Abstract
Introduction
Antiviral therapy may be underutilized in patients at high risk for increased clinical and economic burden (e.g. older adults). We aimed to examine the benefits associated with antiviral treatment of seasonal influenza among treated and untreated Medicare beneficiaries.
Methods
This retrospective study of Medicare Claims Research Identifiable Files identified patients ≥66 years old with an influenza diagnosis in outpatient setting between October 2016–March 2019 (flu seasons 2016–2018). Index date defined as date of first claim with influenza diagnosis; baseline as the 12 months pre-index. Treated patients received antivirals ≤2 days from index. Untreated patients had no antivirals ≤6 months post-index. Treated/untreated patients were 1:1 propensity score matched. Outcomes (death, all-cause and respiratory-related healthcare resource utilization [HCRU] and costs) were assessed until death or up to 6 months post-index. Descriptive statistics were reported; Kaplan-Meier estimation was used for survival over time.
Results
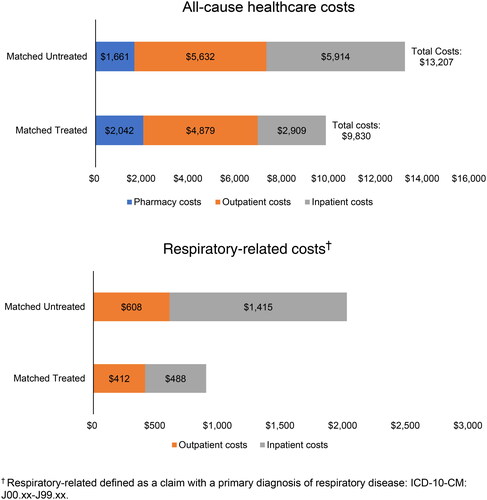
Among 116,901 matched patient pairs, all-cause mortality within 6 months from index diagnosis was 1.6% among treated versus 4.3% among untreated patients. Rates (treated versus untreated) of all-cause inpatient hospitalizations during follow-up were 13.9% versus 22.7% and respiratory-related hospitalizations were 4.2% versus 9.0%. Mean (SD) total all-cause and respiratory-related costs were $9,830 ($18,616.0) and $900 ($4016.4) among the treated, respectively, versus $13,207 ($24,405.1) and $2,024 ($7,623.7) among untreated, respectively. All differences were statistically significant (p < 0.001).
Conclusions
Lack of antiviral treatment is associated with increased mortality, HCRU, and economic burden in older Medicare beneficiaries with seasonal influenza. Future research should investigate whether the choice of antivirals affects influenza burden.
PLAIN LANGUAGE SUMMARY
Previous studies have shown that antiviral drugs help prevent flu-related complications and lower healthcare utilization and costs. However, these previous studies have focused on working aged people with existing health problems. Our study looks at how antiviral treatment can lower the health and financial burden caused by the flu in older adults. Using a Medicare claims database from the 2016–2018 flu season, we identified 116,901 matched (treated versus untreated) patient pairs. All-cause mortality within 6 months from the index diagnosis (defined as the first claim with a flu diagnosis) was 1.6% among treated versus 4.3% among untreated patients. Rates (treated versus untreated) of all-cause inpatient hospitalizations during follow-up (defined as 6 months after the index diagnosis date) were 13.9% versus 22.7% and respiratory-related hospitalizations were 4.2% versus 9.0%. Mean total all-cause and respiratory-related costs were $9,830 and $900 among the treated, respectively, versus $13,207 and $2,024 among untreated, respectively. All differences were statistically significant (p < 0.001). This analysis of older adults with the flu found that prompt antiviral treatment is associated with lower rates of mortality and acute complications, reduced hospitalization, and lower healthcare costs. Use of antiviral treatment for patients at high risk of flu, such as older adults, is warranted.
Introduction
Seasonal influenza is a major cause of morbidity and mortality in the United States (US). The Centers for Disease Control and Prevention (CDC) estimates that the 2018–2019 influenza season was associated with 380,000 influenza-related hospitalizations and 28,000 related deaths in the USCitation1. Recent estimates for the total annual direct medical costs in the US range from $3.2 billion to $5.5 billion Citation2,Citation3.
Adults 65 years and older are at high risk of influenza-related complications, morbidity, and increased healthcare costsCitation4–6. Indeed, in the estimates for the 2018–2019 influenza season, older adults, who made up 16% of the US population in 2018–2019Citation7, account for 57% of the influenza-related hospitalizations and for 75% of the influenza-related deathsCitation1. Moreover, the age group with the largest share of the direct medical costs was 65 years and older (42.7% of the total), driven primarily by hospitalization costs ($1.3 billion)Citation2.
An important public health measure for the prevention of seasonal influenza is vaccinationCitation8–10. Multiple studies have shown that immunization reduces the risk of mortality, hospitalization, and complications associated with influenzaCitation11–13. Despite the recommendation that everyone 65 or older receive the influenza vaccine annuallyCitation8, about 25–30% of the elderly did not receive the influenza vaccine during the most recent influenza seasonsCitation14.
Several antiviral treatment options exist for patients who become infected with influenza. Neuraminidase inhibitors (i.e. zanamivir, oseltamivir, peramivir) and the selective inhibitor of influenza cap-dependent endonuclease (i.e. baloxavir) are all active against both influenza A and B, and currently recommended by the CDCCitation15–21. Clinical studies have shown that when initiated promptly (e.g. within 48 h of symptom onset), antiviral agents, as above, shorten the duration of symptoms and may reduce the incidence and severity of complications of influenzaCitation17,Citation22–26. Despite evidence of potential benefits, antiviral therapy may be underutilized in patients at high risk for complications, even among those who present early for careCitation27.
Real world evidence on antiviral use and clinical and economic burden in influenza is scarce. Recent studies focused on commercially insured patients found that antiviral use is associated with a decrease in influenza-related complications and healthcare utilization and costsCitation28–30. Another analysis investigated influenza burden for Medicare beneficiaries but treatment status was not trackedCitation31.
This study’s aim was to broadly examine the clinical and economic benefits, including reduced acute complications, mortality, healthcare utilization, and costs, associated with antiviral treatment of seasonal influenza among a large, contemporaneous sample of Medicare beneficiaries.
Methods
Study design and data source
This retrospective cohort study examined the burden of influenza among Medicare beneficiaries in the US. The five most recent years (2015–2019) of Medicare Fee-for-Service (FFS) claims from the 100% Research Identifiable Files (RIFs) were used for the analysis. The RIF database is the most comprehensive Medicare database covering 100% of Medicare beneficiaries from all census regions and includes patient-level demographic, enrolment, and fee-for-service administrative claims data across all places of service (e.g. emergency departments, inpatient and outpatient facilities, skilled nursing and hospice facilities, and home health agencies).
Patient identification
This study included Medicare FFS beneficiaries at least 66 years of age with a diagnosis of influenza in the outpatient setting, indicated by at least one outpatient medical claim with an ICD-10-CM diagnosis code for influenza: J09.xx, J10.xx, J11.xx during the identification (ID) period of October through March of each year (seasonal influenza season) between 2016 and 2019 (i.e. three full influenza seasons). The index date for the study was defined as date of the first claim with an influenza diagnosis. If patients had multiple influenza diagnoses during the study period, one occurrence was picked randomly for identification. Patients with multiple episodes of influenza within a given season were observed for the first episode only. Patients were also required to have continuous enrollment in Medicare FFS Parts A/B and Part D during the one year prior to the index date (baseline period) and during the 6 months after index (follow-up period), or until death if occurring earlier. Thus, patients were observed from baseline until the end of follow-up or death, whichever occurred first.
Of the above patients diagnosed with influenza, two groups were created: 1) treated patients who received an approved antiviral medication for influenza (had a claim with a NDC drug code for zanamivir, oseltamivir, peramivir, or baloxavir) within 2 days after the index date; and 2) untreated patients who did not receive treatment within 6 months after the index date. Patients in either group who received antiviral medication for influenza within two months prior to the index date were excluded to ensure the exposure treatment status (i.e. initiators and nonusers of antiviral treatment). Additionally, treated patients were further excluded if they received antiviral medication for prophylaxis as indicated by a total days’ supply of antiviral medication of 10 days or more, within 10 days after the first antiviral claim.
Untreated patients were matched 1:1 to treated patients using propensity score matching (greedy nearest neighbor with caliper width of 0.1 of the standard deviation of the logit of the propensity score)Citation32,Citation33. Propensity score matching allows for more balanced baseline characteristics between the cohorts and, in turn, for the antiviral treatment effect to be observed in the follow-up period with few confounding factorsCitation34,Citation35. The propensity for initiating antiviral medication was estimated using logistic regression with independent variables of year of influenza season, age, gender, geographic region, race, influenza vaccine status, usual physician specialty, Charlson Comorbidity Index (CCI), number of chronic conditions, dual-eligible status, and each individual high-risk condition (asthma, chronic lung disease, heart disease, blood disorders, endocrine disorders, kidney disorders, liver disorders, metabolic disorders, extreme obesity, chronic obstructive pulmonary disease [COPD], immunosuppressive conditions [MS, HIV, RA]). In addition, patients were matched exactly on the year of influenza season and influenza vaccine status. Balance diagnostics after matching were assessed using standardized mean difference.
Measures
Disease burden was assessed during the follow-up period by observing acute complications, mortality, healthcare utilization and costs. Patients who experienced an acute complication of influenza during an inpatient hospitalization were categorized as follows: respiratory tract diagnoses, influenza with other manifestations, neurologic diagnoses, cardiovascular events, endocrine diagnoses, gastrointestinal tract diagnoses, hematologic diagnoses, and other acute diagnoses ()Citation36. Mortality within 6 months from the index diagnosis was observed. Healthcare utilization was assessed by place of service, number of patients with an: inpatient, emergency department (ED), outpatient visits (excluding ED visits), skilled nursing facility, home health agency, and hospice care. Additionally, inpatient length of stay and number of office visits were calculated. Finally, total healthcare costs (all cause and respirator-related) were measured. Patients’ baseline demographics (age, gender, race) were observed. To assess baseline health status of patients, comorbidities (Charlson Comorbidity Index [CCI]) and chronic conditions (as defined by the Healthcare Cost and Utilization Project [HCUP] Chronic Condition Indicator Citation37) as well as conditions contributing to high influenza risk were reported (). Finally, vaccination status (assessed in the same season as and prior to the index influenza diagnosis) and year of influenza season was noted.
Table 1. Baseline characteristics.
Table 2. Healthcare utilization during the 6-month follow-up period.
Statistical analysis
Descriptive statistics for all baseline and outcome measures were reported by treatment status of the matched cohorts. Means and standard deviations were reported for continuous variables; counts and percentages were reported for categorical variables. All outcome measures for matched treated and untreated patients with influenza were compared using t-tests or Chi-square tests for continuous and categorical variables, respectively.
All data transformations and statistical analyses were performed using SAS® version 9.4.
Results
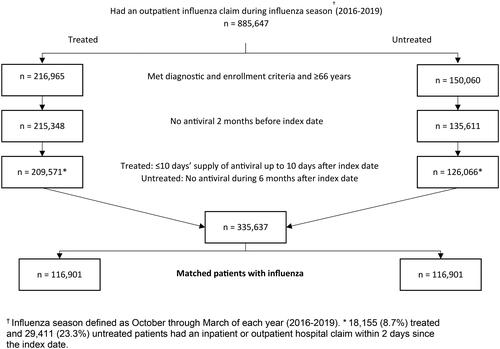
During the three influenza seasons observed between 2016–2019, 335,637 patients (209,571 treated and 126,066 untreated patients) met study inclusion criteria. The final matched cohort contained 116,901 matched patient pairs ().
Figure 1. Patient attrition. During the three influenza seasons observed between 2016–2019, 335,637 patients (209,571 treated and 126,066 untreated patients) met study inclusion criteria. The final matched cohort contained 116,901 matched patient pairs.
Demographics and clinical characteristics
In the matched cohort, the mean (SD) age at index was 75.6 (7.2) years among both treated and untreated patients, with the greatest proportion of patients in the youngest, 66-74 years, age group (52.4% vs. 53.3% in the treated and untreated groups, respectively) (). The majority of patients were female (61.1% vs. 61.5% in the treated and untreated groups, respectively). In addition, at baseline, matched treated and untreated patients had on average 5.8 chronic conditions and 92.0% versus 91.6% had a high-risk condition. In each group, 61.4% received an influenza vaccine prior to influenza diagnosis, and almost 50% of influenza cases were recorded in the 2017–2018 influenza season. For patients with an antiviral, oseltamivir was most often prescribed (99.7%), followed by baloxavir (0.25%), zanamivir (0.01%), and peramivir (percentage not reported due to CMS cell suppression policy).
Acute complications and mortality
Treated patients were less likely to develop complications during the follow-up period (). In particular, the proportion of treated patients who experienced an acute complication was nearly half that for untreated patients (8.3% vs. 15.7%). Antiviral treatment was associated with reduced complication rates across all complication categories. The most prevalent complication type, respiratory-related complications, was observed among 4.7% of treated compared to 9.6% of untreated patients. The cardiovascular event rate in the treated group was 1.3% compared to 2.6% in the untreated group. Neurologic complication rates were 0.67% and 0.97% among treated and untreated patients, respectively. The likelihood of gastrointestinal complication was also about half as much in the treated group as in the untreated group (0.27% vs. 0.45%). Furthermore, the rate of influenza with other acute manifestations was 0.07% among treated and 0.18% among untreated, while the rates of hematologic complications were 0.07% and 0.11% and of endocrine diagnoses were 0.06% and 0.11%, respectively. Other acute diagnoses were present in 4.4% of treated and 9.2% of untreated patients. Within this category, the complication rates for acute kidney failure were 3.2% vs. 6.3%, for sepsis were 2.1% vs. 4.9%, for bacteremia were 0.14% vs. 0.34%, for rhabdomyolysis were 0.09% vs. 0.29%, for complications of transplanted organ were 0.04% vs. 0.10% for treated versus untreated patients, respectively. Complication rates were statistically significantly different between the treated and untreated group in all complication categories.
Figure 2. Acute complications† and mortality. Treated patients had lower rates of acute complications and mortality during the follow-up period (6 months after index date, defined as date of first claim with an influenza diagnosis).
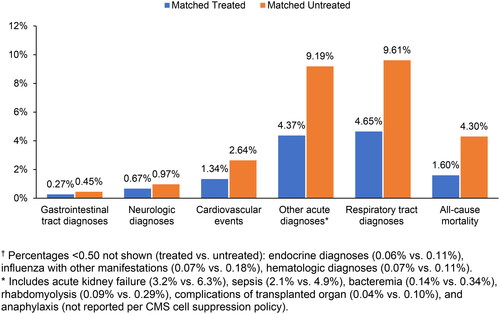
Antiviral treatment was also associated with mortality benefits during the follow-up period (). All-cause mortality within 6 months from index diagnosis was as low as 1.6% among treated patients compared to 4.3% among untreated patients (p < 0.001).
Healthcare utilization and costs
Healthcare utilization was generally statistically significantly lower in treated patients than in untreated patients (). In particular, rates of all-cause inpatient hospitalizations during follow-up were 13.9% versus 22.7% for treated versus untreated patients (p < 0.001) while rates of respiratory-related hospitalizations were 4.2% vs. 9.0% for treated versus untreated patients (p < 0.001). Similar differences were observed for services at other service locations. Treated patients versus untreated patients had lower rates of all-cause ED visits compared to untreated (18.9% vs. 21.5%), as well as lower rates of skilled nursing facility care (2.5% vs. 7.3%), home health agency visits (6.6% vs. 12.3%), and hospice care (1.2% vs. 2.9%). However, the rate of all-cause outpatient hospitalizations (excluding ED visits) was higher in treated compared to untreated patients (63.7% vs. 62.2%), as were mean number of office visits (9.8 visits vs. 9.3). All differences were statistically significant (p < 0.001). The results for respiratory-related utilization and costs showed a similar pattern, with treated patients having lower utilization, except for a slightly higher mean number of respiratory-related office visits, which was lower among untreated patients (1.4 vs. 1.2 visits) (). Healthcare costs were significantly lower in treated patients. Mean (SD) total all-cause and respiratory-related costs were $9,830 ($18,616.0) and $900 ($4,016.4) among the treated patients, respectively, compared to $13,207 ($24,405.1) and $2,024 ($7,623.7) among untreated patients, respectively (p < 0.001 for all cost outcomes) (). Inpatient services accounted for 30% of total all-cause costs for treated patients compared to 45% for untreated patients. Outpatient services accounted for 50% of total all-cause costs for treated patients compared to 43% for untreated patients.
Discussion
Elderly adults (65 years and older) are at higher risk of influenza-related complications, morbidity, and increased healthcare costsCitation4–6. Vaccination is a recommended intervention for prevention of influenza, nevertheless, more than half of the elderly and other persons at high risk do not receive the influenza vaccineCitation8,Citation14. Additionally, prompt antiviral treatment after symptom detection can further alleviate the burden of disease. This study quantifies the significant clinical and economic benefits of antiviral influenza treatment in a large cohort of Medicare beneficiaries from three influenza seasons between 2016 and 2019.
For elderly Medicare beneficiaries diagnosed with influenza, we found that treatment with antiviral medication was associated with lower mortality rates, lower rates of influenza-related complications, lower healthcare utilization, and lower healthcare costs, both all-cause and respiratory-related. Observed differences were statistically significant for nearly all outcome measures assessed in this study. For example, treated patients experienced overall complication rates that were nearly half of rates for untreated patients. This difference was most pronounced for respiratory-related complications, which were also the most common complication observed (4.7% vs. 9.6%). Antiviral treatment was also associated with better survival following an influenza diagnosis, as about 16 out of 1000 treated elderly patients died within 6 months after diagnosis versus about 43 out of 1000 untreated patients. In addition, the data showed that treated patients compared to untreated patients used healthcare resources much less intensively in the 6 months following an influenza diagnosis, particularly for all-cause and respiratory-related hospitalization, which were less than two-thirds (13.9% vs. 22.7%) and half (4.2% vs. 9.0%), respectively. Finally, the mean total all-cause healthcare costs were about 25% lower for treated patients compared to untreated patients ($9,830 vs. $13,207) while respiratory-related costs were less than half for treated versus untreated individuals with influenza ($900 vs. $2,024).
Prior research
Two earlier studies using large commercial claims data examined the association between antiviral treatment and patient outcomes. Spanguolo and colleagues (2016)Citation28 used regression analyses while Wallick and colleagues (2021)Citation29 created a propensity score matched cohort of treated and untreated patients with influenza for the 2006-2010 and 2014-2016 influenza seasons, respectively. Both studies found that antiviral use is associated with a decrease in influenza-related complications and healthcare utilization and costs. However, their data source captures primarily data from a working age population. Neuberger and colleagues (2022) also use a propensity score matched design but their focus is the subpopulation with rheumatoid arthritisCitation30. Their results show that prompt antiviral treatment after influenza diagnosis may reduce healthcare utilization and costs among this particular group of high-risk patientsCitation30. All studies above confirm the results of the present analysis, however, their findings are not directly comparable with ours due to differences in patient cohort.
Results of this study highlight that prompt intervention with antiviral treatment could reduce clinical and economic burden associated with influenza among older adults. These findings underscore the need to increase use of antiviral medications in this population. As older adults are underrepresented in clinical trials and commercial claims data sourcesCitation38,Citation39, this study takes steps towards filling a current research gap by characterizing the clinical and economic burden in this vulnerable population, as well as highlighting a potential avenue (increased use of antivirals) for decreasing this burden.
Limitations
This retrospective cohort study uses administrative claims data. Such data are primarily designed to support reimbursement and may inaccurately represent clinical information. For example, the presence of a diagnosis code on a medical claim does not guarantee the presence of a disease, as the diagnosis code may be miscoded or included as a rule-out criterion. However, Feemster and colleagues (2012) found that diagnosis codes (ICD-9-CM) for influenza detected 73% of laboratory-confirmed influenza cases, and that fewer than 1% of patients without a diagnosis code had laboratory-confirmed influenzaCitation40. Additionally, a claim for an antiviral treatment indicates a drug fill but does not ensure that the treatment was actually taken. The results of this analysis may be conservative if some of the patients who filled an antiviral prescription didn’t actually take the antiviral. Another data-related limitation is that the size of the analyzed populations may have impacted the observed statistically significant differences in outcomes among the treated and untreated patients. Even small differences in the percent of patients experiencing a complication can translate into a large number of patients because influenza affects many older adults. For example, in this study, there were a small proportion of patients who experienced hematologic conditions (0.11 for untreated vs 0.07 for treated patients). However, based on the estimate of 581,594 seniors with flu during 2021–2022 mild influenza seasonCitation41, even this small difference of 0.04 translates to 233 fewer people experiencing the complication, which may be meaningful.
The benefits of real-world data, including the use of large data sets, continue to be uncoveredCitation42,Citation43. Real world evidence is often available more quickly than trial data and can supplement trial data by capturing measures such as adherence, hospitalizations, causes of death, and treatment patterns; this is significant as noted earlier, the current study’s population (older adults) is often underrepresented in clinical trialsCitation38. The trends of greater healthcare utilization and associated costs in untreated patients with influenza is consistent with previously published workCitation28–30, even if the patient cohorts (e.g. younger and commercially insured populations) are not directly comparable. Another limitation of this study is that additional factors, beyond treatment status, that could impact outcomes (e.g. income or education levels, or additional barriers to treatment access) were not examined. Lastly, as this study focused on Medicare beneficiaries 66 years of age or older and who were predominantly white (>90%), results may not be generalizable to uninsured individuals or those with other types of insurance or to individuals of a different age group or race.
Conclusion
Lack of antiviral treatment is associated with increased mortality, healthcare resource utilization, and economic burden in elderly Medicare beneficiaries with seasonal influenza, a population already at risk for increased resource use and associated costs compared to their counterparts without influenza. Promoting antiviral treatment for patients at high risk of influenza like older adults, is warranted. Future research should investigate whether the choice of antivirals affects the clinical and economic burden of influenza.
Transparency
Author contributions
Concept and design (JB, SRR, EC, KB, AS); acquisition of subjects and/or data (SRR, EC, KB, MHT), data analysis (SRR, EC) and interpretation (JB, SRR, EC, KB, AS), and preparation of manuscript (SRR, JB, EC, KB, MHT, SC, AS).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Declaration of financial/other relationships
EC, and MHT are employees of PHAR (Partnership for Health Analytic Research), a health services research company paid to conduct the research described in this manuscript. At the time this study was conducted, SRR and KB were employees of PHAR. JB and AS are employees of Genentech, Inc., the study sponsor.
Availability of data and materials
The data that support the findings of this study originate from Medicare data, which are available from the Centers for Medicare and Medicaid through ResDAC (https://www.resdac.org/).
Additional information
Funding
References
- Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States—2018–2019 influenza season [Internet]. Influenza Flu. 2020 [cited 2020 Oct 20]. Available from: https://www.cdc.gov/flu/about/burden/2018-2019.html.
- Putri WCWS, Muscatello DJ, Stockwell MS, et al. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960–3966. doi: 10.1016/j.vaccine.2018.05.057.
- Ozawa S, Portnoy A, Getaneh H, et al. Modeling the economic burden of adult vaccine-preventable diseases in the United States. Health Aff. 2016;35(11):2124–2132. doi: 10.1377/hlthaff.2016.0462.
- Uyeki TM. Preventing and controlling influenza with available interventions. N Engl J Med. 2014;370(9):789–791. doi: 10.1056/NEJMp1400034.
- Matias G, Taylor R, Haguinet F, et al. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health. 2017;17(1):271. doi: 10.1186/s12889-017-4177-z.
- Czaja CA, Miller L, Alden N, et al. Age-related differences in hospitalization rates, clinical presentation, and outcomes among older adults hospitalized with influenza—U.S. Influenza hospitalization surveillance network (FluSurv-NET). Open Forum Infect Dis. 2019;6:ofz225.
- U.S. Department of Health and Human Services, Administration for Community Living. Profile of Older Americans [Internet]. Adm. Community Living. 2021. Available from: https://acl.gov/aging-and-disability-in-america/data-and-research/profile-older-americans.
- Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2022–23 influenza season. MMWR Recomm Rep. 2022;71(1):1–28. doi: 10.15585/mmwr.rr7101a1.
- Treanor JJ. Influenza vaccination. Solomon CG, editor. N Engl J Med. 2016;375(13):1261–1268. doi: 10.1056/NEJMcp1512870.
- Paules CI, Fauci AS. Influenza vaccines: good, but We can do better. J Infect Dis. 2019;219(Suppl_1):S1–S4. doi: 10.1093/infdis/jiy633.
- Nichol KL, Nordin JD, Nelson DB, et al. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–1381. doi: 10.1056/NEJMoa070844.
- Beyer WEP, McElhaney J, Smith DJ, et al. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013;31(50):6030–6033. doi: 10.1016/j.vaccine.2013.09.063.
- Demicheli V, Jefferson T, Ferroni E, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. [Internet]. 20182(2):CD001269. doi: 10.1002/14651858.CD001269.pub6.
- Centers for Disease Control and Preventionl. Influenza Vaccination Coverage by Season. [Internet]. 2021 [cited 2022 Sep 9]. Available from: https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm.
- Zachary KC. Seasonal influenza in adults: Treatment [Internet]. UpToDate;2021 [cited 2021 Dec 8]. Available from: https://www.uptodate.com/contents/seasonal-influenza-in-adults-treatment.
- Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians [Internet]. Influenza Flu. 2021 [cited 2021 Dec 8]. Available from: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis. 2019;68(6):895–902. doi: 10.1093/cid/ciy874.
- TAMIFLU® (oseltamivir phosphate) - PRESCRIBING INFORMATION. [Internet]. Roche; 2012 [cited 2022 Sep 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021087s06_2lbl.pdf.
- RAPIVABTM (peramivir injection) - PRESCRIBING INFORMATION. [Internet]. BioCryst Pharmaceuticals, Inc; 2014 [cited 2022 Sep 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206426lbl.pdf.
- RELENZA (zanamivir inhalation powder) - PRESCRIBING INFORMATION. [Internet]. GlaxoSmithKline. 2018 [cited 2022 Sep 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021036s030lbl.pdf.
- XOFLUZA (baloxavir marboxil) - PRESCRIBING INFORMATION. [Internet]. Genentech USA, Inc.; 2018 [cited 2022 Sep 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210854s000lbl.pdf.
- Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729–1737. doi: 10.1016/S0140-6736(14)62449-1.
- Venkatesan S, Myles PR, Leonardi-Bee J, et al. Impact of outpatient neuraminidase inhibitor treatment in patients infected with influenza A(H1N1)pdm09 at high risk of hospitalization: an individual participant data metaanalysis. Clin Infect Dis. 2017;64(10):1328–1334. doi: 10.1093/cid/cix127.
- Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913–923. doi: 10.1056/NEJMoa1716197.
- Heneghan CJ, Onakpoya I, Jones MA, et al. Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Health Technol Assess. 2016;20(42):1–242. doi: 10.3310/hta20420.
- Doll MK, Winters N, Boikos C, et al. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother. 2017;72(11):2990–3007. doi: 10.1093/jac/dkx271.
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis. 2018;66(7):1035–1041. doi: 10.1093/cid/cix922.
- Spagnuolo PJ, Zhang M, Xu Y, et al. Effects of antiviral treatment on influenza-related complications over four influenza seasons: 2006–2010. Curr Med Res Opin. 2016;32(8):1399–1407. doi: 10.1080/03007995.2016.1176016.
- Wallick C, Wu N, To TM, et al. Antiviral use is associated with a decrease in the rate of influenza-related complications, health care resource utilization, and costs. J Med Econ. 2021;24(1):386–393. doi: 10.1080/13696998.2021.1889572.
- Neuberger EE, To TM, Seetasith A, et al. Antiviral use and health care use among patients with rheumatoid arthritis and influenza in three influenza seasons, 2016‐2019. ACR Open Rheumatol. 2022;4(7):631–639. doi: 10.1002/acr2.11441.
- Bolge SC, Kariburyo F, Yuce H, et al. Predictors and outcomes of hospitalization for influenza: real-World evidence from the United States medicare population. Infect Dis Ther. 2021;10(1):213–228. doi: 10.1007/s40121-020-00354-x.
- Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057–1069. doi: 10.1002/sim.6004.
- Rosenbaum PR, Rubin DB. The Central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41.
- Littnerová S, Jarkovský J, Pařenica J, et al. Why to use propensity score in observational studies? Case study based on data from the czech clinical database AHEAD 2006-09. Cor Vasa. 2013;55(4):e383–e390. doi: 10.1016/j.crvasa.2013.04.001.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786.
- Chow EJ, Rolfes MA, O'Halloran A, et al. Respiratory and nonrespiratory diagnoses associated with influenza in hospitalized adults. JAMA Netw Open. 2020;3(3):e201323. doi: 10.1001/jamanetworkopen.2020.1323.
- Agency for Healthcare Research and Quality. HCUP Chronic Condition Indicator [Internet]. Healthc. Cost Util. Proj. HCUP. 2015 [cited 2022 May 10]. Available from: www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp.
- Herrera AP, Snipes SA, King DW, et al. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;100 1(Suppl 1):S105–S112. doi: 10.2105/AJPH.2009.162982.
- IBM Watson HealthTM. IBM MarketScan Research Databases for Health Services Researchers [Internet]. Somers, NY: IBM Corporation; 2019 [cited 2019 Oct 15]. Available from: https://www.ibm.com/downloads/cas/6KNYVVQ2.
- Feemster KA, Leckerman KH, Middleton M, et al. Use of administrative data for the identification of laboratory-confirmed influenza infection: the validity of influenza-Specific ICD-9 codes. J Pediatric Infect Dis Soc. 2013;2(1):63–66. doi: 10.1093/jpids/pis052.
- Centers for Disase Control and Prevention. Preliminary Estimated Influenza-Related Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States – 2021-2022 Influenza Season [Internet]. Influenza Flu. 2023 [cited 2024 Jan 24]. Available from: https://www.cdc.gov/flu/about/burden/2021-2022.htm.
- Baser O, Samayoa G, Yapar N, et al. Use of open claims vs closed claims in health outcomes research. JHEOR. 2023;10:44–52. doi: 10.36469/jheor.2023.87538.
- Liu F, Panagiotakos D. Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol. 2022;22(1):287. doi: 10.1186/s12874-022-01768-6.
Appendix Tables
Appendix Table 1.
Acute complications by categories and associated ICD-10 code.
Appendix Table 1.
Appendix Table 2.
ICD-9 and 10 codes for CDC-defined high-risk conditions for influenzaa.