Abstract
Aims
Anemia is the most common extraintestinal complication of inflammatory bowel disease (IBD), with approximately half of cases caused by iron deficiency (ID). Intravenous iron is the preferred ID anemia (IDA) treatment where oral iron is contraindicated, ineffective or not tolerated, or where ID correction is urgent. The objective was to evaluate the cost-utility of ferric derisomaltose (FDI) versus ferric carboxymaltose (FCM) in patients with IBD and IDA in England, in whom IV iron treatment is preferred.
Materials and methods
A patient-level simulation model was developed, capturing quality of life (QoL) differences based on SF-36v2 data from the PHOSPHARE-IBD randomized controlled trial, monitoring and incidence of post-infusion hypophosphatemia, and number of iron infusions required. Analyses were conducted over a five-year time horizon from the Department of Health and Social Care (DHSC) perspective, with healthcare provider and societal perspectives adopted in separate analyses. Future costs and effects were discounted at 3.5% per annum and one-way and probabilistic sensitivity analyses were performed.
Results
FDI increased quality-adjusted life expectancy by 0.075 QALYs versus FCM from 2.57 QALYs to 2.65 QALYs per patient. Patients receiving FDI required 1.63 fewer iron infusions over the five-year time horizon, driving infusion-related cost savings of GBP 496 per patient (GBP 2,188 versus GBP 1,692) from the DHSC perspective. Costs of monitoring and treating hypophosphatemia after FCM were GBP 226, yielding total savings of GBP 722 per patient (GBP 2,414 versus GBP 1,692) over the five-year time horizon. FDI also led to reduced costs versus FCM in the societal and provider analyses and was therefore the dominant intervention across all three perspectives.
Limitations
The analysis did not capture patient adherence, hypophosphatemic osteomalacia, or fractures.
Conclusions
Results showed that FDI improved patient QoL and reduced direct healthcare expenditure versus FCM in patients with IBD and IDA in England.
PLAIN LANGUAGE SUMMARY
Ferric derisomaltose (FDI) is an intravenous iron approved for the treatment of clinically diagnosed iron deficiency in the United Kingdom (UK), and can be an important therapeutic option for patients with inflammatory bowel disease (IBD), who require regular and rapid iron replenishment. Ferric carboxymaltose (FCM) is the sole alternative intravenous iron formulation available in the UK, but is associated with reduced blood phosphate levels, potentially causing fatigue and weakening of the bones. We conducted an economic analysis to weigh the costs and clinical outcomes associated with FDI and FCM in the UK, for patients with IBD and iron deficiency anemia (IDA). The main clinical difference we investigated was reduced blood phosphate levels, which occurred more often after FCM than FDI. We also incorporated recent quality of life data from a clinical study, and calculated the number of infusions (and associated costs) of each iron formulation, that patients would require over five years. Clinical data were obtained from published medical literature, while cost data came from UK sources including the 2022/2023 National Tariff Payment System and the British National Formulary. Our model showed that FDI was associated with quality of life improvements, fewer overall infusions per treatment course, and reduced costs compared to FCM, from the English Department of Health and Social Care perspective, the societal perspective, and the perspective of individual healthcare providers (namely NHS Trusts) within NHS England. FDI is therefore likely to represent the best value intravenous iron for the treatment of IDA with IBD in the UK.
Introduction
Inflammatory bowel disease (IBD) is characterized by gastrointestinal tract inflammation, caused by an immune response to gut microflora in genetically predisposed individualsCitation1, and can be divided into two types: ulcerative colitis (UC) and Crohn’s disease (CD) Citation1. Anemia is the most common extraintestinal complication of IBD, with some reports suggesting that approximately one-third of people living with IBD may be suffering from recurrent anemiaCitation2,Citation3. Anemia arises predominantly due to intestinal blood loss that occurs in IBD, but reduced absorption of vitamins and minerals are also contributory factorsCitation4,Citation5. While anemia of chronic disease (ACD) is also a common cause of anemia in IBDCitation6–8, approximately half of all cases of anemia in patients with IBD are caused by iron deficiency (ID) Citation2, making it the most common cause of anemia secondary to IBD. Decreased hematopoiesis from the bone marrow in this active phase of IBD may play a synergistic role.
Oral iron preparations are widely used to treat iron-deficiency anaemia (IDA). However, given that patients with IBD and IDA already experience malabsorption of vitamins and minerals, oral iron preparations may be ineffectiveCitation9, and have been reported to exacerbate IBD symptoms in some casesCitation10. Intravenous (IV) iron is therefore the recommended IDA treatment where oral iron is contraindicated, ineffective or not tolerated, or where correction of ID is urgentCitation11.
Several IV iron preparations are currently licensed for use within the UK. These include Venofer® (iron sucrose), Cosmofer® (low molecular weight iron dextran), Ferinject® (ferric carboxymaltose (FCM)) and Monofer® (ferric derisomaltose (FDI)) Citation12. FCM and FDI allow for comparatively higher doses to be infused at a faster rate than other IV iron preparations, which is important when correction of ID is urgentCitation13. This factor can lead to improved patient quality of life (QoL) as patients require comparatively fewer infusions, whilst the healthcare service also benefits through reduced resource consumption by patients with IDA. One important side-effect that can occur due to administration of specific IV iron formulations is hypophosphatemia.
FCM has recently been found to result in significantly higher incidence of hypophosphatemia than FDICitation14–17 and other IV iron formulations such as ferumoxytolCitation18,Citation19. The recent PHOSPHARE-IBD double-blind randomized controlled trial (RCT) (NCT03466983) investigated the incidence of hypophosphatemia after administration of FCM and FDI for the treatment of IDA in patients with IBDCitation20. The trial included a primary outcome measure of hypophosphatemia incidence (s-phosphate <2.0 mg/dL) experienced any time from baseline to day 35. Secondary outcome measures included hypophosphatemia incidence at any time from baseline to week 10, incidence of severe hypophosphatemia (s-phosphate ≤1.0 mg/dL) within 35 days from baseline, and time with hypophosphatemia.
Although both iron formulations effectively corrected IDA, the primary endpoint showed a significantly higher incidence of all hypophosphatemia with FCM than FDI (51% versus 8.3%, respectively; p < 0.0001). Additionally, while patient-reported scores from the Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue Scale improved in both groups (demonstrating both numerical and clinical significance), they improved to a lesser extent with FCM than FDI. The magnitude of improvement in FACIT Fatigue Scale scores was inversely associated with the magnitude of decrease in phosphate concentrationCitation21.
Hypophosphatemia after administration of FCM appears to be caused by higher concentrations of the hormone fibroblast growth factor 23 (FGF23), which in turn triggers downstream alterations in mineral metabolism such as increased urinary phosphate, severe reductions in active vitamin D (calcitriol), an acute drop in serum calcium and compensatory secondary hyperparathyroidismCitation17,Citation19,Citation21–23. In cases where hypophosphatemia is persistent or recurrent, it can in turn increase the risk of severe derangements in bone and mineral metabolismCitation24. Consequently, patients who develop severe hypophosphatemia can go on to develop osteomalacia and fractures, which can affect patient QoL and require costly clinical intervention.
Another difference between FCM and FDI is the maximum dose that can be administered in a single infusion. Although infusion times can be shorter with low doses of FCM compared to FDI (e.g. a minimum of 6 min for FCM doses of 500 mg versus 15 min for FDI), patients receiving FCM can only receive a maximum dose of 1000 mg per infusionCitation25. Conversely, FDI can be administered at doses over 1000 mg (up to a maximum of 20 mg/kg bodyweight) per infusion, which can enable iron correction in one infusion in a greater proportion of patients than FCMCitation26.
Given the clinical and posological differences between the iron formulations, an evaluation of their relative cost-utility in treating patients with IBD and IDA is warranted to ensure the best value for healthcare expenditure. Selection of the most cost-effective treatment option may lead to both improved patient outcomes and either a reduction in costs or more efficient use of additional healthcare budget. The objective of the present study was to evaluate the cost-utility of FDI versus FCM in patients with IDA and IBD in the UK.
Methods
Patient-level simulation model
A patient-level simulation model was developed in Microsoft Excel to evaluate the cost-effectiveness of FDI versus FCM from three perspectives: a provider perspective (i.e. an NHS Trust), a national payer perspective (i.e. the English National Health Service and ultimately the Department of Health and Social Care [DHSC]), and a societal perspective, capturing the costs to the DHSC in addition to transportation costs and workplace absenteeism costs arising from attending IV infusions.
The model structure was based on a previously-published patient-level cost-utility model adaptable to various settingsCitation27. The patient-level simulation model structure was selected based on the technical guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit. Notably, the relationship between patient characteristics (baseline hemoglobin and bodyweight) and subsequent model outcomes (iron need and the number of iron infusions required) was non-linear.
Modeled patients were generated with baseline age, hemoglobin, and bodyweight sampled from parametric distributions informed by the baseline characteristics in the PHOSPHARE-IBD RCT (). All distributions were sampled by supplying uniformly-distributed values to the (inverse of the) cumulative distribution functions for the selected parameter distributions. Uniform distribution samples were generated reproducibly using the Visual Basic for Applications Rnd() function, which utilizes a linear congruential pseudorandom number generatorCitation29. Covariance between baseline hemoglobin and bodyweight was not modelled based on a lack of evidence for any correlation between the characteristics. These simulated patient characteristics were then used to calculate a mean iron need using simplified tables of iron need as described in the respective summaries of product characteristicsCitation30. The need for a course of iron treatment was established in each cycle based on a patient-specific retreatment interval (). Costs of administered IV iron and QoL process-related utilities associated with administration of the IV iron formulations were calculated based on the modeled number of infusions administered per cycle (). The proportion of patients experiencing hematological response was determined to be the same in both arms, based on results from the PHOSPHARE-IBD trialCitation21. Treatment-specific disease-related QoL data were also obtained from the results of the trialCitation32. For the probabilistic sensitivity analysis (PSA), Monte Carlo methods were used to investigate the likelihood of alternative patient outcomes based on stochastic model parameters and patient-level heterogeneity. In each model cycle, QoL and cost outcomes were calculated and recorded for each patient. Then, the QoL and costs from each model cycle over the time horizon were aggregated and presented on a cost-utility plane.
Figure 1. Patient-level simulation model schematic.
Note: References have been supplied for the simplified TableCitation29 and Ganzoni TableCitation31
Abbreviations. IBD, inflammatory bowel disease; QoL, quality of life.
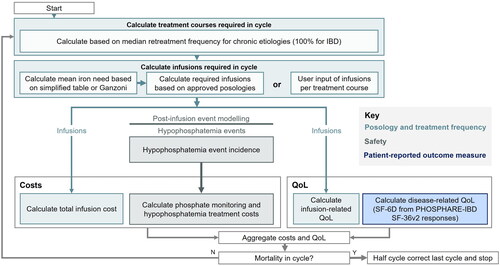
Figure 2. Resource utilisation associated with iron infusions, phosphate monitoring and phosphate replenishment.
Illustrative costs are from the Department of Health and Social Care perspective.
Table 1. Baseline patient characteristics and disease parameters.
Survival, quality-adjusted life years (QALYs), and cost outcomes in 2022 Great British Pounds (GBP) were calculated and summarized across all modeled patients separately for FCM and FDI. These model outcomes were then used to calculate an incremental cost-utility ratio (ICUR) and net monetary benefit (NMB) for FDI versus FCM.
Clinical inputs and patient characteristics
Data regarding the safety and effectiveness of the iron formulations were identified from the published literature. Two previous systematic reviews were used as starting points to identify suitable RCTs: a 2019 systematic literature review and indirect treatment comparison of FDI and FCMCitation33, and a 2020 network meta-analysis that investigated hypophosphatemia risks after IV iron treatmentCitation34. Two further reviews were also used to ascertain relevant clinical information regarding IV-infusion related hypophosphatemia and safetyCitation22,Citation35. Supplementary searches were then performed to identify RCTs published after the search dates employed in the previous systematic literature reviews.
As the PHOSPHARE-IBD trial demonstrated that treatment with FDI or FCM results in similar hematological response, no differences between the formulations were modeled either in terms of the initial increase in hemoglobin levels, nor in the time to recurrence of anemia.
Baseline patient data, including mean age (42.1 years), bodyweight (80.2 kg) and pre-treatment hemoglobin levels (10.5 g/dL), were obtained from the PHOSPHARE-IBD trialCitation21 (). All patients (100%) in the cohort experienced IDA, with a median time to iron retreatment of 16 months (95% confidence interval: 7-24 months)Citation28. The proportion of patients experiencing any hypophosphatemia (s-phosphate <2.0 mg/dL) was also based on data from the PHOSPHARE-IBD trial (data by day 14)Citation21, specifically 2.2% of patients treated with FDI and 45.8% of patients treated with FCM. Patients experiencing hypophosphatemia were conservatively assumed not to develop hypophosphatemic osteomalacia or experience an increased risk of fracture. Finally, background mortality was modelled using UK life tablesCitation36.
Perspective, time horizon, and discounting
Costs were evaluated from the perspective of NHS England (specifically the DHSC) and calculated in 2022 GBP over a five-year time horizon. Costs were then also evaluated separately, using a micro-costing approach, from the perspective of healthcare providers in NHS England (i.e. individual NHS trusts), with costs again calculated in 2022 GBP over a five-year time horizon. This time horizon was considered sufficient to model the chronic nature of the IDA, capturing multiple IDA treatment cycles even at the upper bound of the 95% confidence interval around the retreatment frequency. The model used cycle lengths of one month, allowing for accurate modeling of multiple treatment courses and gradual, intra-annual reversion to baseline hemoglobin (and QoL) levels, whilst reducing the computational burden relative to shorter cycle lengths. Future costs and effects were discounted at 3.5% per annumCitation37. For this study, a willingness-to-pay (WTP) threshold of GBP 20,000 was used, in line with the most conservative end of the range between GBP 20,000 and GBP 30,000 specified in the NICE Guide to the Methods of Technology AppraisalCitation38.
Resource use
For patients treated with FCM, one phosphate test was assumed to be administered after the first IV iron infusion; patients who were found to be hypophosphatemic would then receive either oral or IV phosphate replenishment depending on the severity of hypophosphatemia, followed by a second phosphate test to check recovery of phosphate levels (). In those patients requiring a second IV iron infusion, an additional phosphate test would then also be conducted after the infusion. These assumptions were based on treatment recommendations from the literature and monitoring recommendations from a 2020 MHRA Drug Safety Update describing the risk of symptomatic hypophosphataemia leading to osteomalacia and fractures after treatment with FCMCitation39,Citation40. Conversely, no phosphate monitoring was assumed to take place in patients treated with FDI. Furthermore, the proportion of patients treated with FDI receiving either oral or IV phosphate for hypophosphatemia was assumed to be 0% on the grounds that no phosphate monitoring would take place and no severe—and therefore potentially symptomatic—hypophosphatemia occurred in FDI-treated patients in the PHOSPHARE-IBD trial. In FCM-treated patients, the proportion with moderate hypophosphatemia receiving oral phosphate replenishment was assumed to be 100% in line with a hypophosphatemia management algorithm proposed by Kassianides and BhandariCitation39. The mean number of oral phosphate doses required to treat patients experiencing moderate hypophosphatemia was calculated to be 105 based on data from a previously-conducted systematic review and meta-analysisCitation41. For FCM-treated patients experiencing severe hypophosphatemia, a mean of 4.4 phosphate infusions was assumed to be administered based on data from real-world study conducted in a UK tertiary centreCitation42. The proportion of modeled patients receiving either FDI or FCM who subsequently experienced either any or severe hypophosphatemia can be found in , alongside the serum phosphate levels used to define each category of severity for hypophosphatemia.
Health-related quality of life data
Quality of life data was based on the PHOSPHARE-IBD RCTCitation32, which used the SF-36v2 outcome measure to obtain health state utilities from patients with IBD and IDA across 20 outpatient hospital clinics across Austria, Denmark, Germany, Sweden and the UKCitation20. Patients in this trial received either FDI or FCM at baseline and at day 35, using identical weight- and hemoglobin-based regimes. Differences in QoL for FDI and FCM recipients were calculated based on the reported SF-6D utility values (Supplementary Figure 1). Specifically, SD-6Dv2 health state utility values (HSUVs) were derived from ten items from the SF-36v2, which were combined to form six dimensions of health, with 5-6 health levels within each dimension. UK-specific utility weights were then used to derive the final SF-6Dv2 HSUVs, which involved a summation of the weighted score on each dimension. All weights were negative in value, detracting from the initial utility score of 1 representing perfect health. A further utility decrement was added on if the worst score was experienced on any dimension, as detailed by Mulhern et al. in their derivation of a UK-specific value set for the SF-6Dv2Citation43. The resulting utility values were assumed to capture all disease-related differences in QoL, including any differences in QoL arising from the significant differences in the incidence of hypophosphatemia with FCM versus FDI, and any differences in the effects of IDA and IBD on QoL. In addition to these disease-related HSUVs from PHOSPHARE-IBD, an additional “process disutility” was associated with receiving additional iron infusions. Process (dis-)utilities typically arise from patients experiencing reductions in quality of life as a direct result of receiving treatment (i.e. interacting with the healthcare system) rather than as a result of the underlying course of the disease or treatment-related adverse eventsCitation44. A published diminishing marginal disutility model (DMDM) was used to capture infusion-related QoL, and patients receiving more IV iron infusions therefore experienced a marginal disutility that diminished logarithmically as the number of infusions increased. This was designed to reflect how patients receiving multiple IV iron infusions may, over time, become accustomed to the infusion process and therefore perceive each additional infusion as having a smaller detrimental effect on QoL than the lastCitation45. The rationale for incorporating the DMDM was to more accurately reflect the patient experience and perception of IV iron infusions and not to overestimate the QoL benefit of any reduction in the number of infusions required.
National payer perspective costing
The main source of costs for delivery of FDI and FCM was the 2022/2023 National Tariff Payment System, with the July 2022 revised cost uplift factor includedCitation46.
In the primary base case analysis, the DHSC perspective was adopted when considering costs. Here, the cost of each iron infusion was calculated using the five healthcare resource group (HRG) codes for IDA, specifically SA05G-L (“Iron Deficiency Anaemia” with varying comorbidity and complication levels). The five codes were weighted by recent activity data from the National Cost Collection for the NHS, ultimately yielding a cost of GBP 322.09 per infusion. Market Forces Factors (MFFs) were not applied to the base tariffs, leading to a conservative analysis with regard to the absolute and incremental costs of treatment with FDI versus FCM.
Costs of administering intravenous phosphate were similarly calculated using the five HRG codes for “Fluid or Electrolyte Disorders, without Interventions”, specifically KC05J-N. The final cost per phosphate infusion was determined to be GBP 304.00. Additionally, serum phosphate tests were assumed to cost GBP 12.50 each, calculated based on a laboratory test costing GBP 4.00, alongside 10 min of Band 6 nurse time (at a rate of GBP 51 per hour), in line with costs obtained from the Personal Social Services Resource Unit (PSSRU)Citation47. Finally, oral phosphate costs per dose were GBP 0.16, based on the BNF price and dosage data for Phosphate Sandoz effervescent tabletsCitation48. All costs were presented in 2022 GBP.
Provider perspective micro-costing
Micro-costing is a cost estimation method in which the costs of all labour and materials used in the treatment of a patient are enumerated, and is therefore well-suited for evaluating costs from a healthcare provider perspective (e.g. individual NHS trusts)Citation49. This in turn allows for a more precise assessment of the economic costs of competing health technologies, and subsequent efficient allocation of scarce resources at the provider levelCitation50. In the present analysis, the model captured three aspects relating to the administration of IV iron: IV iron list prices, HCP time, and ancillary costs.
The cost of each iron formulation was obtained from the March 2023 version of the British National Formulary (BNF), which reported a consistent per-gram price of GBP 169.50 for FDI across vial sizes of 100 mg, 500 mg, and 1000 mgCitation51. The per-gram price of FCM differed by vial size; 100 mg and 500 mg vial sizes were priced at GBP 191.00 per gram, while the 1000 mg vial size was priced at GBP 154.23 per gramCitation52. A weighted average cost of FCM was therefore derived using these per-gram prices combined with processed pharmacy stock control data from the NHS Business Services Authority (specifically the Secondary Care Medicines Data) in Q4, 2022Citation52,Citation53. The final weighted FCM price per gram was GBP 167.60 (Supplementary Table S1).
For HCPs, the hourly cost of an NHS Band 6 nurse was obtained from the PSSRUCitation47, and applied for time spent before, during, and immediately after an iron infusion in line with the administration and monitoring times outlined in the respective summaries of product characteristics (SmPCs). Ancillary items included a giving set, cannula and dressing which were costed at GBP 4.44, GBP 2.94, and GBP 0.43, respectively. Ancillary item costs were obtained from a UK medical equipment supply company with experience in fulfilling NHS purchase ordersCitation54.
Societal perspective costing
The societal perspective analysis was formulated to capture direct medical costs from the national payer perspective in addition to costs of personal social services (PSS) borne by the NHS and costs of absenteeism from the workplace arising from time spent attending—and travelling to and from—iron infusion appointments. Costs of transportation borne by the NHS were assumed to be incurred in 3% of outpatient appointments at a cost of GBP 76 per round-trip, based on a 2021 NHS England reportCitation55. Costs of workplace absenteeism were based on a combination of 34.8 min of round-trip travel time based on a recent analysis of travel time for IV iron infusions, in addition to 15 min of preparation time, infusion time in line with the summaries of product characteristics, and 30 min of post-infusion observation timeCitation56. The average cost of labour per hour was taken to be GBP 24.47 based on 2023 unit labour costs data from the UK Office for National StatisticsCitation57.
Sensitivity analysis
Sensitivity analyses were conducted to investigate the extent to which model outcomes were affected by changes to specific model parameters. One-way sensitivity analyses (OWSA) included modifying the distributional and correlative assumptions around baseline bodyweight and hemoglobin levels. FDI and FCM costs per iron infusion and discount rates were also varied for the purposes of the OWSA. A PSA consisting of 1000 Monte Carlo iterations was then conducted. Here, key model parameters were sampled to investigate the effects of uncertainty surrounding pre-determined model inputs.
Results
Base case
The primary (national payer perspective) base case analysis was run with 1,000 patients. Relative to FCM, FDI resulted in a QALY gain of 0.075 QALYs, from 2.57 QALYs to 2.65 QALYs () per patient. Patients treated with FDI required 1.63 fewer iron infusions versus FCM over the five-year time horizon, resulting in infusion-related cost savings of GBP 496 per patient (GBP 2,188 for FCM versus GBP 1,692 for FDI). Hypophosphatemia monitoring and treatment costs after FCM treatment were GBP 226 resulting in total cost savings of GBP 722 per patient (GBP 2,414 for FCM versus GBP 1,692 for FDI) over the five-year time horizon (). FDI was therefore the dominant intervention, demonstrating increased clinical benefit at a lower cost than FCM.
Table 2. Key health economic results from the base case, micro-costing analysis, and societal perspective analysis.
Table 3. Cost breakdown for the base case, micro-costing analysis, and societal perspective analysis.
Provider perspective micro-costing analysis
The micro-costing analysis showed cost savings of GBP 276 per patient for FDI over a five-year time horizon (GBP 1,586 FCM versus GBP 1,310 for FDI ()). The cost of FCM was marginally lower than FDI at GBP 961 versus GBP 980; however, total HCP time costs were higher with FCM at GBP 346 versus GBP 289 with FDI. Ancillary costs were also marginally higher for FCM (GBP 53) than FDI (GBP 41). Based on these figures, considering exclusively iron procurement and administration, FDI would result in a small saving compared to FCM (GBP 51); however, the additional costs associated with hypophosphatemia after administration of FCM (GBP 226) resulted in overall cost savings of GBP 276 with FDI compared to FCM ().
Societal perspective analysis
The societal perspective analysis showed an additional cost saving of GBP 63 with FDI versus FCM beyond the primary analysis from the national payer perspective, and reported total costs of GBP 2,692 with FCM versus GBP 1,907 with FDI and cost savings of GBP 785 with FDI versus FCM. The modeled QALY gains with FDI versus FCM were the same as in the national payer perspective analysis, thereby yielding a result in which FDI was also dominant relative to FCM from the societal perspective.
Probabilistic sensitivity analyses
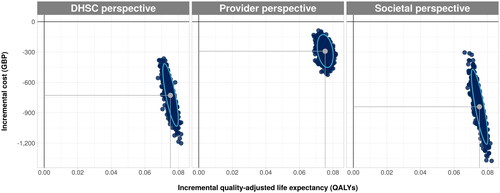
In all PSA iterations, ICURs fell within the south-eastern quadrant of the cost-utility plane, demonstrating an increase in QALY gains and cost savings for FDI from the national payer, provider, and societal perspectives (). FDI was therefore 100% likely to be cost-effective at all WTP thresholds between GPB 0 and GBP 50,000.
Figure 3. Scatterplot from probabilistic sensitivity analysis showing the incremental cost and quality-adjusted life year outcomes with ferric derisomaltose versus ferric carboxymaltose from the DHSC, provider, and societal perspectives.
Abbreviations. DHSC, Department of Health and Social Care; GBP, pounds sterling; QALYs, quality-adjusted life years.
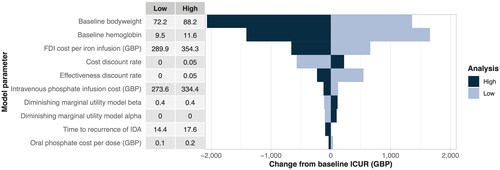
One-way sensitivity analysis
For the one-way sensitivity analyses (OWSA), the ten parameters with the largest impact on the final base case ICUR are presented in . Baseline bodyweight was associated with the greatest variation in the final ICUR compared to the base case results. For instance, a baseline bodyweight of 72.18 kg increased the ICUR by GBP 1359 per QALY gained, while a baseline bodyweight of 88.22 kg resulted in a reduction in the ICUR of GBP 2,070 per QALY gained. Baseline hemoglobin was associated with the second greatest variation in the final ICUR; a hemoglobin level of 9.45 g/dL increased the ICUR by GBP 1,658 per QALY gained, while a hemoglobin level of 11.55 g/dL decreased the ICUR by GBP 1,411 per QALY gained.
Discussion
In the present study, a previously-published, patient-level simulation model showed that FDI would lead to reduced costs and be more effective than FCM from the provider, DHSC, and societal perspectives. These results were driven primarily by data from the PHOSPHARE-IBD study of IDA patients with IBD and IDACitation20. Over a five-year time horizon, FDI was projected to result in a QALY gain of 0.075 QALYs versus FCM (from 2.57 QALYs to 2.65 QALYs) per patient. From the DHSC perspective, FDI also led to reduced costs of GBP 722 per patient (GBP 2,414 versus GBP 1,692) within the same five-year period. Based on these results, FDI was the dominant intervention compared to FCM, and was also less likely to result in patients experiencing hypophosphatemia. Relatedly, a significant factor that drove the higher costs of FCM was the need for hypophosphatemia monitoring in line with recommendations from the recent MHRA Drug Safety Update on FCMCitation40 and, in patients who went on to develop hypophosphatemia, phosphate replenishment. Specifically, FCM treatment resulted in hypophosphatemia-related costs of GBP 226, compared with no such costs in the FDI arm. Furthermore, patients would require 1.63 fewer infusions over the five-year time period if receiving FDI rather than FCM, resulting in reduced costs associated with administration of IV iron. Patients receiving FDI would also incur lower costs of transportation to and from hospital and would give rise to reduced absenteeism-related losses as a result of fewer hospital visits for treatment. These phenomena were captured in an analysis from the societal perspective in which the cost savings with FDI increased by GBP 63 relative to the DHSC perspective analysis.
To our knowledge, this is the first cost-utility analysis (as distinct from cost-effectiveness) of FDI versus FCM to be conducted in a European healthcare setting. The cost results were in line with much of the established health economic literature on the cost of FDI versus FCM, with one study demonstrating the cost savings arising primarily from the reduced number of infusions required with FDI versus FCM in patients with IBD and IDA across Norway, Sweden and FinlandCitation13. Similarly, another study assessing the cost-effectiveness of FDI versus FCM in a general IDA population in the UK found FDI to be the dominant strategy, whilst reducing the number of infusions requiredCitation58. The healthcare systems and patient populations differed across these previous studies; however, the results demonstrate that FDI is likely to be cost saving and/or cost-effective versus FCM across a wide range of etiologies and in diverse healthcare systems. It is also worth noting that cardiovascular (CV) event incidence and hypersensitivity reactions (HSRs)—both of which can occur after administration of IV irons—were not modeled as part of this analysis. However, recent indirect comparisons suggest that the incidence of both CV events and HSRs is rare after administration of IV iron formulationsCitation59,Citation60.
Conversely, there are three previous studies that investigate the cost-effectiveness of FDI and FCM in patients with IBD and IDA, finding FCM to be cost-effective versus FDICitation30,Citation61,Citation62. The studies all present cost-per-responder analyses covering Sweden, Switzerland and the UK. While these studies report results contrary to those in the present analysis, the reason for the discrepancy stems from the source of the efficacy data employed; specifically, all three studies relied on a network meta-analysis (NMA) as the source of the efficacy data of each iron formulationCitation63. Based on the current literature, NMAs should account for variations in doses and baseline patient characteristics across studiesCitation64. Notably, however, the NMA in question did not account for heterogeneity, and was not adjusted with regards to dose differences nor baseline hemoglobin levels across the patient samples for included studies. Thereby, the analysis overlooked meaningful predictors of hematological response, especially given the well-established dose-response relationship in the administration of IV iron. Higher average doses of iron were administered in the studies of FCM compared with the single study of FDI included in the NMA, and patients therefore experienced commensurately larger increases in hemoglobin levels with FCM. The assumed differences in efficacy, and therefore also the modeled cost-effectiveness of FCMCitation30,Citation61,Citation62 were therefore likely attributable simply to FCM being delivered at higher doses than FDI, rather than any fundamental differences in the pharmacodynamic properties or efficacy of the iron formulations. Indeed, the head-to-head PHOSPHARE-IBD RCT showed no significant differences in hematological response between FDI and FCMCitation21.
The majority of limitations of the present analysis were attributable to a lack of publicly available data, which resulted in either proxy data being incorporated into the model, or certain aspects being omitted entirely (provided said aspects would likely have a limited impact on the final results). For example, where possible, UK-specific data for the clinical and cost inputs for the model were used. However, the PHOSPHARE-IBD RCT used to inform the disease-related QoL estimates was not UK-specific, although it did include a subset of patients in the UK; twenty outpatient hospitals enrolled patients for the trial spanning Austria, Denmark, Germany, Sweden and the UK. The UK IDA-IBD patient population may therefore differ from the overall PHOSPHARE-IBD population, and this should be considered when interpreting the findings. A similar limitation was also present in the data source for infusion-associated disutilities, which were sourced from a time trade-off study conducted in Chinese patientsCitation27,Citation45.
The micro-costing analysis was also subject to data limitations, with data on the costs of ancillary items (giving sets, cannulas, needles, etc.) notably being unavailable from publicly-available NHS sources. These data were therefore obtained from a UK medical supply company with a history of fulfilling NHS purchase orders. Whilst this data source is likely to reflect costs similar to those paid by the NHS for ancillary items, bulk-ordering and privately-negotiated discounts could not be captured accurately and the ancillary cost estimates may therefore represent an overestimation of the actual costs borne by the NHS; results should be interpreted with this in mind.
The analysis also omitted downstream sequelae of hypophosphatemia such as incidence of osteomalacia and fractures. While hypophosphatemic osteomalacia was one of the key foci of the 2020 MHRA Drug Safety Update on FCM, the incidence data were based on spontaneous event reporting, making modelling challengingCitation40. Nevertheless, considering the significantly higher rates of hypophosphatemia with FCM versus FDI, incidence of osteomalacia would also likely be higher in patients receiving FCM versus FDI and the present analysis was therefore likely to have been conservative in this regard. Data on fracture risk was covered similarly in the MHRA Drug Safety Update (i.e. based on spontaneous event reporting), and robust data showing significantly elevated risk of fractures, radiological signs of osteomalacia, and kidney stones in patients treated with FCM versus FDI has only recently started to emergeCitation40,Citation65.
Patients with hypophosphatemia may also experience symptoms such as fatigue, weakness and irritabilityCitation41,Citation66. Whilst the effects of fatigue and weakness on patient QoL are likely to have been well-captured in the present analysis through the incorporation of the SD-6Dv2 HSUVs and specifically the vitality domains of the SF-36v2, irritability is less likely to have been captured holistically. As with osteomalacia, it is probable that had irritability been captured within the model, FDI would be deemed to be even more cost-effective than the results presented here suggest.
A further limitation pertaining to the lack of robust data was that adherence and appointment attendance rates were also not captured in the present analysis. As the analysis demonstrated that FDI increased the proportion of patients able to achieve iron replenishment with a single infusion relative to FCM, incorporating adherence would be likely to affect the results although characterizing the effect on QALY outcomesCitation26. Relatedly, the model did not capture any clinical discretion regarding the decision to administer more than one dose of iron; where any outstanding iron need after a first iron infusion is only a small fraction of the overall calculated iron need, clinicians may exercise the discretion to forego the second iron infusion.
One final data-related limitation was with regards to resource utilisation, specifically that the number of iron infusions administered over the five-year period may have been overestimated due to a strict alignment of the number of infusions in each treatment course with the SmPCs. Reduced patient compliance in real-world clinical scenarios may lead to fewer infusions being received during each treatment course, but the lack of publicly-available, UK-specific compliance data in the literature led to the omission of compliance from the final analysis.
A key strength of the study was that the main resource use and costing data used were UK-specific, with data sources used including the BNF, 2022/2023 National Tariff Payment System, and product SmPCs. Proxy model inputs for costs, clinical and QoL data were therefore only used where it was deemed necessary due to a lack of more relevant data sources. In these cases, special consideration was given to ensure that as many sourced model inputs as possible were UK-specific or UK-inclusive. The projected cost savings and QALY gains associated with FDI are therefore likely to be as representative of the UK IDA-IBD patient population and NHS as permitted by the available data and literature.
The analyses included a micro-costing analysis to evaluate the economic benefit of FDI versus FCM from the healthcare provider perspective. The results from this analysis demonstrated that FDI was also the dominant strategy when provider-level costs of IV iron, consumables, and HCP time were captured in the cost-utility model. The micro-costing analysis thereby demonstrated that the key finding of FDI being the dominant intervention was consistent even from an alternative payer perspective (i.e. that of the healthcare provider).
Finally, the model utilised in the present analysis captured patient-level heterogeneity, first-order uncertainty (i.e. random walk), and stochastic variation at the parameter levelCitation65. This approach resulted in a more comprehensive representation of the uncertainty around individual patient trajectories versus a cohort-level modeling approachCitation67. The additional incorporation of hypophosphatemia and its associated impact on QoL, treatment and monitoring costs represented a meaningful advance on previous health economic studies of FDI versus FCM that have focussed exclusively on differences in the posology of the iron formulations and modeled differences in hematological response.
Conclusion
The analysis showed that FDI would improve QoL and reduce direct healthcare expenditure versus FCM in patients with IBD and IDA in the UK. Cost savings with FDI were driven by reductions in the number of infusions required and the lack of need for monitoring and treatment of hypophosphatemia.
Transparency
Author contributions
RFP, NK, TI, and AD conceived of and designed the analysis. RFP developed the simulation model and conducted the statistical and health economic analyses with clinical input from NK, TI, and AD. WA conducted sensitivity analyses, generated tables and figures, and prepared the first draft of the manuscript, which was revised critically for intellectual content by all authors. All authors approved the final version to be published. All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work. One of the reviewers has disclosed that they have been involved in research into the safety of these drugs. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Previous presentations
Select analyses presented here were presented previously at ISPOR Europe 2022 (Vienna, November 6 to November 9, 2022) as poster EE395.
Supplemental Material
Download MS Word (93.3 KB)Declaration of financial/other relationships
RFP is a full-time employee, director, and shareholder in, and WA is a full-time employee of, Covalence Research Ltd, which received consultancy fees from Pharmacosmos A/S to develop the patient-level simulation model and analysis and prepare the manuscript. TI has attended paid advisory boards for Pharmacosmos A/S and/or its subsidiaries and has received fees for presenting at academic meetings. NK has received speaker honoraria and support for meetings and/or travel from Pharmacosmos A/S, and has participated in an advisory board for Pharmacosmos A/S. AD has received speaker honoraria and support for meetings and/or travel from Pharmacosmos A/S and/or its subsidiaries.
Additional information
Funding
References
- McDowell C, Farooq U, Haseeb M. Inflammatory bowel disease. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematol. 2019;142(1):30–36. doi: 10.1159/000496728.
- Gasche C, Lomer MCE, Cavill I, et al. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53(8):1190–1197. doi: 10.1136/gut.2003.035758.
- Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(12):1545–1553. doi: 10.1002/ibd.20285.
- Hodges P, Sauriol D, Man SF, et al. Vitamin and iron intake in patients with Crohn’s disease. J Am Diet Assoc. 1984;84(6):664–669. doi: 10.1016/S0002-8223(21)08097-4.
- Madu AJ, Ughasoro MD. Anaemia of chronic disease: an in-depth review. Med Princ Pract. 2017;26(1):1–9. doi: 10.1159/000452104.
- Murawska N, Fabisiak A, Fichna J. Anemia of chronic disease and iron deficiency anemia in inflammatory bowel diseases: pathophysiology, diagnosis, and treatment. Inflamm Bowel Dis. 2016;22(5):1198–1208. doi: 10.1097/MIB.0000000000000648.
- Martin J, Radeke HH, Dignass A, et al. Current evaluation and management of anemia in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2017;11(1):19–32. doi: 10.1080/17474124.2017.1263566.
- Erichsen K, Hausken T, Ulvik RJ, et al. Ferrous fumarate deteriorated plasma antioxidant status in patients with crohn disease. Scand J Gastroenterol. 2003;38(5):543–548. doi: 10.1080/00365520310000771.
- Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. PubMed [Internet]. [cited 2022 Oct 12]. Available at https://pubmed.ncbi.nlm.nih.gov/8592524/.
- Baird-Gunning J, Bromley J. Correcting iron deficiency. Aust Prescr. 2016;39(6):193–199. doi: 10.18773/austprescr.2016.069.
- Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. JPAC - transfusion guidelines. 2014. [cited 2022 Oct 12]. [Internet]. Available at https://transfusionguidelines.org.uk/.
- Pollock RF, Muduma G. An economic analysis of ferric derisomaltose versus ferric carboxymaltose in the treatment of iron deficiency anemia in patients with inflammatory bowel disease in Norway, Sweden, and Finland. Clinicoecon Outcomes Res. 2021;13:9–18. doi: 10.2147/CEOR.S284959.
- Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26(4):266–275. doi: 10.1097/MNH.0000000000000329.
- Emrich IE, Lizzi F, Siegel JD, et al. Hypophosphatemia after high-dose iron repletion with ferric carboxymaltose and ferric derisomaltose—the randomized controlled HOMe aFers study. BMC Med. 2020;18(1):178. doi: 10.1186/s12916-020-01643-5.
- Glaspy JA, Lim-Watson MZ, Libre MA, et al. Hypophosphatemia associated with intravenous iron therapies for iron deficiency anemia: a systematic literature review. Ther Clin Risk Manag. 2020;16:245–259. doi: 10.2147/TCRM.S243462.
- Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432–443. doi: 10.1001/jama.2019.22450.
- Adkinson NF, Strauss WE, Macdougall IC, et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: a randomized trial. Am J Hematol. 2018;93(5):683–690. doi: 10.1002/ajh.25060.
- Wolf M, Chertow GM, Macdougall IC, et al. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3(23):e124486. doi: 10.1172/jci.insight.124486.
- Pharmacosmos A/S. A randomized, double-blinded, comparative trial comparing the incidence of hypophosphatemia in relation to repeated treatment courses of iron isomaltoside and ferric carboxymaltose in subjects with iron deficiency anaemia due to inflammatory bowel disease. 2021. [Internet]. [cited 2022 Oct 11]. Available at https://clinicaltrials.gov/ct2/show/NCT03466983.
- Zoller H, Wolf M, Blumenstein I, et al. Hypophosphataemia following ferric derisomaltose and ferric carboxymaltose in patients with iron deficiency anaemia due to inflammatory bowel disease (PHOSPHARE-IBD): a randomised clinical trial. Gut. 2022;72(4):644–653. doi: 10.1136/gutjnl-2022-327897.
- Schaefer B, Tobiasch M, Wagner S, et al. Hypophosphatemia after intravenous iron therapy: comprehensive review of clinical findings and recommendations for management. Bone. 2022;154:116202. doi: 10.1016/j.bone.2021.116202.
- Huang LL, Lee D, Troster SM, et al. A controlled study of the effects of ferric carboxymaltose on bone and haematinic biomarkers in chronic kidney disease and pregnancy. Nephrol Dial Transplant off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2018;33:1628–1635.
- Schaefer B, Zoller H, Wolf M. Risk factors for and effects of persistent and severe hypophosphatemia following ferric carboxymaltose. J Clin Endocrinol Metab. 2021;107(4):1009–1019. doi: 10.1210/clinem/dgab852.
- Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose: a review of its use in iron-deficiency anaemia. Drugs. 2009;69(6):739–756. doi: 10.2165/00003495-200969060-00007.
- Dahlerup JF, Jacobsen BA, van der Woude J, et al. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand J Gastroenterol. 2016;51(11):1332–1338. doi: 10.1080/00365521.2016.1196496.
- Hu S, Liu L, Pollock RF, et al. Intravenous iron for the treatment of iron deficiency anemia in China: a patient-level simulation model and cost-utility analysis comparing ferric derisomaltose with iron sucrose. J Med Econ. 2022;25(1):561–570. doi: 10.1080/13696998.2022.2065092.
- Kulnigg S, Teischinger L, Dejaco C, et al. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol. 2009;104(6):1460–1467. doi: 10.1038/ajg.2009.114.
- Walidainy H, Zulfikar Z. An improved design of linear congruential generator based on wordlengths reduction technique into FPGA. IJECE. 2015;5(1):55. doi: 10.11591/ijece.v5i1.pp55-63.
- Aksan A, Schoepfer A, Juillerat P, et al. Iron formulations for the treatment of iron deficiency anemia in patients with inflammatory bowel disease: a cost-effectiveness analysis in Switzerland. Adv Ther. 2021;38(1):660–677. doi: 10.1007/s12325-020-01553-1.
- Table 12. Ganzoni formula for iron isomaltoside 1000 dosing [Internet]. Canadian Agency for Drugs and Technologies in Health; 2020. [cited 2023 Oct 31]. Available at https://www.ncbi.nlm.nih.gov/books/NBK564172/table/pe.app5.tab4/.
- Bennett CL, Westerman IL. Economic analysis during phase III clinical trials: who, what, when, where, and why. Oncol Williston Park N. 1995;9:169–175.
- Pollock RF, Muduma G. A systematic literature review and indirect comparison of iron isomaltoside and ferric carboxymaltose in iron deficiency anemia after failure or intolerance of oral iron treatment. Expert Rev Hematol. 2019;12(2):129–136. doi: 10.1080/17474086.2019.1575202.
- Bellos I, Frountzas M, Pergialiotis V. Comparative risk of hypophosphatemia following the administration of intravenous iron formulations: a network meta-analysis. Transfus Med Rev. 2020;34(3):188–194. doi: 10.1016/j.tmrv.2020.07.002.
- Blumenstein I, Shanbhag S, Langguth P, et al. Newer formulations of intravenous iron: a review of their chemistry and key safety aspects - hypersensitivity, hypophosphatemia, and cardiovascular safety. Expert Opin Drug Saf. 2021;20(7):757–769. doi: 10.1080/14740338.2021.1912010.
- WHO. Life tables by country (GHE: life tables). [Internet]. [cited 2022 Oct 21]. Available at https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-life-tables-by-country.
- NICE. Guide to the methods of technology appraisal 2013: process and methods [PMG9] [Internet]. London and Manchester: National Institute for Health and Care Excellence; 2013. [cited 2022 Dec 5]. Available at https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
- 5 The reference case. | Guide to the methods of technology appraisal 2013 | Guidance | NICE [Internet]. NICE; [cited 2022 Oct 18]. Available at https://www.nice.org.uk/process/pmg9/chapter/the-reference-case.
- Kassianides X, Bhandari S. Hypophosphataemia, fibroblast growth factor 23 and third-generation intravenous iron compounds: a narrative review. Drugs Context. 2021;10:1–29. doi: 10.7573/dic.2020-11-3.
- Medicines and Healthcare products Regulatory Agency. Drug safety update: ferric carboxymaltose (Ferinject▼): risk of symptomatic hypophosphataemia leading to osteomalacia and fractures [Internet]. GOV.UK; 2020. [cited 2021 Aug 12]. Available at https://www.gov.uk/drug-safety-update/ferric-carboxymaltose-ferinject-risk-of-symptomatic-hypophosphataemia-leading-to-osteomalacia-and-fractures.
- Schaefer B, Tobiasch M, Viveiros A, et al. Hypophosphataemia after treatment of iron deficiency with intravenous ferric carboxymaltose or iron isomaltoside-a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87(5):2256–2273. doi: 10.1111/bcp.14643.
- Fragkos KC, Sehgal V, Rogers J, et al. Hypophosphataemia after intravenous iron therapy with ferric carboxymaltose—real world experience from a tertiary Centre in the UK. GastroHep. 2020;2(5):205–214. doi: 10.1002/ygh2.415.
- Mulhern BJ, Bansback N, Norman R, et al. Valuing the SF-6Dv2 classification system in the United Kingdom using a discrete-choice experiment with duration. Med Care. 2020;58(6):566–573. doi: 10.1097/MLR.0000000000001324.
- Brennan VK, Dixon S. Incorporating process utility into quality adjusted life years: a systematic review of empirical studies. PharmacoEconomics. 2013;31(8):677–691. doi: 10.1007/s40273-013-0066-1.
- Hu S, Wu D, Wu J, et al. Disutilities associated with intravenous iron infusions: results from a time trade-off survey and diminishing marginal utility model for treatment attributes in China. Patient Relat Outcome Meas. 2023;14:253–267. doi: 10.2147/PROM.S400389.
- NHS England and NHS Improvement. National tariff payment system. [Internet]. London: NHS England and NHS Improvement; 2022/23. [cited 2022 May 26]. Available at https://www.england.nhs.uk/publication/national-tariff-payment-system-documents-annexes-and-supporting-documents/.
- Jones KC, Burns A. Unit costs of health and social care 2021. Kent, UK: Personal Social Services Research Unit; 2021.
- NICE. Phosphate [Internet]. [cited 2022 Oct 13]. Available at https://bnf.nice.org.uk/drugs/phosphate/medicinal-forms/.
- Xu X, Grossetta Nardini HK, Ruger JP. Micro-costing studies in the health and medical literature: protocol for a systematic review. Syst Rev. 2014;3(1):47. doi: 10.1186/2046-4053-3-47.
- Potter S, Davies C, Davies G, et al. The use of micro-costing in economic analyses of surgical interventions: a systematic review. Health Econ Rev. 2020;10(1):3. doi: 10.1186/s13561-020-0260-8.
- NICE. Ferric derisomaltose. [Internet]. [cited 2022 Oct 13]. Available at https://bnf.nice.org.uk/drugs/ferric-derisomaltose/medicinal-forms/.
- NICE. Ferric carboxymaltose [Internet]. [cited 2022 Oct 13]. Available at https://bnf.nice.org.uk/drugs/ferric-carboxymaltose/medicinal-forms/.
- NHS Open Data Portal. Secondary Care Medicines Data (SCMD): important update. [Internet]. 2020. [cited 2023 Feb 27]. Available at https://opendata.nhsbsa.net/dataset/secondary-care-medicines-data.
- HCE. NHS Orders. [Internet]. [cited 2022 Oct 18]. Available at https://www.hce-uk.com/NHS-Orders.
- NHS England. Improving non-emergency patient transport services: report of the non-emergency patient transport review; 2021. [Internet]. [cited 2023 Nov 27]. Available at https://www.england.nhs.uk/wp-content/uploads/2021/08/B0682-fnal-report-of-the-non-emergency-patient-transport-review.pdf.
- Ahmed W, Pöhlmann J, Brewerton S, et al. HSD114 reductions in travel time and carbon dioxide emissions arising from patient transportation to hospitals in England for treatment with intravenous iron: an analysis comparing ferric derisomaltose with ferric carboxymaltose. Value Health. 2022;25(12):S295. doi: 10.1016/j.jval.2022.09.1457.
- Office for National Statistics. Labour costs and labour income, UK; 2023. [Internet]. [cited 2023 Nov 27]. Available at https://www.ons.gov.uk/economy/economicoutputandproductivity/productivitymeasures/datasets/labourcostsandlabourshare.
- Pollock RF, Muduma G. A patient-level cost-effectiveness analysis of iron isomaltoside versus ferric carboxymaltose for the treatment of iron deficiency anemia in the United Kingdom. J Med Econ. 2020;23(7):751–759. doi: 10.1080/13696998.2020.1745535.
- Pollock RF, Biggar P. Indirect methods of comparison of the safety of ferric derisomaltose, iron sucrose and ferric carboxymaltose in the treatment of iron deficiency anemia. Expert Rev Hematol. 2020;13(2):187–195. doi: 10.1080/17474086.2020.1709437.
- Pollock RF, Kalra PA, Kalra PR, et al. A systematic review, meta-analysis, and indirect comparison of blindly adjudicated cardiovascular event incidence with ferric derisomaltose, ferric carboxymaltose, and iron sucrose. Adv Ther. 2022;39(10):4678–4691. doi: 10.1007/s12325-022-02242-x.
- Aksan A, Beales ILP, Baxter G, et al. Evaluation of the cost-effectiveness of iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease in the UK. Clinicoecon Outcomes Res. 2021;13:541–552. doi: 10.2147/CEOR.S306823.
- Ramirez de Arellano A, Norton N, Enkusson D, et al. Cost-effectiveness of intravenous iron formulations in patients with iron deficiency anaemia and inflammatory bowel disease, in a Swedish regional setting using real-world tender prices. GastroHep. 2022;2022:e9991311–10. doi: 10.1155/2022/9991311.
- Aksan A, Işık H, Radeke HH, et al. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(10):1303–1318. doi: 10.1111/apt.14043.
- Watt JA, Giovane CD, Jackson D, et al. Incorporating dose effects in network meta-analysis. BMJ. 2022;376:e067003. doi: 10.1136/bmj-2021-067003.
- Zoller H, Pammer LM, Schaefer B, et al. Incidence of fractures after intravenous iron: a retrospective analysis comparing ferric carboxymaltose and ferric derisomaltose. ASH; 2023. [cited 2023 Nov 10]. Available at https://ash.confex.com/ash/2023/webprogram/Paper174508.html.
- Ifie E, Oyibo SO, Joshi H, et al. Symptomatic hypophosphataemia after intravenous iron therapy: an underrated adverse reaction. Endocrinol Diabetes Metab Case Rep. 2019;2019:19.
- Davis S, Stevenson M, Tappenden P, et al. NICE DSU technical support document 15: cost-effectiveness modelling using patient-level simulation. Sheffield; 2014.