU (inpatient stays, length of stay [LOS] for each inpatient hospital admission [discharge date – admit date + 1], outpatient visits, ER visits, and prescriptions), direct medical costs assessed between those with and without non-CNS-related AEs and those with and without CNS-related AEs, and acute (30-day) costs. Due to the variable follow-up, HCRU and costs were reported as PPPY. Total healthcare costs (in 2020 US dollars) were calculated as the sum total of medical and pharmacy costs (costs were adjusted to the Consumer Price Index at Q2 2020 and from the perspective of a commercial payer)Citation20. Costs reflect all payments made to providers of care from both the plan (plan and coordination of benefits) and the patient out of pocket (copayment, coinsurance, deductible). Acute (30-day) costs for patients with clinical events were evaluated for 30 days following the occurrence of a clinical event. For patients without a clinical event, acute (30-day) costs were evaluated for 30 days following a randomly generated date that served as a proxy for the occurrence of a clinical event.
Statistical analysis
Descriptive statistics were used to summarize patient demographics, clinical characteristics, unadjusted measures of HCRU, direct healthcare costs, acute 30-day costs, and treatment discontinuation for the overall patient sample and for patients who experienced an AE during the follow-up period vs those who did not (≥1 non-CNS-related AE vs no AEs and ≥1 CNS-related AE vs no CNS-related AEs). Standard summary statistics for continuous variables were presented as mean (standard deviation [SD]), median, and interquartile range values and compared between groups using t-tests or Mann–Whitney tests; categorical variables were presented as number and percentage and compared between groups using chi-square tests or Fisher’s exact tests.
Inverse probability weights (IPWs) were calculated using separate multivariable logistic regression models to estimate the probability of experiencing non-CNS-related AE and a CNS-related AE and, during follow-up, conditional on patients’ covariates and index ARA therapy. The estimated probabilities were used to compute separate IPWs for each patient. To test whether weighting resulted in the balance of baseline covariates, the covariates were compared between patients with ≥1 non-CNS-related AE versus no AE and patients with ≥1 CNS-related AE versus no CNS-related AE using standardized differences. Variables for which the standardized difference exceeded 0.10 were included as covariates in weighted models to account for potential residual confounding. Mean HCRU and costs PPPY among patients with and without AEs were estimated using IPW multivariable generalized linear models (GLMs) that were adjusted for baseline patient covariates found to be imbalanced between groups after weighting. Overall, the IPW GLM was used to estimate and compare the mean PPPY HCRU (hospitalizations, ER visits, and outpatient visits) and total direct healthcare costs for patients with AEs vs those with no AEs. All counts of AEs were counted for healthcare visits. Mean PPPY costs were calculated for the entire duration of the follow-up for all patients divided by the total patient years.
Results
Baseline demographics
A total of 605 patients (apalutamide, n = 156; enzalutamide, n = 449) were included in the study. Most patients were ≥65 years (94%), were White (56%), and had Medicare insurance (86%). A plurality resided in the South (47%). Almost all patients were medically castrated (99%), and the mean Charlson Comorbidity Index (CCI) score was 2.28. More than half of patients (55%, n = 332) had non-CNS-related AEs and nearly one-third of patients (32%, n = 191) had CNS-related AEs. Among patients who received apalutamide or enzalutamide, more patients experienced non-CNS-related AEs than CNS-related AEs within each treatment group (apalutamide, 56% vs 34%; enzalutamide, 54% vs 31%, respectively). The most common pre-index comorbidities in the overall population were hypertension (80%), diabetes (38%), and peripheral vascular disease (24%).
The mean age of patients with non-CNS-related AEs (n = 332) and without non-CNS-related AEs (n = 273) was 77.5 and 76.7, respectively, with a mean CCI score in each respective subgroup of 2.48 and 2.05. The mean age of patients with CNS-related AEs (n = 191) and without CNS-related AEs (n = 414) was 78.4 and 76.6, respectively, with a mean CCI in each respective subgroup of 2.65 and 2.11. Patients’ baseline demographics are presented in Supplemental Table 2.
Median time from index to first occurrence of non-CNS-related AE
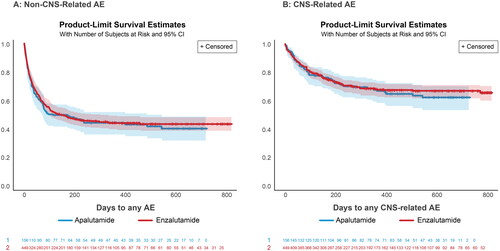
Median time from treatment initiation to non-CNS-related AE was estimated to be similar between both treatment groups (116 days for apalutamide, 117 days for enzalutamide) (). Median time from treatment initiation to CNS-related AE was also estimated to be similar in both groups (337 days for apalutamide, 250 days for enzalutamide) (). Patients most frequently reported AEs of pain (12%), arthralgia/joint pain (11%), and fractures (7%). The most commonly reported CNS-related AEs were fatigue/asthenia (14%), dizziness (7%), and weakness (6%) (Supplemental Table 3).
PPPY AE count estimates
The mean PPPY AE count estimates for non-CNS-related AE and CNS-related AEs were similar for both apalutamide and enzalutamide groups as shown in . Overall, the mean (SD) and median PPPY visits wherein an AE was coded were 19.73 (64.17) and 3.04, respectively, for non-CNS-related AE and 5.48 (33.03) and 0.00, respectively, for CNS-related AEs. The mean (SD) PPPY AE count estimates for AEs (e.g. falls, fatigue, headache, cardiovascular events, and hypertension) were similar across both treatment groups. Although there was a greater count estimate of fatigue and hypertension in the apalutamide group versus the enzalutamide group, the enzalutamide group had a greater count estimate of falls.
Table 1. PPPY AE count estimates.
Predictors for AEs
Having a high CCI score was a significant predictor of having non-CNS-related AEs (hazard ratio [HR]: 95% confidence interval [CI]: 1.56 [1.08, 2.26]), while being aged >75 years was a significant predictor of having CNS-related AEs (HR: 95% CI: 1.48 [1.06, 2.09]). Having baseline AEs was not a significant predictor for having new AEs or CNS-related AEs.
HCRU and direct medical costs
Among patients with non-CNS-related AEs versus those without non-CNS-related AEs, 34% vs 8% had an ER event, 100% versus 98% had an outpatient event, and 25% versus 2% had an inpatient event, with a mean LOS of 13.2 days versus 2.8 days among hospitalized patients. Among patients with CNS-related AEs versus those without CNS-related AEs, 41% versus 14% had an ER event, 100% versus 99% had an outpatient event, and 38% versus 4% had an inpatient event, with a mean LOS of 14.1 days versus 6.3 days among hospitalized patients. The HCRU of non-CNS-related AEs and CNS-related AEs is summarized in .
Table 2. HCRU among patients with and without non-CNS-related AEs and CNS-related AEs.
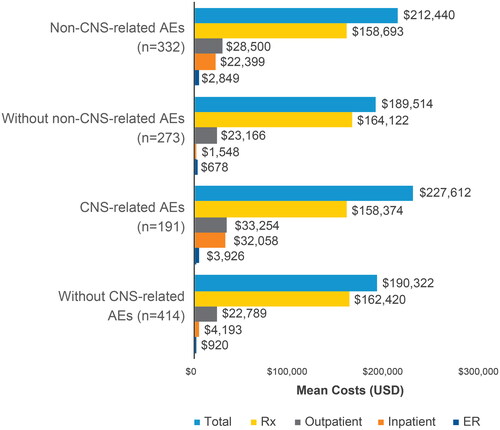
Patients with non-CNS-related AEs had increased total direct medical costs (unadjusted PPPY) of $212,440 (SD: $132,303) compared to patients without non-CNS-related AEs ($189,514 [SD: $78,836]). Patients with CNS-related AEs had increased costs compared to patients without CNS-related AEs ($227,612 [SD: $149,250] vs $190,322 [$87,320], respectively). Across the four subgroups of patients with and without non-CNS-related AEs or CNS-related AEs, prescription costs were the highest contributor to total costs followed by costs due to outpatient, inpatient, and ER visits (). AEs were not associated with differences in prescription costs, which were similar between patients with and without non-CNS-related AEs ($158,693 vs $164,122, respectively) and between patients with and without CNS-related AEs ($158,374 vs $162,420, respectively) (). The adjusted PPPY total cost difference (using IPW GLM) between patients with and without non-CNS-related AEs was $30,765 (p = 0.0018) (Supplemental Table). The adjusted PPPY total cost difference between patients with and without CNS-related AEs was $40,689 (p = 0.0017). Higher total costs in patients with AEs or CNS-related AEs, when compared to patients without AEs or CNS-related AEs, could mainly be attributed to PPPY inpatient costs. The unadjusted mean PPPY inpatient cost was $20,851 higher for patients with AEs than without AEs and $27,865 higher for patients with CNS-related AEs than without CNS-related AEs.
Figure 2. CPI-adjusted PPPY mean costs for non-CNS-related AEs and CNS-related AEs. Abbreviations. AE, adverse event; CNS, central nervous system; CPI, Consumer Price Index; ER, emergency room; PPPY, per patient per year; Rx, prescription; USD, United States dollars.
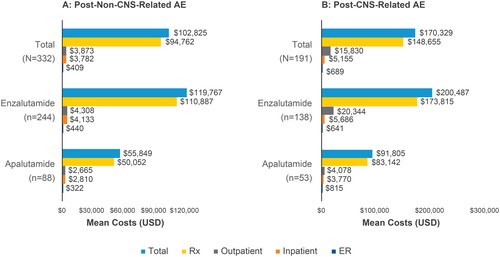
The total mean acute 30-day cost was greater in patients following CNS-related AEs than in patients following non-CNS-related AEs ($170,329 vs $102,825, respectively) (). Out of these total costs, prescription costs incurred the greatest cost at 30 days following non-CNS-related AEs ($94,762) and CNS-related AEs ($148,655). The total mean acute 30-day cost was higher in the enzalutamide group than the apalutamide group following non-CNS-related AEs ($119,767 vs $55,849, respectively) and in the group with CNS-related AEs ($200,487 vs $91,805, respectively). Treatment with enzalutamide also incurred higher prescription claims costs compared to apalutamide following non-CNS-related AEs ($110,887 vs $50,052, respectively) and following any CNS-related AEs ($173,815 vs $83,142, respectively) ().
Discussion
The potential for an AE is an important consideration when selecting therapies for any disease, especially nmCRPC, wherein men are often older and have pre-existing comorbidities. Furthermore, management of these AEs and the added HCRU and costs will significantly increase payer burden. This study found that non-CNS-related AEs and CNS-related AEs were common among nmCRPC patients treated with apalutamide and enzalutamide, where patients most frequently experienced pain, arthralgia, haematuria, fatigue, dizziness, and weakness. Findings from our study also suggest that patients treated with these agents who developed non-CNS-related AEs or CNS-related AEs contribute to a significantly higher HCRU and costs compared to those who did not develop non-CNS-related AEs or CNS-related AEs. Among patients who did not experience a non-CNS-related or CNS-related AE, prescription costs were higher, likely due to the longer duration of therapy of the index drug.
This study confirms the findings of a previous real-world retrospective analysis of commercial MarketScan data from 2012 to 2017 among patients with nmCRPC who initiated second-line treatment with enzalutamide, bicalutamide, or abiraterone for ≥1 year, which found that non-CNS-related AEs and CNS-related AEs were associated with increased HCRUCitation16. Patients with non-CNS-related AEs and CNS-related AEs experienced more inpatient, ER, and outpatient visits on an average annual basis than those without non-CNS-related AEs and those without any CNS-related AEsCitation16. Shah et al. estimated $18,500 more in annual total medical costs for patients with CNS-related AEs compared to patients without CNS-related AEs (p < 0.05)Citation16. This is less than our finding of $40,689 in increased annual cost for patients with CNS-related AEs compared to patients without CNS-related AEs (p < 0.05); the difference may be attributed to the study timeframes, medications evaluated, and population criteria. In the Shah study, the incidence of non-CNS-related AEs and CNS-related AEs also increased treatment discontinuation rates by 48% and 38%, respectivelyCitation16. In another retrospective analysis that included patients with metastatic PC, the incidence of CNS-related AEs 12 months after treatment with enzalutamide, bicalutamide, or abiraterone was >30%Citation19. While Pilon et al. did not evaluate HCRU and costs in nmCRPC patientsCitation19, the high incidence rates of AEs associated with enzalutamide and abiraterone were consistent with our findings. Although Shah et al. evaluated the impact in patients who initiated second-line treatment, only one other study assessed the incidence and management of AEs in nmCRPC patients receiving apalutamide and enzalutamideCitation16,Citation21. Hussain et al. found that among nmCRPC patients with second-generation ARAs who experienced AEs, 10.4% discontinued treatment and 4.8% required hospitalizations; the results were consistent with our findingsCitation21.
Limitations
Limitations include the lack of clinical information in administrative claims, resulting in the definition of nmCRPC being solely based on diagnosis, procedure, and drug codes. Some clinical events may not have been captured since they do not generate medical claims; however, from the perspective of a commercial payer, this may not have a substantial impact on the utility of the results as many of the non-serious AEs would likely be treated with over-the-counter medications and thereby not contribute to cost to a payer. There was also a lack of generalizability to the entire nmCRPC patient population since the database only included individuals with commercial insurance or Medicare Advantage. Darolutamide was not included due to its approval in late July 2019, near the end of the study period, resulting in insufficient data to conduct an analysis. Given the relatively recent approval of apalutamide in nmCRPC, the sample size was small compared to enzalutamide (156 vs 449, respectively). Given the limitations of the study data source, AE occurrence could not be attributed to a specific treatment; rather, diagnoses related to AEs were studied without ascertainment of the underlying cause. Additionally, as AEs were assessed as non-CNS-related versus no non-CNS-related AEs and CNS-related versus no CNS-related AEs, patient counts are not available for patients who had both non-CNS-related and CNS-related AEs or neither. Associations between specific AEs or CNS-related AEs and HCRU and costs could not be established. Furthermore, we only counted new AEs during the treatment period to avoid double-counting them with patients’ pre-existing conditions. Hence, we could not determine whether a specific pre-existing condition was associated with an increased risk of an AE. Additionally, the time to first occurrence of an AE does not distinguish between AEs that may occur in a shorter time frame (e.g. diarrhoea) vs those that may occur after a longer period of time (e.g. cardiovascular events).
Conclusions
More than half (55%) of nmCRPC patients treated with apalutamide or enzalutamide had at least 1 AE and nearly one-third (32%) had a CNS-related AE. There is a significantly increased HCRU and cost burden to the healthcare system among nmCRPC patients treated with novel ARAs developing non-CNS-related AEs, with a more pronounced cost burden in patients developing CNS-specific AEs. Further evaluation would be warranted to confirm these events are related to ARAs. Additional studies are also warranted with recently approved agents, including darolutamide.
Transparency
Author contributions
All authors were involved in the concept and design of the study, the analysis and interpretation of the data, the drafting or critical revision for intellectual content of the manuscript, and the final approval of the manuscript for publication. All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Excel (467.5 KB)Acknowledgements
The authors acknowledge Hubert Kusdono for medical writing and Jessica Beifuss and Kylie Matthews for copyediting and manuscript preparation assistance, all from Xcenda. Xcenda was contracted by Bayer to assist with the completion of this study.
Declaration of financial/other relationships
SA, JY, JP, SXK, and JP are employees of and own stock in Bayer Healthcare Pharmaceuticals. SJF has a consultancy agreement with Bayer Healthcare Pharmaceuticals as well as with Janssen, Pfizer, Astellas, Merck, AstraZeneca, Sanofi, and Myovant.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Key statistics for prostate cancer [Internet]. American Cancer Society; 2021 cited 2022 Feb 25]. Available from: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708.
- SEER cancer stat facts: prostate cancer [Internet]. National Cancer Institute; [cited 2021 Aug 18]. Available from: https://seer.cancer.gov/statfacts/html/prost.html.
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x.
- Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17 (Suppl 2):S72–S79. doi: 10.3747/co.v17i0.718.
- Macomson B, Lin JH, Tunceli O, et al. Time to metastasis or death in non-metastatic castrate resistant prostate cancer (nmCRPC) patients by National Comprehensive Cancer Network (NCCN) risk groups. J Clin Oncol. 2017;35(15_suppl):5027–5027. doi: 10.1200/JCO.2017.35.15_suppl.5027.
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone metastasis-free survival in men with castration-resistant prostate cancer: results of a global phase 3, randomized, placebo-controlled trial. Lancet. 2012;379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9.
- Goldberg H. Expanding treatment options in non-metastatic castrate-resistant prostate cancer [Internet]. UroToday; January 22, 2022 [cited 2022 Feb 25]. Available from: https://www.urotoday.com/library-resources/mcrpc-treatment/114580-expanding-treatment-options-in-non-metastatic-castrate-resistant-prostate-cancer.html.
- Saad F, Bögemann M, Suzuki K, et al. Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors. Prostate Cancer Prostatic Dis. 2021;24(2):323–334. doi: 10.1038/s41391-020-00310-3.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. doi: 10.1016/j.eururo.2020.09.042.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–1418. doi: 10.1056/NEJMoa1715546.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150–158. doi: 10.1016/j.eururo.2020.08.011.
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–2474. doi: 10.1056/NEJMoa1800536.
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197–2206. doi: 10.1056/NEJMoa2003892.
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–1246. doi: 10.1056/NEJMoa1815671.
- Shah A, Shah R, Kebede N, et al. Real-world incidence and burden of adverse events among non-metastatic prostate cancer patients treated with secondary hormonal therapies for androgen deprivation therapy. J Med Econ. 2020;23(4):330–346. doi: 10.1080/13696998.2019.1705313.
- Halabi S, Jiang S, Terasawa E, et al. Indirect comparison of darolutamide versus apalutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer. J Urol. 2021;206(2):298–307. doi: 10.1097/JU.0000000000001767.
- Wang L, Paller C, Hong H, et al. Comparison of treatments for nonmetastatic castration-resistant prostate cancer: matching-adjusted indirect comparison and network meta-analysis. J Natl Cancer Inst. 2022;114(2):191–202. doi: 10.1093/jnci/djab071.
- Pilon D, Behl AS, Ellis LA, et al. Assessment of real-world Central nervous system events in patients with advanced prostate cancer using abiraterone acetate, bicalutamide, enzalutamide, or chemotherapy. Am Health Drug Benefits. 2017;10(3):143–153.
- U.S. Bureau of Labor Statistics. CPI Home: U.S. Bureau of Labor Statistics. Bls.gov. Published 2023. https://www.bls.gov/cpi/.
- Hussain A, Jiang S, Varghese D, et al. Real-world burden of adverse events for apalutamide- or enzalutamide-treated non-metastatic castration-resistant prostate cancer patients in the United States. BMC Cancer. 2022;22(1):304. doi: 10.1186/s12885-022-09364-z.