Abstract
Aim
To evaluate the cost-effectiveness of dapagliflozin added to standard of care (SoC) versus SoC in heart failure with reduced ejection fraction (HFrEF) and without type 2 diabetes mellitus (T2DM) patients from the Qatari healthcare perspective.
Materials and Methods
A lifetime Markov model was developed to evaluate the cost-effectiveness of adding dapagliflozin to SoC based on the findings of Petrie et al. 2020, which were based on the DAPA-HF trial. The model was constructed based on four health states: “alive with no event”, “urgent visit for heart failure”, “hospitalization for heart failure”, and “dead”. The model considered 1,000 hypothetical HFrEF and without T2DM patients using 3-month cycles over a lifetime horizon. The outcome of interest was the incremental cost-effectiveness ratio (ICER) per quality-adjusted life-year gained (QALY) and years of life lived (YLL). Utility and cost data were obtained from published sources. A scenario analysis was performed to replace the transition probabilities of events in people without T2DM with the transition probabilities of events irrespective of T2DM status, based on findings of the DAPA-HF trial. Sensitivity analyses were conducted to confirm the robustness of the conclusion.
Results
Adding dapagliflozin to SoC was estimated to dominate SoC alone, resulting in 0.6 QALY and 0.8 YLL, at a cost saving of QAR771 (USD211) per person compared with SoC alone, with total healthcare costs of QAR42,413 (USD 11,620) versus 43,184 (USD11,831) per person, respectively. When replacing the transition probabilities of events in people without T2DM with the transition probabilities of events in people irrespective of T2DM status, dapagliflozin was cost-effective at ICER of QAR5,212 (USD1,428) per QALY gained and QAR3,880 (USD1,063) per YLL. In the probabilistic sensitivity analysis, dapagliflozin combined with SoC was cost saving in over 49% of the cases and cost-effective in over 43% of the simulated cases against QALYs gained and YLL.
Limitations
Data from clinical trials were used instead of local data, which may limit the local relevance. However, evidence from the local Qatari population is lacking. Also, indirect costs were not included due to a paucity of available data.
Conclusions
Adding dapagliflozin to SoC is likely to be a cost-saving therapy for patients with HFrEF and without T2DM in Qatar.
PLAIN LANGUAGE SUMMARY
Heart failure with reduced ejection fraction is a type of heart failure characterized by left ventricular ejection fraction of 40% or less. Dapagliflozin is a novel therapy for this condition, which was initially designed to treat type 2 diabetes mellitus. It is unclear whether dapagliflozin is a cost-effective option for patients with heart failure with reduced ejection fraction and without type 2 diabetes. A lifetime Markov model was developed to evaluate the cost-effectiveness of adding dapagliflozin to standard of care from the Qatari healthcare perspective. Model results suggest that adding dapagliflozin to standard of care dominated standard of care alone, resulting in a gain of 0.8 years of life lived, a gain of 0.6 quality-adjusted life-years, and a cost saving of 211 United States dollars per person.
Introduction
Heart failure is one of the most disabling cardiovascular syndromes, affecting approximately 65 million people worldwide.Citation1,Citation2 The prevalence of heart failure has increased along with population aging;Citation1,Citation3 1.3–6.7% greater in Asia than in Western countries.Citation3 Compared to these countries, heart failure affects patients a decade younger in the Middle East.Citation4 Heart failure is a progressive disease and is characterized by recurrent admission, reduced quality of life, and increased mortality, which in turn leads to a considerable health and economic burden on the healthcare system.Citation3 Based on 2014 global estimates, the annual economic burden attributable to heart failure was USD 108 billion, 60% and 40% of which were attributed to direct and indirect costs, respectively.Citation5
The fundamental goal of managing patients with heart failure with reduced ejection failure (HFrEF) is to prevent clinical symptoms and hospitalization, reduce mortality, and improve quality of life.Citation6 Both pharmacologic and device therapies are offered to the patients based on their symptoms and disease stages.Citation7 Pharmacological therapies include beta-blockers (BB), mineralocorticoid receptor antagonists MRAs (e.g. spironolactone), angiotensin-converting enzyme inhibitors (ACEi), angiotensin-receptor blockers (ARBs), or angiotensin receptor–neprilysin inhibitors (ARNis).Citation7 Dapagliflozin, a member of the sodium-glucose transport protein 2 inhibitor (SGLT2i) class, is a novel therapy for HFrEF that was designed initially to treat type 2 diabetes mellitus (T2DM).Citation8 The Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 trial (DECLARE-TIMI 58) demonstrated a reduction in the rate of heart failure hospitalization with dapagliflozin plus SoC relative to SoC in patients with T2DM and multiple risk factors or established cardiovascular (CV) disease.Citation8 The results of this study led to the conduction of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, which tested dapagliflozin in patients with HFrEF irrespective of their diabetic status.Citation9 The DAPA-HF trial, in alignment with the results of the DECLARE-TIMI 58 trial, revealed that adding dapagliflozin to SoC reduced hospitalization for heart failure and cardiovascular mortality by 30% and 18%, respectively, compared to SoC alone in patients with HFrEF.Citation9
Few studies have revealed that adding dapagliflozin to SoC among patients with HFrEF is cost-effective irrespective of diabetes status, and most were conducted in Western countries.Citation10–13 Because patients with T2DM are 2–4 times more likely to develop CVD compared with those without T2DM, and T2DM has been associated with a 75% increase in mortality rate, where CVD accounts for a large proportion of the excess mortality,Citation14,Citation15 it remains unclear whether dapagliflozin is cost-effective among patients with HFrEF and without T2DM at baseline. It is crucial to allocate resources efficiently, design strategies focused on patients without T2DM, and aid policymakers and clinicians in prioritizing interventions to treat HFrEF, especially in resource-constrained settings and stretched health system budgets. This interest in non-diabetic HFrEF patients is particularly of significance in settings like the main cardiology setting in Qatar, where prescribing of SGLT2i is restricted to the heart failure team in the absence of diabetes. Therefore, this study aimed to evaluate the cost-effectiveness of dapagliflozin added to SoC against SoC alone in patients with HFrEF and without T2DM.
Methods
Model structure
A lifetime Markov model was developed to simulate the disease progression in patients with HFrEF and without T2DM to evaluate the cost-effectiveness of adding dapagliflozin to SoC based on the findings of Petrie et al. 2020, which were based on the DAPA-HF trial.Citation9,Citation16 The authors developed the model, explored assumptions, and identified appropriate data sources after reviewing the relevant literature, including four key studies.Citation11,Citation13,Citation17,Citation18 The model was constructed based on four health states; “alive with no event”, “alive with urgent visit for heart failure” (i.e. patients are not hospitalized), “hospitalization for heart failure”, and “dead” (). These employed health states accommodate the chronic and progressive nature of HFrEF, with a 3-month cycle length.Citation11,Citation19,Citation20 The structuring of the model with the most relevant health states was guided by published studies in heart failure.Citation21,Citation22 Hussain et al. described the main events associated with patients with HFrEF, which included hospitalization, urgent care, and death. These health states are consistent with those of interest to the cardiology setting in Qatar and were further validated by the cardiologists involved in the current study. The states used were also aligned with those used in relevant cost-effectiveness studies of SGLT2i for HFrEF.Citation11,Citation20,Citation23,Citation24
Figure 1. A lifetime Markov model for patients with heart failure with reduced ejection fraction and without type 2 diabetes mellitus.
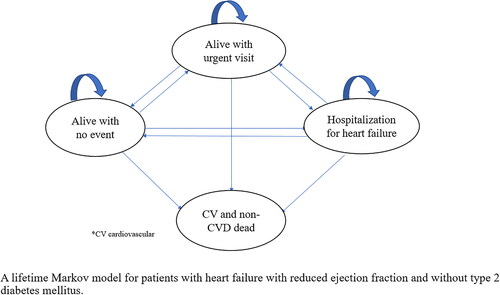
All patients without any clinical events and using (i) dapagliflozin added to SoC or (ii) SoC alone entered the state of “alive with no event” at the first cycle of the Markov follow up. The patients then can stay in the “alive with no event” state or progress to the state of “alive with urgent visit for heart failure”, “hospitalization for heart failure”, or “dead” (due to CV or non-CV causes) based on the given transition probabilities among the health states. Patients in the “alive with urgent visit for heart failure” state would either stay in the same health state, move to “alive with no event” “hospitalization for heart failure” or die due to CV or non-CV causes, while patients with “hospitalization for heart failure” would either stay in the same health state (i.e. the patients will be readmitted to the hospital in the next cycle), move to “alive with no event”, alive with urgent visit for heart failure or die due to CV or non-CV causes. In the base-case analysis, consistent with the population of Petrie et al. 2020, the model assumed that all patients had started treatment at the age of 66 years and progressed from stable HFrEF without acute events through the Markov model. After reviewing the relevant literature,Citation11,Citation17,Citation20 the authors developed the model using a 3-month cycle until either death or a lifetime horizon was reached, whichever came first.
There is no available data on the lifetime expectancy horizon for patients with HFrEF in Qatar. The study model, therefore, utilized the life expectancy that was commonly used in relevant cost-effectiveness models in the literature in patients with HFrEF, where patients were followed until 89 years old. Patients were allowed to enter the model at the age of 66 years, reflecting the mean age reported in the Petrie et al. studyCitation9, and they would exist the model when they reached 89 years old. Overall, the model follows individuals for lifetime years, until they die, or until they reach 89 years of age in the model, whatever was reached first.Citation23,Citation25–28
A willingness-to-pay (WTP) threshold of USD 150,000 per additional year of life lived (YLL) or quality-adjusted life-year (QALY) gained was used as a reference threshold for cost-effectiveness in Qatar.Citation29–31
The Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS) was followed.Citation32
Perspective
The study was conducted from the perspective of the public and primary healthcare provider in Qatar, Hamad Medical Corporation (HMC), i.e. the Heart Hospital.Citation33 The Heart Hospital is the only public specialized healthcare provider for CVD in Qatar, with 116 beds. It provides services for various cardiac complications including critical care, heart failure, general cardiology, and cardiac surgery.
Cohort population
The model simulated a hypothetical population of 1,000 individuals that reflects the patient characteristics included in the Petrie et al. study, an exploratory analysis of the DAPA-HF trial,Citation9 where no significant differences in any of the patient baseline characteristics were identified between the study treatment groups. The trial had a mean age of 66 years with HFrEF of ≤40% EF and New York Heart Association (NYHA) class II, III, or IV symptoms. All patients included in the model were required to receive standard heart failure care, consistent with that in HMC, including device therapy (i.e. implantable cardioverter defibrillator (ICD)) and drug therapy (i.e. ACEi/ARBs, MRAs, or ARNi, and beta-blockers). Also consistent with HMC practices, the dapagliflozin dose in the DAPA-HF trail was the standard 10 mg daily.
Model input parameters
Transition probabilities:
Powered studies for the events in patients with HFrEF and without T2DM are unavailable in the literature. Therefore, the rates of alive with no event, urgent visit for heart failure, hospitalization for heart failure event, and CV and non-CV deaths were obtained from the findings of Petrie et al. 2020.Citation16 The data by the Petrie et al. study is based on the subgroup analysis of the DAPA-HF trial, which provides the only source of data regarding the fatal and non-fatal CVD events in HFrEF without T2DM. For this model, 3-month transition probabilities were derived from annual event rates by assuming a constant hazard.Citation34 For the first cycle, the risk of events was based on event probabilities estimated from the Petrie et al. 2020, study,Citation16 while for all subsequent cycles, transition probabilities were adjusted according to age-related trends estimated by the Ministry of Development Planning and Statistics, Qatar,Citation35,Citation36 to account for the risk of CVD and non-CVD deaths according to age- and sex-specific mortality rates. Here, we initially used the age- and sex-specific mortality rates for the general population from the Ministry of Development Planning and Statistics, Qatar, as contemporary age- and sex-specific mortality rates for Qataris with HFrEF and without T2DM are lacking.Citation37 Then, these rates were adjusted for patients with HFrEF based on data from a study by Bertoluci et al. which reported that the relative risk for CVD patients is 2.0.Citation15 To estimate the mortality rates by single year of age, mortality rates for an age group were first plotted against the midpoint age for the age group (e.g. 50 for the age group 45–54 years). Polynomial functions were then applied to model the mortality rates for age in single years (Electronic Supplementary Material (ESM) ).
The functions below were used to transform probabilities into transition probabilities using the formula by Briggs et al.:Citation34
r = (- ln(1 − p))/t, where r is the 3-month rate, p is the proportion of event, and t is the duration of the trial.
tp = 1 − e−rt, where r is the 3-month adjusted rate, and t is the cycle length.
The transition probabilities can be found in .
Table 1. Model inputs for the base-case analysis.
Cost estimates
The current model was designed based on a Qatari public hospital system perspective; thus, only direct medical costs were included. Resource categories were aligned with published clinical practice guidelinesCitation39 and a recent systematic review of cost-effectiveness evaluations of SGLT2i in heart failure.Citation40 Costs included urgent visits for heart failure, hospitalization for heart failure, CV death, and pharmaceutical use. According to the pharmacy department at HMC, the 3-month weighted average combination cost of 10 mg daily dapagliflozin dose added to SoC was calculated to be Qatari Rial (QAR) 557 (United States Dollars, USD 153) per person. SoC cost was QAR 176 (USD 48) per person, calculated based on proportional costs of ACEi, BB, and spironolactone. Costs of acute events, including costs of urgent visit for heart failure, hospitalization for heart failure, and CV death were QAR 2,583 (USD 708), QAR 22,329 (USD 6,118), and QAR 6,396 (USD 1,752) per person, respectively. Clinical event costs were based on the finance department of HMC, which were available as per resource category, calculated based on a micro-costing approach of involved direct medical resources. Further description of the costs of events is provided in ESM . In accordance with a recent multinational cost-effectiveness analysis of empagliflozin in HFrEF, by Tafazzoli et al.,Citation41 the model assumed no cost for non-CV death. We assumed that for patients who are alive with no event, a hospital stay is not required, and patients were assumed to incur only medication and follow-up visit costs, with no additional disease management cost.
The cost of a worsening with hospitalization for a heart failure event was assumed to be the cost of hospitalization, while the cost of being alive with urgent visit was assumed to be the cost of urgent care received during emergency care only without requiring inpatient hospitalization. Of note, costs of adverse events were not included in the analysis because there were no observed differences in any adverse event in the Petrie et al. trial.Citation16 Also, all patients in this model were assumed to receive the same initial monitoring parameters; therefore, monitoring costs were not included in the analysis.
Cost values were reported in 2023 QAR (Qatari Riyal), with the US$ equivalence being provided based on 2023 exchange rates (1 QAR = 0.27 US$). All costs were inflated to QAR 2023 year values using the Qatari Health Price Index and were presented in QAR and USD values.Citation42 In line with similar studies, the long-term costs and outcomes were annually discounted starting in the second year of the model, and a 3% rate was used as suggested by recent global recommendations and previous studies.Citation11,Citation17,Citation20,Citation29,Citation43–46
Utility
Utility scores estimate the strength of people’s preferences for different health states. Utility scores fall between 0 and 1, with 1 indicating a perfect health state and 0 indicating death.Citation47 The outcomes in this cost-effective analysis are the QALYs gained and the YLL. Utility data among Qatari patients with heart failure are not available, and in the literature, there are no utility data for patients with heart failure who are non-T2DM. Thus, utilities for each health state in the model were estimated from multicenter international studies.Citation10,Citation38 These studies were considered due to their relevance to the Qatari setting, large sample size, and the provision of comprehensive utilities in patients with HFrEF with different health states. The utility values of patients with HFrEF was based on the Euro-QoL-5 Dimensions, 5 Levels (EQ-5D) questionnaire, validated and widely used in patients with CV disease.Citation48,Citation49
The probability, cost, and utility inputs of the study model at its base case are all presented in .
Sensitivity and scenario analyses
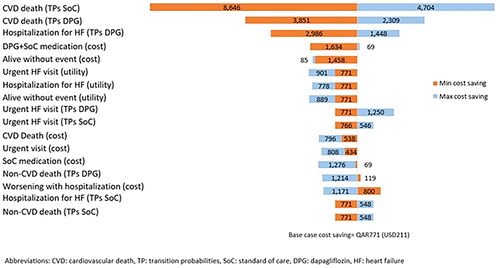
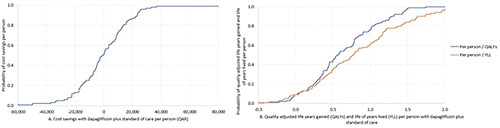
One-way and probabilistic sensitivity analyses (PSA) were conducted to quantify the variations in estimating results caused by additional parameter uncertainties. A one-way sensitivity analysis was performed to examine the impact of parameter uncertainty, using the lower and upper bound of the parameter distributions to ascertain the key determinants of cost savings. All model inputs presented in were included in the one-way sensitivity analysis, where an uncertainty range of ±25% was assigned to the base-case values. Results from the one-way sensitivity analysis were presented in a Tornado diagram. Briefly, the numbers in the bars of a Tornado diagram represent the impact of each input variable on the output variable (i.e. cost saving). The length of each bar represents the magnitude of the impact, with longer bars reflecting a larger influence on the cost saving. Values appearing to the left of the base case represent a negative impact, indicating that as the value of the input variable decreases, it has a decreasing effect on the cost saving. While values appearing to the right of the base-case reflect a positive impact, indicating that as the value of the input variable increases, it has an increasing effect on the cost saving. In the PSA, the transition probabilities, cost, and utility model inputs were allowed to vary simultaneously within a predefined uncertainty range. The PSA consisted of a Monte Carlo simulation with 1,000 iterations, consistent with the hypothetical study population size.Citation50 Key input parameters and respective distributions are given in ESM . Results of PSA were presented in a cost-effectiveness probability curve (CEPC) and a cost-effectiveness plane (CEP). Both graphs are helpful to summarize the impact of uncertainty on the findings of a cost-effectiveness analysis, frequently expressed as an incremental cost-effectiveness ratio (ICER) or net monetary benefit. CEPC plots a range of cost-effectiveness or net monetary benefit thresholds on the horizontal axis against the probability that the intervention will be cost-effective at a threshold on the vertical axis.Citation34,Citation51 At the same time, CEP plots the incremental effectiveness of an intervention (relative to a comparator) against the incremental cost of the intervention. It demonstrates the uncertainty and the magnitude of the estimates, and each point on the plot is from a particular random model re-run derived from the PSA.Citation34,Citation51 Also, a linear regression tornado analysis was conducted to investigate the relationship between model inputs and outcome and its direction. While the regression coefficient does not show the consistency of the relationship, it shows the strength of the relationship. A positive regression coefficient means that as that input increases, the output increases; and a negative regression coefficient means that as that input increases, the output decreases. In addition, multiple scenario analyses were conducted:
Table 2. Base-case analysis outcomes.
Shortening the Markov time horizon to 5, 10, 15, and 20 years.
Changing the discount rates to 0% (undiscounted), 1% and 5%.
Canceling the age-related death trends.
Replacing ACEi with ARB (i.e. valsartan).
Replacing ACEi with sacubitril and valsartan.
Changing the relative risk of increased cardiovascular mortality by +15%.
Changing the relative risk of increased cardiovascular mortality by –15%.
Replacing the transition probabilities of events in people without T2DM with the transition probabilities of events in people irrespective of T2DM status based on findings of the DAPA-HF trial.Citation9
Replacing the transition probabilities of events in people without T2DM with the transition probabilities of events in people with T2DM based on Petrie et al., 2020, findings.Citation16
Model validation
The model was built in Microsoft Excel. The Assessment of the Validation Status of Health-Economic decision models (AdViSHE) and TECHnical VERification (TECH-VER) tools were followed, which provide a framework to improve the efficiency and credibility of the model and assist in identifying errors in a systematic way.Citation52,Citation53 The conceptual model, input data, and model outcomes were validated by an expert in pharmacoeconomics modeling (DB). In addition, the model was examined for face validity to evaluate the appropriateness of model inputs and was also varied to assess if expected effects were predicted, and the manual review of formulae and cross-check of all inputs was performed. External validation was also considered by comparing the findings of our model with those reported in international studies.Citation54,Citation55 We also followed the three “orders” of cost-effectiveness model validation described by McCabe et al.Citation56 These include (i) first-order validation that requires expert concurrence, (ii) second-order validation to compare the model predictions with data used to estimate the model parameters, and (iii) third-order validation to compare the model prediction with “other” observed data, i.e. data not used in the model construction.
Results
Base-case analysis
In the hypothetical population of 1,000 patients, adding dapagliflozin to SoC in patients with HFrEF and without T2DM yielded an additional 0.6 QALY and 0.8 YLL, at a cost saving of QAR 771 (USD 211) per person, compared with SoC alone, with total healthcare cost of QAR 42,413 (USD 11,620) versus 43,184 (USD 11,831) per person, respectively. Dapagliflozin, combined with SoC, resulted in an additional 1.17 lives without event per person compared to SoC. Additionally, dapagliflozin, combined with SoC, prevented 0.05 alive with urgent visits, 0.02 deaths from CVD, and 0.01 non-CVD deaths, per person, compared to SoC. reported the health outcomes and costs of the study comparators.
Sensitivity analyses
One-way sensitivity analysis
Results from the one-way sensitivity analysis are presented in the Tornado diagram in . The results showed that the transition probabilities of CVD death in both groups, transition probabilities of hospitalization for HFrEF in dapagliflozin plus SoC, and the cost of dapagliflozin plus SoC were the main drivers of the cost saving.
Probabilistic sensitivity analysis
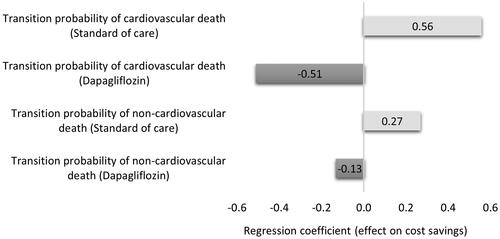
The CEP for QALYs showed that dapagliflozin combined with SoC in patients with HFrEF and without T2DM was cost saving in 49.8% of the cases and cost-effective in 44.8% of the simulated cases. At the same time, the CEP for YLL showed that dapagliflozin combined with SoC is cost saving in 49.8% of the cases and cost-effective in 43.2% of the cases ( and ). The regression tornado analysis demonstrated that the transition probabilities of death due to CVD with dapagliflozin plus SoC and SoC groups were the key drivers influencing the QALYs and YLL outcomes, while the transition probability of hospitalization for heart failure with dapagliflozin plus SoC and the transition probability of non-CVD death with both groups were the minor drivers, respectively ( and ). While with cost savings outcome, the transition probabilities of CVD death in both groups were the main influential factors, while the transition probability of non-CVD death with dapagliflozin was the least influential factor ().
Figure 3. (A) Cost-effectiveness plane per quality-adjusted life years, (B) Cost-effectiveness plane per life years lived.
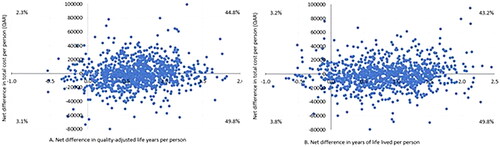
Figure 5. A regression tornado diagram indicating the main drivers of model variables behind the outcomes (quality-adjusted life years).
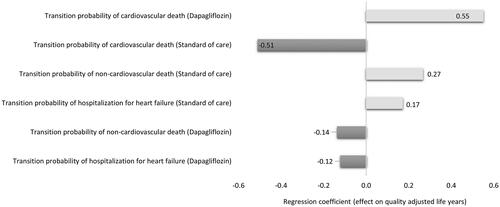
Figure 6. A regression tornado diagram indicating the main drivers of model variables behind the outcomes (years of life lived).
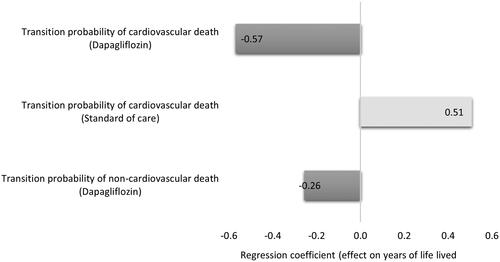
Figure 7. A regression tornado diagram indicating the main drivers of model variables behind the outcomes (cost saving).
Included inputs in the probabilistic sensitivity analysis, their uncertainties, and the outcomes are presented in .
Table 3. Sensitivity analyses results.
Scenario analysis
Variations in the model did not alter the overall base-case cost saving in the majority of scenario cases, except in the following cases, where dapagliflozin combined with SoC became cost-effective:
When removing age-related death trends, dapagliflozin was cost-effective at an ICER of QAR 5,645 (USD 1,547) per QALY gained and 4,295 (USD 1,177) per YLL.
When reducing the time horizon to 5 and 10 years, dapagliflozin was cost-effective at ICER of QAR 4,992 (USD 1,368) per QALY gained and 3,708 (USD 1,016) per YLL. When reducing the time horizon to 15 and 20 years, dapagliflozin was cost-effective at ICER of QAR 5,397 (USD 1,479) per QALY gained and 3,999 (US 1,095) per YLL. When replacing the transition probabilities of events in people without T2DM with transition probabilities of events in people with T2DM, dapagliflozin was cost-effective at ICER of QAR 5,212 (USD 1,428) per QALY gained and QAR 3,880 (USD 1,063) per YLL.
When replacing the transition probabilities of events in people without T2DM with transition probabilities of events in people with T2DM, dapagliflozin was cost-effective at ICER of QAR 5,023 (USD 1,376) per QALY gained and 3,759 (USD 1,030) per YLL.
All results of the scenario analyses are shown in .
Table 4. Outcomes of scenario analysis.
Model validation
The AdViSHE and TECH-VER tools and CHEERS checklist are reported in ESM and . Our model was validated by comparing the findings of the model versus the Petrie et al. study findingsCitation16 as well as international references.Citation54,Citation55 The validation suggests that our model may overestimate the outcomes in patients with HFrEF.
Discussion
Although a growing body of evidence supports the role of SGLT2i in HFrEF, prescribing barriers, such as prior authorization formulary restriction, may hinder the widespread use of these medications.Citation57 Our analysis attempts to provide insight into prescribing dapagliflozin to patients with HFrEF without T2DM. To the best of our knowledge, this study is the first in Qatar and the literature to provide new insights about the benefits of adding dapagliflozin to SoC for patients with HFrEF and without T2DM to improve QALYs and decrease overall healthcare costs. Our findings revealed that the addition of dapagliflozin to SoC in patients with HFrEF and without T2DM might be cost saving compared with SoC alone, resulting in a reduced cost of QAR 771 (USD 211) per person, with a total healthcare costs of QAR 42,413 (USD 11,620) versus 43,184 (USD 11,831) per person, respectively, in the model’s 1,000 patients population. Our sensitivity analyses indicated that the economic advantage of dapagliflozin is maintained at both lower and higher limits of input uncertainties, suggesting that study findings were not sensitive to underlying uncertainty, and providing some reassurance to the potential generalizability of our conclusions. The PSA not only addresses the confidence interval of the point estimate of an ICER or cost saving but also, in combination with CEPCs and CEPs, provides the decision-maker with further confidence that the base case results are not arbitrary, where the dapagliflozin plus SoC was shown to be between cost saving and cost-effective in approximately 50% of simulated cases. Additionally, when testing results against the scenario of including the T2DM status in the population, the conclusion remains in favor of dapagliflozin combined with SoC.
Our economic analysis of using the combination of dapagliflozin and SoC strategy is necessary to get an overall picture of costs versus benefits, where investing in the strategy is likely to result in long-term health and economic gains, i.e. YLL and QALYs. As per our study, added YLL, QALYs and healthcare savings can be achieved, advantaging dapagliflozin combined with SoC for HFrEF. While these gains may be limited in proportional terms (i.e. YLL and QALYs), they are considerable in absolute terms and translate to hundreds of dollars in economic value. The issue with high healthcare expenditure is that it is viewed as just expenditure. Instead, devoting funds to such interventions should be perceived as an investment rather than an expenditure. These investments are vital and will be paramount for Middle Eastern countries like Qatar, where benefits cannot be underestimated as the healthcare expenditure is the highest in the Middle EastCitation58 and the HFrEF prevalence is rising in the younger working age groups at a much more rapid rate than in Western countries.Citation3 To also note, in relation to QALY outcomes, the current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA), as well as the European Society of Cardiology (ESC) for the management of heart failure,Citation7,Citation39 have relatively little to say about quality of life outcomes. For instance, the ESC guidelines reported that cardiac resynchronization therapy leads to significant improvements in QALYs in patients with heart failure, with one-third of the change in QALYs attributable to increased longevity and two-thirds attributable to improved quality of life.Citation59 Treatment for HFrEF may have an immense impact on healthcare budgets as many patients might be eligible for lifelong treatments, necessitating the prioritization of cost-effective interventions. In our study, we included all relevant unit costs for HFrEF, such as costs of medications, worsened with and without hospitalization for heart failure, CV death, and non-CV death, which are of particular importance to patients with HFrEF, given the expected increase in heart failure prevalence, and where most of the costs are incurred within secondary care.
Although our study is the first cost-effectiveness study among patients with HFrEF and without T2DM, our results are consistent with other cost-effectiveness analyses in patients with HFrEF, irrespective of T2DM status. Prior studies were conducted from the perspective of different healthcare systems, including the United Kingdom, Germany, Spain, China, and Australia, which estimated that adding dapagliflozin to SoC was cost-effective.Citation10–13 Just like the current study, the major limitations of these studies were primarily that, in all of them, clinical events of dapagliflozin plus SoC stemmed from the DAPA-HF trial, a single randomized clinical trial. Additionally, previous studies adopted a healthcare perspective, and thus, only direct medical costs were included. Also similar to the current study, their studies were run over a lifetime horizon to capture the long-term implications of HFrEF management, but with varying cycle lengths. For instance, Phil McEwan et al. and Isaza et al. used a monthly cycle, while Yao et al. used a 3-month cycle.Citation10,Citation11,Citation13 This is while noting that the annual healthcare cost of dapagliflozin in our study was considerably higher (QAR 42,413 (USD 11,620) per person) compared with other healthcare systems, which included patients regardless of their T2DM status (USD 4,192 in the US, USD 302-780 in Europe, USD 257 in China, and USD 563 in Australia).Citation10–12 Also, consistent with our study, all studies performed numerous sensitivity and scenario analyses to explore the model uncertainties on outcomes. Our findings were also similar to previous studies regarding the reductions in CV death being the main driver of cost, yielding greater YLL and QALY gains in patients managed with dapagliflozin.
With regards to the Asia–Pacific region, Savira et al.Citation12 and Krittayaphong et al.Citation17 suggested that adding dapagliflozin to SoC among patients with HFrEF with or without T2DM may be cost-effective from the Australian and Thai healthcare system perspectives. Likewise, clinical events were acquired from a single trial (i.e. DAPA-HF) and adopted a healthcare perspective. Both studies were conducted over a lifetime with yearly cycles in Savira et al.Citation12 and 3-month cycles in Krittayaphong et al.Citation17. Moreover, sensitivity and scenario analyses were conducted to address the study uncertainty. However, Yao et al.Citation11 reported a relatively low probability of cost-effectiveness for adding dapagliflozin to SoC among the Chinese population with HFrEF. These inconsistent findings could be due to differences in study assumptions, model inputs, and WTP thresholds across studies. For instance, the utility parameters associated with the heart failure health state used in Yao et al. study (i.e. 0.127–0.204) were lower than those used in other studies (i.e. 0.508–0.833), including our research.Citation10–12,Citation60
Our model captured disease progression in HFrEF patients transitioning between the “alive with no event”, “urgent visit for heart failure”, and “hospitalization for heart failure” health states, characterized by the NYHA functional class as reported in DAPA-HF trial, which is an established measure of health status in HFrEF, rather than using the Kansas City Cardiomyopathy Questionnaire (KCCQ-TSS) reported in a prior study,Citation10 which is a patient-reported outcome. Also, time-dependent disease severity using KCCQ quartiles may lead to an opposite pattern to that seen with NYHA, i.e. a progressive deterioration over the long term.Citation10
It is conventional to use data from clinical trials to estimate the transition probabilities to model a time-dependent NYHA class. For instance, King et al. evaluated the cost-effectiveness of sacubitril-valsartan combination therapy compared with enalapril using the NYHA transitions derived from the PARADIGM-HF trial. These transitions improve the severity of heart failure over time, given the dynamic and chronic nature of the disease.Citation24
According to the findings of our one-way sensitivity analyses via tornado diagram, we found that the transition probabilities of CV death in both groups and the transition probability of hospitalization for heart failure with dapagliflozin combined with SoC were the top drivers of the cost-saving variations. These findings were also aligned with the results of our regression analyses, depicting the effect of key parameters on cost savings. The findings are expected because the reduction in hospitalization and CV death with dapagliflozin plus SoC versus SoC alone were one of the most promising efficacy outcomes in favor of dapagliflozin as shown in the Petrie et al. 2020, study.Citation16
Here, while a favorable economic benefit was still maintained with the use of dapagliflozin as add-on therapy when we shortened the time horizon (i.e. 5, 10, 15, and 20 years), such findings suggest that medication duration is an essential input for consideration.
There is no approved WTP cost-effectiveness threshold in Qatar. While the World Health Organization suggests using 1 to 3 times the GDP per capita as the threshold value in a country, it is acknowledged that this is arbitrary and not based on any methodological justification.Citation61 In addition, Qatar’s average 2023 GDP per capita (PPP) was approximately USD 94,028;Citation62 one of the world’s highest. Thus, adopting the WHO recommendation for calculating the WTP will result in a range of values that is too wide to be directly useful, i.e. USD 64,781 to 194,343. In this study, we adopt a threshold value of USD 150,000, which is increasingly accepted as a higher threshold value in the literature and within the range suggested by WHO for Qatar.Citation63
HFrEF is a chronic condition necessitating lifelong management. Add this to the economic burden associated with the management of HErEF, our study empowers effective and affordable services via informed decisions that best optimize resource use, including medications. While currently, in Qatar, citizens receive healthcare services, including medications free of charge, and expatriates are required to co-pay 20% for the cost of medications, the very recent law No. 22 for the Year 2021 has re-constituted the future direction in healthcare reimbursement. According to this, free treatment at government health facilities will only be available to nationals. Non-nationals can only be treated via out-of-pocket and private health insurance.Citation64 This increased consideration of affordability influences decisions on treatment choices. Indeed, the delayed implementation of SGLT2i in Qatar compared to ACEi/ARBs is disappointing. For example, rates of SGLT2i initiation are much higher among endocrinologists and internists compared with cardiologists, and less than 15% of eligible patients were prescribed an SGLT2i in Qatar.Citation65 The proven efficacy of dapagliflozin, and the cost saving based on our study, would help to remove restrictions on SGLT2i prescription imposed by the Pharmacy and Therapeutics committee at HMC and, ultimately, improve access to dapagliflozin.
The main strength of this study is that, to our knowledge, it is the first analysis of the cost-effectiveness of dapagliflozin combined with SoC for patients with HFrEF. Also, we used real-world individual-level data to estimate resources and associated costs. Another notable strength, our model encompasses all potential consequences of dapagliflozin combined with SoC in HFrEF, allowing for a more accurate representation of real-life practice and the overall cost of resource utilization. Added to the strengths of the current study is the estimation of the humanistic outcome of strategies (i.e. QALYs gained). QALYs capture how many extra years of life of a reasonable quality an individual might gain from the strategy. This is particularly important when considering strategies for chronic diseases such as heart failure.
This study has several limitations. Based on non-published data from the advanced heart failure outpatient clinic at our cardiology center in Qatar, our patients seem to be a decade younger than those enrolled in the DAPA-HF trial (56 versus 66 years old, respectively). Other potentially outstanding differences include atrial fibrillation (17% versus 38%) and diabetes (64% versus 42%) co-morbidities, the use of mineralocorticoid receptor inhibitors (55% versus 71%) and diuretics (74% versus 39%), and the levels of B-type natriuretic peptide (3700 versus 1440 pg/ml). Given that patients enrolled in the DAPA-HF are older and have more patients with atrial fibrillation and NYHA class III, our patients may be considered healthier than those enrolled in the DAPA-HF trial. Thus, the impact of dapagliflozin may be potentially more prominent in the DAPA-HF trial population. Another limitation is that the transition probabilities were obtained from Petrie et al. 2020 (16) based on the DAPA-HF trial. In the trial, while the subgroup results were consistent with those in the main analysis, a level of uncertainty is suspected due to the limited sample size. However, this is not uncommon in health economics, especially where our base-case modeling was supported by several one-way and probabilistic sensitivity analyses to confirm robustness against additional input uncertainties. Furthermore, because of the lack of utility data for the HFrEF without T2DM, the model used utility in general patients with HFrEF irrespective of diabetes status, which may over- or under-estimate the actual utility in those without T2DM. However, sensitivity analyses were designed to address this limitation. Another limitation that may lead to uncertainty is the necessity to extrapolate beyond the median follow-up time of approximately 18.2 months in the DAPA-HF trial. Similarly, the sensitivity analysis concerning the follow-up horizon of the model confirmed its robustness. Moreover, relying on data from clinical trials instead of local data may limit the local relevance. However, evidence on the effect of dapagliflozin use in HFrEF among the local Qatari population is lacking, whereby relying on international clinical trials is justified and is best practice in health economics, pending evidence of robustness via sensitivity analyses, especially given the standardized dosing of drugs and the similar SoC practices. Furthermore, our validation suggests that our model may overestimate the outcomes in patients with HFrEF. This overestimation could potentially mislead decision-makers. However, to address this concern, sensitivity analyses were performed to examine the model inputs and assess the robustness of the results. Additionally, our model only focused on the CV outcomes of HFrEF patients without T2DM, and did not include the renal outcome as another clinically important outcome. Here, however, given the renal protective effect associated with dapagliflozin, it is expected that the results of adding dapagliflozin to SoC, when kidney-related health states are considered in the model, would further increase the dapagliflozin’s economic benefits.Citation66 Also, a limitation is the lack of data on the lifetime expectancy horizon for patients with HFrEF in Qatar. We, therefore, utilize the life expectancy that was commonly utilized in relevant cost-effectiveness models in the literature in patients with HFrEF, where patients were followed until around 89 years old.Citation23,Citation24,Citation26–28 To note, in these studies, unlike in our current study, populations were sicker, including diabetic patients. For this reason, as life expectancy is an average age, it is possible that not all patients will die by 89 years, just like in our study model. Nevertheless, long Markov follow-ups, like in the current study, are associated with considerable uncertainties. Because of this, it is questionable that there can be survival by the end of a lifetime horizon, especially where (i) survival in patients with HFrEF becomes generally unlikely to persist over a lifetime, and (ii) the difference in mortality between interventions over a lifetime becomes highly doubtful. It is for this reason that we have tested the scenarios of shorter time horizons, whereby a favorable economic benefit was still maintained with the use of dapagliflozin as add-on therapy when we shortened the time horizon, i.e. 5, 10, 15, and 20 years. Here, however, it seems that the model requires over 10 years of follow up for cost and outcome differences to sufficiently accumulate for significant variations in costs and outcomes to be captured. The differences between the findings when changing the time horizon from 10 years to 15 years may be attributed to the fact that this shift includes a more substantial portion of the long-term dynamics of the interventions being evaluated. Additionally, locally-specific health utility values for HFrEF patients in Qatar are unavailable; hence, these were drawn from international studies.Citation10,Citation38 While the utility estimate varies across different countries, it is highly associated with the quality of life index of the country.Citation67 Within this context, studies from the US, Germany, and Spain were used, which have a comparable quality of life index to that in the Qatari setting,Citation68 being one of the wealthiest countries in the world with one of highest gross domestic incomes per capita.Citation62 Also, indirect costs were not included due to a paucity of available data. However, since patients with HFrEF had improved QALYs outcomes, including indirect costs is not expected to influence the overall conclusion of the analysis. Finally, the present study did not compare the economic outcomes associated with different SGLT2is, which is due to the lack of head-to-head comparisons of individual SGLT2is among heart failure patients, and addressing the cost-effectiveness of different SGLT2is was beyond the scope of this study. Future research, therefore, should explore the variation in the health outcomes and economic costs for heart failure patients using different SGLT2is in HFrEF patients without T2DM.
Conclusions
Taking into consideration the study’s limitations and the healthcare perspective, findings enable enhanced effective and affordable decision-making that suggests the addition of dapagliflozin to SoC as a positive value for investment, being cost saving under our baseline assumptions and cost-effective when assumptions were varied from the baseline in HFrEF patients, whether without T2DM, with T2DM irrespective of the T2DM status. Within this context, in Qatar, which has the highest healthcare expenditure in the Middle East, added to an HFrEF prevalence that is at a higher rate than the international average, potential benefits to the healthcare system cannot be underestimated. Further research should explore the impact of the cost-effectiveness of different SGLT2i in HFrEF.
Transparency
Author contributions
DA conceived the study, developed the model, performed the analyses, and wrote the first draft of the manuscript. DB contributed to model development, analyses, interpretation of data, and revised the manuscript. SC, RK, MA, PA, WE, JS, RN contributed to the interpretation of data, and revised the manuscript. All authors read and approved the final manuscript.
Reviewer comments
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (77.1 KB)Declaration of funding
No funding was received for undertaking this study. Open Access funding was provided by the Qatar National Library, Qatar.
Declaration of financial/other relationships
Authors have no conflicts of interest to disclose.
References
- Maddox TM, Januzzi JLJ, Allen LA, et al. Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American college of cardiology solution set oversi. J Am Coll Cardiol. 2021;77(6):772–810. doi: 10.1016/j.jacc.2020.11.022.
- Groenewegen A, Rutten FH, Mosterd A, et al. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–1356. doi: 10.1002/ejhf.1858.
- Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi: 10.15420/cfr.2016:25:2.
- Elasfar AA, Alhabeeb W, Elasfar S. Heart failure in the Middle east arab countries: current and future perspectives. J Saudi Heart Assoc. 2020;32(2):236–241. https://pubmed.ncbi.nlm.nih.gov/33154923 doi: 10.37616/2212-5043.1040.
- Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368–376. doi: 10.1016/j.ijcard.2013.12.028.
- Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)developed with the special contribution o. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. Sep doi: 10.1093/eurheartj/ehab368.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. Nov doi: 10.1056/NEJMoa1911303.
- McEwan P, Darlington O, McMurray JJV, et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail. 2020;22(11):2147–2156. doi: 10.1002/ejhf.1978.
- Yao Y, Zhang R, An T, et al. Cost-effectiveness of adding dapagliflozin to standard treatment for heart failure with reduced ejection fraction patients in China. ESC Heart Fail. 2020;7(6):3582–3592. doi: 10.1002/ehf2.12844.
- Savira F, Wang BH, Kompa AR, et al. Cost-effectiveness of dapagliflozin in chronic heart failure: an analysis from the Australian healthcare perspective. Eur J Prev Cardiol. 2020;28(9):975–982. Jul;2047487320938272. doi: 10.1177/2047487320938272.
- Isaza N, Calvachi P, Raber I, et al. Cost-effectiveness of Dapagliflozin for the treatment of heart failure with reduced ejection fraction. JAMA Netw Open. 2021;4(7):e2114501. Jul doi: 10.1001/jamanetworkopen.2021.14501.
- Dal Canto E, Ceriello A, Rydén L, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26(2_suppl):25–32. doi: 10.1177/2047487319878371.
- Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9(1):25. https://pubmed.ncbi.nlm.nih.gov/28435446 doi: 10.1186/s13098-017-0225-1.
- Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323(14):1353–1368. doi: 10.1001/jama.2020.1906.
- Krittayaphong R, Permsuwan U. Cost-utility analysis of add-on dapagliflozin treatment in heart failure with reduced ejection fraction. Int J Cardiol. 2021;322:183–190. doi: 10.1016/j.ijcard.2020.08.017.
- Nguyen B-N, Mital S, Bugden S, et al. Cost-effectiveness of dapagliflozin and empagliflozin for treatment of heart failure with reduced ejection fraction. Int J Cardiol. 2023;376:83–89. doi: 10.1016/j.ijcard.2023.01.080.
- Krittayaphong R, Permsuwan U. Cost-utility analysis of Sacubitril-Valsartan compared with enalapril treatment in patients with acute decompensated heart failure in Thailand. Clin Drug Investig. 2021;41(10):907–915. doi: 10.1007/s40261-021-01079-6.
- Jiang Y, Zheng R, Sang H. Cost-effectiveness of adding SGLT2 inhibitors to standard treatment for heart failure with reduced ejection fraction patients in China. Front Pharmacol. 2021;12:733681. doi: 10.3389/fphar.2021.733681.
- Hussain A, Misra A, Bozkurt B. Endpoints in heart failure drug development. Card Fail Rev. 2022;8:e01. doi: 10.15420/cfr.2021.13.
- Zannad F, Garcia AA, Anker SD, et al. Clinical outcome endpoints in heart failure trials: a European society of cardiology heart failure association consensus document. Eur J Heart Fail. 2013;15(10):1082–1094. doi: 10.1093/eurjhf/hft095.
- van der Pol S, Degener F, Postma MJ, et al. An economic evaluation of sacubitril/valsartan for heart failure patients in The Netherlands. Value Health. 2017;20(3):388–396. Mar doi: 10.1016/j.jval.2016.10.015.
- King JB, Shah RU, Bress AP, et al. Cost-effectiveness of Sacubitril-Valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2016;4(5):392–402. doi: 10.1016/j.jchf.2016.02.007.
- Backholer K, Hirakawa Y, Tonkin A, et al. Development of an Australian cardiovascular disease mortality risk score using multiple imputation and recalibration from national statistics. BMC Cardiovasc Disord. 2017;17(1):17. doi: 10.1186/s12872-016-0462-5.
- Abushanab D, Marquina C, Morton JI, et al. Projecting the health and economic burden of cardiovascular disease among people with type 2 diabetes, 2022–2031. Pharmacoeconomics. 2023;41(6):719–732. doi: 10.1007/s40273-023-01258-7.
- Yan BW, Spahillari A, Pandya A. Cost-effectiveness of quadruple therapy in management of heart failure with reduced ejection fraction in the United States. Circ Cardiovasc Qual Outcomes. 2023;16(6):e009793. doi: 10.1161/CIRCOUTCOMES.122.009793.
- Marquina C, Talic S, Vargas-Torres S, et al. Future burden of cardiovascular disease in Australia: impact on health and economic outcomes between 2020 and 2029. Eur J Prev Cardiol. 2022;29(8):1212–1219.
- Abushanab D, Al-Badriyeh D, Liew D, et al. First-line treatment with empagliflozin and metformin combination versus standard care for patients with type 2 diabetes mellitus and cardiovascular disease in Qatar. A cost-effectiveness analysis. Curr Probl Cardiol. 2021;47(6):100852. doi: 10.1016/j.cpcardiol.2021.100852.
- Kaddoura R, Abushanab D, Arabi AR, et al. Cost-effectiveness analysis of sacubitril/valsartan for reducing the use of implantable cardioverter-defibrillator (ICD) and the risk of death in ICD-eligible heart failure patients with reduced ejection fraction. Curr Probl Cardiol. 2022;47(12):101385. doi: 10.1016/j.cpcardiol.2022.101385.
- Al-Badriyeh D, Hssain AA, Abushanab D. Cost-effectiveness analysis of out-of-hospital versus in-hospital extracorporeal cardiopulmonary resuscitation for out-hospital refractory cardiac arrest. Curr Probl Cardiol. 2022;47(12):101387. doi: 10.1016/j.cpcardiol.2022.101387.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi: 10.1016/j.jval.2021.11.1351.
- Hamad Medical Corporation. [Internet]. Available from https://www.hamad.qa/EN/Pages/default.aspx.
- Briggs A, Claxton K, Sculpher M. Decision modeling for health economics evaluation. Briggs A, Claxton K, Sculpher M, editors. Oxford: Oxford University Press; 2006.
- Ministry of Development Planning and Statistics. Births & Deaths In the State of Qatar, [Internet]. 2016; [cited 2021 Dec 10]. Available from: https://www.psa.gov.qa/en/statistics/Statistical Releases/Population/BirthsDeaths/2016/Birth_death_2016_EN.pdf.
- Abushanab D, Liew D, Marquina C, et al. Cost-Effectiveness of empagliflozin and metformin combination versus standard care as first-Line therapy in patients with type 2 diabetes mellitus. Endocr Pract. 2022;28(1):16–24. doi: 10.1016/j.eprac.2021.07.018.
- Ministry of Development Planning and Statistics. Births & Deaths In the State of Qatar, 2016; [cited 2020 April]. Available from: https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Population/BirthsDeaths/2016/Birth_death_2016_EN.pdf.
- Adena MA, Hamann G, Sindone AP. Cost-Effectiveness of ivabradine in the treatment of chronic heart failure. Heart Lung Circ. 2019;28(3):414–422. doi: 10.1016/j.hlc.2018.01.011.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2022;145(18):e895–1032.
- Tan YJ, Ong SC, Kan YM. Is using sodium-glucose cotransporter-2 inhibitors to treat adults with chronic heart failure cost-effective? A systematic review of cost-effectiveness studies. Appl Health Econ Health Policy. 2023;21(6):857–875.
- Tafazzoli A, Reifsnider OS, Bellanca L, et al. A european multinational cost-effectiveness analysis of empagliflozin in heart failure with reduced ejection fraction. Eur J Health Econ. 2022;24(9):1441–1454. doi: 10.1007/s10198-022-01555-6.
- Qatar Inflation Rate [Internet]. 2021; [cited 2021 Oct 15]. Available from: https://tradingeconomics.com/qatar/inflation-cpi.
- Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020;35(1):107–114. doi: 10.1093/heapol/czz127.
- Adel A, Abushanab D, Hamad A, et al. Assessment of dasatinib versus nilotinib as upfront therapy for chronic phase of chronic myeloid leukemia in Qatar: a cost-effectiveness analysis. Cancer Control. 2021;28:10732748211001796. doi: 10.1177/10732748211001796.
- Abushanab D, Rouf PA, Al Hail M, et al. Cost-effectiveness of oral versus intravenous ibuprofen therapy in preterm infants with patent ductus arteriosus in the neonatal intensive care setting: a cohort-based study. Clin Ther. 2021;43(2):336–348.e7. https://www.sciencedirect.com/science/article/pii/S014929182030552X doi: 10.1016/j.clinthera.2020.12.004.
- Abushanab D, Alsoukhni O, AbouNahia F, et al. Clinical and economic analysis of morphine versus fentanyl in managing ventilated neonates with respiratory distress syndrome in the intensive care setting. Clin Ther. 2019;41(4):714–727.e8. doi: 10.1016/j.clinthera.2019.02.009.
- Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21. doi: 10.1093/bmb/ldq033.
- Goldsmith KA, Dyer MT, Schofield PM, et al. Relationship between the EQ-5D index and measures of clinical outcomes in selected studies of cardiovascular interventions. Health Qual Life Outcomes. 2009;7(1):96. https://pubmed.ncbi.nlm.nih.gov/19941657 doi: 10.1186/1477-7525-7-96.
- Dyer MTD, Goldsmith KA, Sharples LS, et al. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8(1):13. https://pubmed.ncbi.nlm.nih.gov/20109189 doi: 10.1186/1477-7525-8-13.
- Oberle W. Monte Carlo Simulations: number of Iterations and Accuracy. 2015; [cited 2023 Jun 10]. Available from: https://apps.dtic.mil/sti/pdfs/ADA621501.pdf.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes (4th ed.). Oxford, New York: Oxford University Press; 2015. 464 p.
- Vemer P, Corro Ramos I, van Voorn GAK, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–361. Apr doi: 10.1007/s40273-015-0327-2.
- Büyükkaramikli NC, Rutten-van Mölken MPMH, Severens JL, et al. TECH-VER: a verification checklist to reduce errors in models and improve their credibility. Pharmacoeconomics. 2019;37(11):1391–1408. doi: 10.1007/s40273-019-00844-y.
- Cai R-P, Xu Y-L, Su Q. Dapagliflozin in patients with chronic heart failure: a systematic review and Meta-Analysis. Cardiol Res Pract. 2021;2021:6657380–6657312. doi: 10.1155/2021/6657380.
- Docherty KF, Jhund PS, Anand I, et al. Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA-HF. Circulation. 2020;142(17):1623–1632. doi: 10.1161/CIRCULATIONAHA.120.047480.
- McCabe C, Dixon S. Testing the validity of cost-effectiveness models. Pharmacoeconomics. 2000;17(5):501–513. doi: 10.2165/00019053-200017050-00007.
- Aggarwal R, Vaduganathan M, Chiu N, et al. Out-of-pocket costs for SGLT-2 (Sodium-Glucose transport protein-2) inhibitors in the United States. Circ Heart Fail. 2022;15(3):e009099. doi: 10.1161/CIRCHEARTFAILURE.121.009099.
- Balkhi B, Alshayban D, Alotaibi NM. Impact of healthcare expenditures on healthcare outcomes in the Middle East and North Africa (MENA) region: a cross-Country comparison, 1995–2015. Front Public Heal. 2020;8:624962.
- Heidenreich PA, Fonarow GC, Breathett K, et al. ACC/AHA clinical performance and quality measures for adults with heart failure: a report of the American college of cardiology/American heart association task force on performance measures. Circ Cardiovasc Qual Outcomes. 2020;13(11):e000099.
- Parizo JT, Goldhaber-Fiebert JD, Salomon JA, et al. Cost-effectiveness of dapagliflozin for treatment of patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(8):926–935. doi: 10.1001/jamacardio.2021.1437.
- Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1):1447828. https://pubmed.ncbi.nlm.nih.gov/29564962 doi: 10.1080/16549716.2018.1447828.
- Qatar GDP Per Capita 1970–2023. Available from https://www.macrotrends.net/countries/QAT/qatar/gdp-per-capita.
- Thresholds for the cost–effectiveness of interventions: alternative approaches. 2023; [cited 2023 Jun 7]. Available from: https://apps.who.int/iris/handle/10665/271648.
- Qatar New Healthcare Insurance Law. 2024; [cited 2024 Jan 9]. Available from: https://www.trade.gov/market-intelligence/qatar-new-healthcare-insurance-law#:∼:text=All%20non%2DQatari%20nationals%20and,to%20receive%20basic%20medical%20services.&text=Qatari%20natio.
- Zaghloul N, Awaisu A, Mahfouz A, et al. A 5-year trend in the use of sodium-glucose co-transporter 2 inhibitors and other oral antidiabetic drugs in a Middle Eastern country. Int J Clin Pharm. 2022;44(6):1342–1350. doi: 10.1007/s11096-022-01464-x.
- Sugiyama S, Jinnouchi H, Kurinami N, et al. Impact of dapagliflozin therapy on renal protection and kidney morphology in patients with uncontrolled type 2 diabetes mellitus. J Clin Med Res. 2018;10(6):466–477. https://pubmed.ncbi.nlm.nih.gov/29707088 doi: 10.14740/jocmr3419w.
- Mielck A, Vogelmann M, Leidl R. Health-related quality of life and socioeconomic status: inequalities among adults with a chronic disease. Health Qual Life Outcomes. 2014;12(1):58. doi: 10.1186/1477-7525-12-58.
- Quality of Life Index by Country 2023. 2023.