 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and purpose
The incidence of end-stage renal disease (ESRD) in Sudan is increasing, affecting the economic status of patients, caregivers and society. This study aimed to measure ESRD’s costs, including direct and morbidity indirect expenditures, and to investigate any associated factors and financial consequences.
Materials and methods
This cross-sectional study used a standardized questionnaire to collect data from 150 ESRD patients who had been receiving dialysis for at least one year before the time of data collection at 13 specialized renal centres in Khartoum state. Data about sociodemographic, clinical, and economic factors were gathered, and their relationship to the cost of ESRD was examined using both bivariate (Man Whitney test, Kruskal Wallis test and Spearman correlation) and multivariate analytical procedures (multivariate linear regression).
Results
This study reported a median direct per capita ESRD cost of 38 600 SDG ($1 723.2 PPP) annually with an interquartile range of 69 319.3 SDG ($3 094.6 PPP). The median morbidity indirect cost was estimated to be 0.0 ± 3 352 SDG ($ 0.0 ± 149.6 PPP) per annum. In 28.8% of cases, the patients were their family’s primary income earner and over 85% were covered by medical insurance. Our study found that none of the study variables were significantly associated with the total cost of ESRD.
Conclusion and limitations
Our findings point out considerable direct out-of-pocket expenses and productivity losses for patients and their households. However, these results should be carefully applied for comparison between the different countries due to differences in the cost of medical interventions and insurance coverage. Further longitudinal studies and studies on health finance and insurance policies are recommended.
PLAIN LANGUAGE SUMMARY
As medical care costs continue to rise in Sudan, this study aimed to estimate direct and indirect costs associated with end-stage renal disease (ESRD) from the patient’s perspective. Accurate information on the cost of illness (COI) helps policymakers prioritize healthcare services and resources, optimize public well-being, and determine health policy effectiveness. Future research should include a longitudinal design and understand ESRD costs from caregivers’ and healthcare providers’ perspectives.
1. Introduction
Chronic kidney disease (CKD) is a spectrum of abnormalities in the structure and function of the kidneys that last for three months or moreCitation1. Diagnosis of CKD relies on two main criteria: a glomerular filtration rate (GFR) of 60 ml/min or less per 1.73 m2 Citation2 -indicating reduced kidney function- and evidence of kidney damage, often assessed through elevated urine albumin or protein to creatinine ratio (ACR) exceeding 30 mg/g)Citation3. GFR is the most reliable measure of renal functionCitation2.
The progression of CKD can eventually lead to irreversible End-Stage Renal Disease (ESRD), which has become a significant public health concern in Sub-Saharan Africa in recent years. The precise prevalence of ESRD in the region is not well-established due to the lack of reliable health information systems. The overall prevalence of CKD was estimated to be 13.9% based on data from 13 Sub-Saharan countriesCitation4. End-stage renal disease necessitates life-sustaining therapy- also called renal replacement therapy- in the form of dialysis or kidney transplantation. Both are expensive and burdensome financially in Sub-Saharan AfricaCitation4,Citation5. The term "burden of disease" refers to a broad range of factors that negatively influence health at the population, regional, community or individual levelCitation6. In the context of CKD and ESRD, the burden includes the direct medical cost associated with investigations, management, and treatment encompassing expenses related to medical imaging, lab tests, drugs, surgical procedures (e.g. fistulas) and dialysis. Additionally, there are direct non-medical costs incurred by patients and their caregivers, such as transportation and food expenses and other expenses that are non-medical but related to the disease. On the other hand, indirect costs encompass the economic losses caused by loss of productivity or missed work daysCitation7.
Annually, CKD and ESRD patients face significant financial burdens including the expenses of CKD identification and management, ESRD treatment, and comorbid diseases management. Furthermore, these diseases result in substantial productivity losses for patients and their caregivers due to absenteeism, presentism and premature death. The progression and the growing prevalence of chronic kidney disease raise concerns about our capacity to manage its economic burden on patients, caregivers, and society. Research results on direct and indirect expenses associated with CKD and end-stage renal disease are highly variable, with the most comprehensive information focusing on direct healthcare costs of patients who progressed to end-stage renal disease. Addressing these gaps in knowledge is crucial for guiding clinical practice and policy developmentCitation8. Cost analysis is a valuable instrument for the decision-making process in the healthcare sector. It is vital to comprehend and deal with the definitions, categories, and types of study approaches addressing the Cost of Illness (COI), to thoroughly analyze the cost firmly. Precise and accurate information on the cost of illness enables policymakers to create, prioritize, and distribute healthcare services and resources according to budgetary limits; optimizing public well-being and achieving economic efficiencyCitation9,Citation10. Cost estimates also provide valuable insights into the need for prioritizing policies that address the disease and its associated factors on the health policy agenda, evaluating the effectiveness of health policies, programs, and interventions, and facilitating cross-national comparisons of disease outcomes and treatment options. Based on the patient’s perspectives, this study aimed to estimate direct and morbidity indirect costs (loss of productivity) of ESRD in Khartoum state using an adapted standardized cost of illness tool and to investigate the factors that may affect the cost of illness.
2. Materials and methods
2.1. Study design and setting
A cross-sectional, health facility-based study was conducted at 13 renal centers in Khartoum state, Sudan in 2020. These centers provide dialysis services to registered renal patients as well as emergency cases. Among the 32 renal centers in Khartoum state, only 13 centers were selected for the study as these were the ones that had patients attending follow-up visits during the study period. Renal centers included in the study were Alwaledeen, Tropical disease, Alnaw, Ombada, Chinese Friendship, Military Hospital, Academy, Elshahida Salma, Bashaier, Ibnsina, Elsafia, Renal Transplant Association, and Ahmed Gasim.
2.2. Study participants
The study sample consisted of 150 ESRD males and females who were on dialysis for a minimum of one year before the time of data collection in 2020. Data collection took place over one month, and the cost estimation was conducted for the preceding year. Patients below 18 years of age, those severely ill -who couldn’t properly communicate and respond appropriately-, or those who declined to participate were excluded from the study.
2.3. Sample size and sampling technique
The study sample was calculated using the single proportion formula (Cochran equation)Citation11, using a 95% level of confidence, a proportion of 0.091 (based on the global prevalence of ESRD in 2017Citation12), and an accepted sample error of 0.05:
n = sample size
t = standard error associated with the chosen level of confidence (%95)
p = proportion of the population (prevalence)
q = 1-p
d = accepted sample error
The calculated sample size was 127. Accounting for a 10% non-response rate, the final sample size was set at 150 patients. The sample was distributed proportionally based on the average patient frequency at each facility, with the interval for each center determined as shown in . For each renal facility, random days of the week were selected to conduct the interviews. Each center had a list of patient appointments for each day of the week. We utilized the patients’ list of each selected day as the sample frame and then approached the patients as they arrived for their consultations by selecting systematically from the list until the recruitment target was reached.
Table 1. Number of end-stage renal disease patients in the 13 dialysis centres in Khartoum state and the size of the sample chosen from each centre.
2.4. Study tools
A face-to-face interview questionnaire was used. Informed consent was obtained on the same day the interview was performed. A standardized adapted questionnaire developed by the Koninklijke Nederlandse Chemische Vereniging (KNCV) or Royal Dutch Chemical Association (Tuberculosis Foundation), the World Health Organization, and the Japan Anti-Tuberculosis Association was used after validation and pilotingCitation13. We reviewed patients’ medical files to record patients’ medical information. The questionnaire was divided into three sections, and the first section contained sociodemographic and economic data about ESRD patients and their primary income earners. The second section contained indicators to measure the direct cost from the patient’s perspective, including tests, imaging, drug, travel, food, food supplements, accommodation, caregiver, and hospitalization costs, as well as costs related to dialysis and follow-up tests. The third section contained indicators to measure indirect cost (loss of productivity). The annual morbidity indirect cost for each patient and caregiver was calculated based on the Human Capital Approach by multiplying the number of days lost per year by their daily income. Additionally, information regarding illness duration, comorbidities, and economic status of ESRD patients were investigated due to their potential impact on the outcomes. We used purchasing power parity (PPP) in 2020 which was 22.4 to change the currency from Sudanese pounds to United States dollars. We used STROBE reporting guidelines for cross-sectional studies to ensure proper reporting for the studyCitation14.
2.5. Statistical analysis
Statistical Package for the Social Sciences (SPSS) ® version 28 was used for data entry and statistical analysis. Percentages, frequencies, means, and standard deviations were used to characterize demographic and medical information of ESRD patients’ features, clinical information, and different aspects of ESRD cost. The direct cost was calculated by summation of its components which includes: diagnostic cost, dialysis cost, direct caregiver cost, hospitalization cost, supplement cost, follow-up cost, medications cost, and fistula cost. The indirect cost for the patient has been estimated by first knowing the time spent by the patient in diagnosis, dialysis, and follow-up visits and finding out the number of days lost per year due to these components. Then we multiplied the number of days lost per year by the income of the patient per day to find the indirect cost for each working patient per year, the same method was used for the caregiver indirect cost, then we calculated the mean, standard deviation, the median and the interquartile range. Missing data for food and travel costs, accounting for 12.6% and 6.6% of the total data respectively, was imputed using the sample mean due to difficulties in recalling the costs. Man Whitney test, Kruskal Wallis test and Spearman correlation were applied to examine relationships among demographic, medical, social, and economic characteristics with ESRD cost. Variables with p value of <0.2 with the total cost in the bivariate analysis were included in the multivariate analysis using a linear regression model except for “stopping school and work due to renal failure” because they don’t include all the study population. A 2-sided P value <0.05 was considered statistically significant.
3. Results
3.1. ESRD socio-economic patients` and household characteristics
We interviewed 150 ESRD patients, of whom 90.7% lived in urban areas. The most frequent age group was 51–60 years (26%), and the majority of patients were males (61.5%). (). Over three-quarters (85%) of patients had medical insurance, with the majority (92.9%) having public insurance, and less than 15% were uninsured. At the time of data collection, nearly half of the study members were off work for one year, with two-thirds attributing their absence to renal disease. () The patient’s household earned an average income of 6,881.8 SDGs ($307.2 PPP) per month, and in 28.8% of cases, the patients were the family’s primary income earner. Non-patient household income, governmental assistance, welfare payments, and other sources provided the rest. ()
Figure 1. Average income sources in USD among households of families of end stage renal disease patients in Khartoum, Sudan 2019 (n = 150).
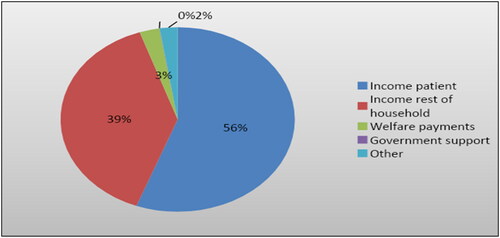
Table 2. Socio-demographic characteristics, insurance status and employment analysis and their association with total ESRD cost rank using analysis of Man Whitney testa, Kruskal-Wallis testb, and spearman correlationc test in Khartoum, Sudan 2019 (n = 150).
3.2. ESRD patients’ clinical information
The mean duration of ESRD among the participants was (4.1 ± 3.8) years, and nearly half had a history of renal impairment before ESRD. The majority of patients (70.7%) had concomitant hypertension, while (15%) had concomitant diabetes. All patients received their regular dialysis sessions at public facilities, with (65.8%) receiving them at the same facility every time. Including transportation and waiting time, a dialysis visit took (5.6 ± 2.0) hours on average. () Almost two-thirds of ESRD patients (60.7%) spent (30.1 ± 52.4) days of hospitalization on average annually, with a mean frequency of (2.4 ± 3.5) times in the last year. ()
Table 3. Renal failure duration, comorbidities and dialysis information and their association with total ESRD cost rank using analysis of Man Whitney testa, Kruskal-Wallis testb, and spearman correlationc test in Khartoum, Sudan 2019 (n = 150).
Table 4. Hospitalization information among ESRD patients and their association with total ESRD cost rank using analysis of man whitney testa, and spearman correlationc test in Khartoum, Sudan 2019 (n = 150).
3.3. ESRD cost estimation and analysis
ESRD's total direct cost median was found to be 38,600 ± 69 319.3 SDGs, with the highest cost attributed to the fistula insertion (11,400), followed by diagnostic procedures (7,850) and dialysis (7,280), respectively. () Patients of ESRD and their caregivers lost an average of (54.1 ± 49.1) and (33.4 ± 42.1) working days per year, respectively. Therefore, the median morbidity indirect cost of ESRD was (0.0 ± 3 352.2) SDGs annually. () This cost is only calculated for those who had work and the small median of the indirect cost occurred because most of the patients were taking permissions and their salary did not reduce because of the hospital visit, Also most of the caregivers (67%) did not have work at that time so their daily income was found to be 0.The median of the annual ESRD total cost was (43 535.9 ± 70 645) SDGs. ()
Table 5. Annual average total and categories of direct cost, indirect cost (loss of productivity), working days loss and annual total cost categories in SDGs ($PPP), among patients and caregivers of end-stage renal disease in Khartoum, Sudan 2019 (n = 150).
3.4. Associations between study variables
The data on total ESRD costs exhibited a right-skewed distribution (skewness= 11.4) and thus, non-parametric tests were conducted. In bivariate analysis ESRD cost rank showed an association with patient gender (p = 0.0.001) (), insurance status (p-value 0.003), stopping work, school or housework due to renal failure (p-value 0.026) (), history of hospitalization (p-value 0.006), and the presence of caregiver with the patient during hospitalization (p-value 0.007) (). However, after adjustment, using a multivariate linear regression model including potential predictors (with p-value less than 0.2 in the bivariate analysis) none of these variables was associated with ESRD cost (). We categorized the duration of renal failure into four distinct groups (). However, our analysis revealed that the duration of renal failure did not significantly impact the total cost of ESRD.
Table 6. Multivariate linear regression model for potentially significant predictors (P-value <0.2) associated with the total ESRD cost among patients of end-stage renal disease in Khartoum, Sudan 2019 (n = 150).
4. Discussion
This cross-sectional descriptive study was conducted to estimate the direct and indirect cost of ESRD in Khartoum and explored potential associations. Previous research has also utilized cross-sectional descriptive studies in similar contextsCitation8,Citation15. The existing literature on the economic burden of ESRD in Sudan is severely lacking, with only one study conducted at a single hospitalCitation15. To the best of our knowledge, our study is one of few studies addressing the cost of illness of ESRD in Sudan. This demonstrates the significance of our study and the important gap it aims to fill.
The study focuses on Khartoum State, the capital of Sudan. As the epicenter of socio-economic and healthcare activities, Khartoum holds a central position influencing trends that may be reflective of broader national patterns. By offering an insight into the cost of end-stage renal disease, our findings can inform researchers and policymakers in Sudan and similar regions, facilitating evidence-based decision-making and resource allocation to improve renal healthcare outcomes in resource-constrained settings. However, potential discrepancies in study populations are imperative. Variations in health systems, demographic profiles, and economic conditions among countries can impact the interpretation of our results.
In our study sample of ESRD patients, males were predominant, consistent with findings from several Sudanese studies, where the male-to-female ratio was 1.6:1Citation15,Citation16. Several studies also showed male gender dominance among ESRD patientsCitation17–20. However, females were shown to have a high frequency in many countries, like the USA, Finland, and China, with the gender gap becoming more evident as people ageCitation21.
The age distribution of the study sample indicated an average age of 47 ± 14.3 years. This is slightly lower than that found in a study conducted in Khartoum to assess the causes of ESRD, where the mean age was found to be 49 ± 15.8 yearsCitation16. A systematic review of 16 papers from North America, Canada, South America, Pacific Asia, the Middle East, and Europe revealed an overall mean age of 50.07 yearsCitation19. The lower age distribution observed in our study may be indicative of a more rapid progression of chronic kidney disease to ESRD. This could potentially be attributed to delays in disease detection, patient referral or the implementation of preventive measures.
Hypertension was the most prevalent comorbidity among ESRD patients in our study (70.7%), followed by diabetes mellitus (15%). Also, in other studies conducted in Sudan, Sub-Saharan Africa, and India, hypertension was identified as the primary comorbidity related to ESRDCitation16,Citation20. This may be attributed to the interesting fact that chronic kidney disease can be both a cause and a consequence of hypertensionCitation22–24. However, chronic glomerulonephritis was at the top in NigeriaCitation25. In China, studies revealed various comorbidity patternsCitation17,Citation26. It is necessary to address the comorbidities associated with end-stage renal disease (ESRD) in various populations, as effectively managing and preventing these conditions can potentially help in better management and reducing the prevalence of ESRD in these populationsCitation23,Citation27.
In our study, more than half (60.7%) of the participants were hospitalized during the last few years, with an average of 2.4 visits per year and 30.1 days of total hospitalization. A relevant study conducted in Guangzhou City, southern China, reported lower hospitalization ratesCitation18. The difference in the quality of ESRD patients’ care could account for the discrepancy in hospitalization rates between Sudan and China.
The health insurance coverage rate in Khartoum is 71.5%Citation28. The vast majority of participants in this study had health insurance (85%). This difference in health insurance coverage may reflect the understanding of the financial strains associated with ESRD, suggesting that ESRD patients are more likely to purchase insurance packages as they are aware of the benefits they will receive. It is worth mentioning that managing ESRD is costly and has a real impact on patients, their families’ financial capabilities, and the overall healthcare systemCitation15.
Concerning cost valuation and analysis, patients and caregivers lost an average of 54.1 and 33.4 work days, respectively. Thus, annual indirect costs averaged 7,634.8 SDGs ($340.8 PPP) for patients, 1,289.9 SDGs ($57.6 PPP) for caregivers, and 8,740 SDGs ($390.2 PPP) for the total indirect costs. The variability of indirect costs between caregivers and patients may be attributed to the fact that unemployed or less salaried caregivers usually accompany the patient during clinical visits or hospital admission. Comparatively, a study done in Spain estimated the total indirect Cost of ESRD to be 8,929.0 EUR annuallyCitation29. A discrepancy in average salaries in Sudan and Spain may explain this significant variance in indirect costs between the two studies.
According to the study, ESRD's average direct cost is 586,315 SDGs ($26,174.8 PPP) per year, with a median of 38,600 SDGs ($1,723.2 PPP) for medical and non-medical expenses. This result is notably different from a study conducted locally at the Ibn Sina Renal Centre, where the average was estimated at 594,119.2 annually with a median of 3,859.1 USDCitation17. This might be due to the difference in health insurance coverage, which was only 67% in Ibn Sina’s study compared to 85% in this study. Higher direct costs were reported in similar studies in China (128,365.0 USD)Citation17,Citation18 and Spain (37,968 EUR)Citation29. This might be explained by the difference in time, the perspectives of cost measurements, the economic status of the country, and the currency exchange rate.
The study revealed high out-of-pocket expenses for ESRD. In Sudan, public health services in governmental hospitals or centers are usually not available due to many problems associated with the health system. Moreover, many essential drugs for those patients are not available in governmental pharmacies where health insurance covers 75% of the medication cost. As a result, patients are forced to seek treatment at private centers and purchase their medications from private pharmacies, where health insurance coverage is not applicable. This, coupled with the high cost of medical procedures, may explain the unexpected finding of high out-of-pocket expenses incurred by patients seeking treatment in the public health system.
These high out-of-pocket expenses for ESRD can be challenging for some patients to afford, especially if their income has decreased as a result of missing work days. In our study, almost half of the patients were unable to work for a year at the time when we collected the data. Likewise, a study conducted in Sudan found a high rate of unemployment among patients undergoing hemodialysisCitation16. The difficulty in covering medication costs may result in patients not adhering to their prescribed medications and dialysis treatmentsCitation30,Citation31. Moreover, these high out-of-pocket expenses can lead to catastrophic health expenditures for households, pushing them into poverty or worsening their existing poverty levels. As a result, families may be forced to make difficult choices between necessary medical care and meeting other basic needs such as foodCitation32,Citation33.
Sociodemographic characteristics did not show significant associations with the total cost of ESRD in our study, contrary to findings from a study conducted at the Ibn Sina Centre in Sudan which revealed that being a female was associated with higher ESRD costs, while residency or education did not have a significant impactCitation15. In Ethiopia, only older age was found to be associated with higher costsCitation7. Similarly, age affected the costs in a Chinese studyCitation18. These differences may be due to variations in healthcare systems, cultural factors or sample sizes in the different studies. According to the data, there was no association between the cost of ESRD and the clinical characteristics of the patients. However, a study conducted in Ethiopia revealed that a higher number of visits per month resulted in increased costs of ESRDCitation7. While in China, higher costs were associated with the presence of comorbiditiesCitation18.
The findings of this study should be viewed in light of some limitations, which include potential recall bias in cost reporting and a limited perspective focused only on patient costs. Also selection bias may occur because if patients of specific treatment type or disease severities are scheduled on specific days this may impact the outcome. Therefore, to address these issues, future research should include a longitudinal design and cost perspectives such as insurance company spending, hospital spending, and government spending should be measured to thoroughly understand the ESRD cost.
5. Conclusion
Findings reveal recognizable high direct expenses of ESRD and significant productivity losses for patients and their households which contributed to a noteworthy indirect cost of ESRD.
The care of ESRD patients requires a multidisciplinary approach, especially considering comorbidities, to mitigate the financial burden. Strengthening the health finance system, expanding health insurance coverage, providing medical and social assistance, and promoting research are crucial steps to address the economic burden and improve the quality of life of ESRD patients.
Although this study represents ESRD patients from all public dialysis centers in Khartoum state, caution should be exercised when comparing results with other countries due to variations in cost, prices of medical interventions, and currency values.
Transparency
Declaration of funding
This study wasn’t funded by any organization.
Declaration of financial/other section
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author contributions
HAH and OAE made the study plan. All authors conducted the analysis, interpreted the results, and drafted, revised, and approved the final manuscript, first authors had equal contributions and order was made on an alphabetic basis. The authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Acknowledgements
This work was under the supervision of the Student Association of Medical Education and Research (SAMER) and Khartoum Medical Student Association (KMSA), University of Khartoum, Khartoum, Sudan
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Consent and ethical approval
All methods of ethical approval followed the Declaration of Helsinki by the research ethics committee at the Ministry of Health in Khartoum State. Permission was then taken from dialysis centres’ administration. ESRD patients gave their voluntary informed consent after the research objectives and processing of data collection were clarified in simple terms. Participants were not harmed by any means and the confidentiality of their information was ensured throughout different stages of this research. The researchers declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author (SOE) upon reasonable request.
References
- Benjamin O, Lappin SL. End-Stage renal disease. 2021 Sep 16. In: StatPearls [internet]. Treasure Island (FL): statPearls Publishing; 2022 Jan. PMID: 29763036.
- Delanaye P, Jager KJ, Bökenkamp A, et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785–1805. doi:10.1681/ASN.2019030238.
- Ren F, Li M, Xu H, et al. Urine albumin-to-creatinine ratio within the normal range and risk of hypertension in the general population: a meta-analysis. J Clin Hypertens (Greenwich). 2021 Jul;23(7):1284–1290. Epub 2021 Jun 5. PMID: 34089300; PMCID: PMC8678728. doi:10.1111/jch.14263.
- Arogundade FA, Omotoso BA, Adelakun A, et al. The burden of end-stage renal disease in Sub-saharan africa. Clin Nephrol. 2020;93(1):3–7. doi:10.5414/CNP92S101.
- Asgeirsdottir TL, Asmundsdottir G, Heimisdottir M[, et al. Cost-effectiveness analysis of treatment for end-stage renal disease. Laeknabladid. 2009 Nov;95(11):747–753.
- Hessel F. Burden of disease. In: Kirch W, editor. Encyclopedia of public health. Dordrecht: Springer; 2008. doi:10.1007/978-1-4020-5614-7_297.
- Kassa DA, Mekonnen S, Kebede A, et al. Cost of hemodialysis treatment and associated factors among end-stage renal disease patients at the tertiary hospitals of Addis Ababa city and Amhara region, Ethiopia. Clinicoecon Outcomes Res. 2020;12:399–409. doi:10.2147/CEOR.S256947.
- Wang V, Vilme H, Maciejewski ML, et al. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol. 2016;36(4):319–330. JulPubMed PMID: 27475662. doi:10.1016/j.semnephrol.2016.05.008.
- Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20(4):327–337. doi:10.3350/cmh.2014.20.4.327.
- Glied S, Smith PC. The oxford handbook of health economics. Oxford University Press; 2011. Available from: https://global.oup.com/academic/product/the-oxford-handbook-of-healtheconomics-9780199675401?cc=us&lang=en&
- Cochran WG. 1977). Sampling techniques. (3rd ed.). New York: John Wiley & Sons.
- Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020 Feb 29;395(10225):662–664. doi:10.1016/S0140-6736(19)32977-0.Epub 2020 Feb 13. PMID: 32061314.
- Tool to estimate patient costs_Questionnaire_final.doc. https://view.officeapps.live.com/op/view.asp?src=https://stoptb.org/wg/dots expansion/tbandpoverty/assets/documents/5 tool to estimate patient costs questionnaire final. doc&dorigin=browselink.
- Von Elm E, Altman DG, Egger M, et al. STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oc;t 20;370(9596):1453–1457. PMID: 18064739 doi:10.1016/S0140-6736(07)61602-X.
- Yousif AO, Idris AKM, Awad MM, et al. Out‐of‐pocket payments by end‐stage kidney disease patients on regular hemodialysis: cost of illness analysis, experience from Sudan. Hemodial Int. 2021;25(1):123–130. doi:10.1111/hdi.12895.
- Banaga AS, Mohammed EB, Siddig RM, et al. Causes of end stage renal failure among haemodialysis patients in Khartoum state/Sudan. BMC Res Notes. 2015;8(1):502. doi:10.1186/s13104-015-1509-x.
- Zhang H, Zhang C, Zhu S, et al. Direct medical costs of end stage kidney disease and renal replacement therapy: a cohort study in guangzhou city, Southern China. BMC Health Serv Res. 2020;20(1):122. doi:10.1186/s12913-020-4960-x.
- Wong CK, Chen J, Fung SK, et al. Direct and indirect costs of end-stage renal disease patients in the first and second years after initiation of nocturnal home haemodialysis, hospital haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2019;34(9):1565–1576. doi:10.1093/ndt/gfy395.
- Kennedy C, Ryan S, Kane T, et al. The impact of change of renal replacement therapy modality on sleep quality in patients with end stage renal disease: a systematic review and meta-analysis. J Nephrol. 2018;31(1):61–70. doi:10.1007/s40620-017-0409-7.
- Suja A, Anju R, Anju V, et al. Economic evaluation of end stage renal disease patients undergoing hemodialysis. J Pharm Bioallied Sci. 2012;4(2):107–111. doi:10.4103/0975-7406.94810.
- Mills KT, Xu Y, Zhang W, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88(5):950–957. doi:10.1038/ki.2015.230.
- Peixoto AJ, Orias M, Desir GV. Does kidney disease cause hypertension? Curr Hypertens Rep. 2013 Apr;15(2):89–94. PMID: 23344662. doi:10.1007/s11906-013-0327-6.
- Whelton PK, Klag MJ. Hypertension as a risk factor for renal disease. Review of clinical and epidemiological evidence. Hypertension. 1989 May;13(5 Suppl):I19–27. PMID: 2490824. doi:10.1161/01.hyp.13.5_suppl.i19.
- Barri YM. Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep. 2008 Feb;10(1):39–45. PMID: 18367025. doi:10.1007/s11906-008-0009-y.
- Arogundade F, Sanusi A, Hassan M, et al. The pattern, clinical characteristics and outcome of ESRD in Ile-Ife, Nigeria: is there a change in trend? Afr Health Sci. 2011;11(4):594–601.
- Cao Y, Li W, Yang G, et al. Diabetes and hypertension have become leading causes of CKD in chinese elderly patients: a comparison between 1990- 1991 and 2009-2010. Int Urol Nephrol. 2012;44(4):1269–1276. AugPubMed PMID: 22648290 doi:10.1007/s11255-012-0194-0.
- Martínez‐Maldonado M. Role of hypertension in the progression of chronic renal disease. Nephrol Dial Transplant. May 2001;16Issue(suppl_1):63–66. volume Pages doi:10.1093/ndt/16.suppl_1.63.
- Al Saran K, Sabry A. The cost of hemodialysis in a large hemodialysis center. Saudi J Kidney Dis Transpl. 2012;23(1):78.
- Salim AMA, Hamed FHM. Exploring health insurance services in Sudan from the perspectives of insurers. SAGE Open Med. 2018;6:2050312117752298. doi:10.1177/2050312117752298.
- Villa G, Rodríguez-Carmona A, Fernández-Ortiz L, et al. Cost analysis of the spanish renal replacement therapy programme. Nephrol Dial Transplant. 2011;26(11):3709–3714. doi:10.1093/ndt/gfr088.
- Hirth RA, Greer SL, Albert JM, et al. Out-of-pocket spending and medication adherence among dialysis patients in twelve countries. Health Aff (Millwood). 2008;27(1):89–102. doi:10.1377/hlthaff.27.1.89.
- Dodd R, Palagyi A, Guild L, et al. The impact of out-of-pocket costs on treatment commencement and adherence in chronic kidney disease: a systematic review. Health Policy Plan. 2018;33(9):1047–1054. doi:10.1093/heapol/czy081.
- Nguyen KT, Khuat OT, Ma S, et al. Effect of health expenses on household capabilities and resource allocation in a rural commune in vietnam. PLoS One. 2012;7(10):e47423. doi:10.1371/journal.pone.0047423.

