Abstract
Aim
Bosentan, ambrisentan, and macitentan are endothelin receptor antagonists (ERAs), currently available in Australia for treatment of pulmonary arterial hypertension (PAH). This study assessed the comparative adherence of these ERAs for PAH in Australian patients.
Methods
This retrospective, observational study used data for adults with PAH from the Services Australia 10% Pharmaceuticals Benefits Scheme (PBS) dataset (01/2006-10/2020). The primary outcome was treatment adherence (i.e. receiving ≥80% of ERA doses over 12 months). Secondary outcomes were time to treatment change (add-on or switch) and overall survival.
Results
The study included 436 patients who took bosentan (n = 200), ambrisentan (n = 69), or macitentan (n = 167). Treatment adherence was significantly greater in patients who received macitentan (65.3%) versus ambrisentan (56.5%) and bosentan (58.0%), with odds ratios (ORs; 95% CI) of 0.51 (0.30–0.88; p = 0.016) for bosentan versus macitentan and 0.48 (0.24–0.96; p = 0.037) for ambrisentan versus macitentan. The median time to treatment change was 47.2 and 43.4 months for bosentan and ambrisentan, respectively (not calculated for macitentan because of insufficient duration of data).
Limitations and conclusions
Real-world data for Australian patients with PAH showed that treatment adherence for ERAs was suboptimal. Adherence was higher for macitentan compared with ambrisentan and bosentan.
Introduction
Pulmonary arterial hypertension (PAH) is a life-threatening disease characterized by proliferative remodeling of the small pulmonary arteries [Citation1]. The actual prevalence of PAH has not yet been established, but a study based on data from 2003 through 2009 in Western Australia reported a prevalence of 151 cases per million people [Citation2], and an estimated 2,000 Australians have been diagnosed with PAH [Citation3]. The number of patients living with the disease in Australia, however, is likely much higher. According to the Pulmonary Hypertension Society of Australia and New Zealand (PHSANZ) registry, the mean (range) age of patients with PAH is 60 (45–71) years and most patients (79%) are female [Citation4].
Currently available treatments for PAH target dysfunction in either the prostacyclin, nitric oxide, or endothelin-1 pathways [Citation5]. At present, five drug classes that target these pathways are recommended for PAH treatment: endothelin receptor antagonists (ERAs; e.g. macitentan, ambrisentan, and bosentan), phosphodiesterase type 5 (PDE-5) inhibitors (e.g. sildenafil and tadalafil), prostanoids (e.g. epoprostenol, iloprost, beraprost, and treprostinil), soluble guanylate cyclase stimulators (e.g. riociguat), and selective prostacyclin receptor agonists [Citation6,Citation7]. Both bosentan—the first ERA to be synthesized—and macitentan are dual endothelin-A and -B receptor antagonists, while ambrisentan is a selective endothelin-A receptor antagonist [Citation8,Citation9]. Bosentan was also the first ERA introduced as a treatment for PAH in Australia in 2004, followed by ambrisentan in 2009, and macitentan in 2014 [Citation4].
For patients with PAH, combination therapy featuring an ERA and a PDE-5 inhibitor has demonstrated superior efficacy than ERA monotherapy in recent network meta-analyses [Citation7,Citation10,Citation11]. Additionally, current pulmonary hypertension treatment guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) recommend initial combination therapy for patients with PAH [Citation9].
Poor treatment adherence may be common in PAH, and medication interruptions can have negative consequences, including increased risk of hospitalization [Citation12,Citation13]. However, there are limited data on adherence to PAH therapy. Grady et al. noted a 47.9% rate of high adherence, 40.3% rate of moderate adherence, and 11.8% rate of low adherence [Citation14]. Frequent dosing, longer duration of PAH-specific therapy, combination therapy versus monotherapy, and poor use of compliance aids (e.g. pill organizers) are among factors that were associated with low adherence. In a study by Studer et al. adherence to combination therapy was nearly double that of monotherapy (90.2% vs 46.6%) [Citation15]. Adherence rates for sildenafil and tadalafil vary among studies (94% in 1 study [Citation16] and 46.8% in another [Citation17]).
Although substantial evidence supports the efficacy and tolerability of ERAs [Citation7,Citation18], there is paucity of data regarding adherence to ERA therapy. Therefore, this study was conducted with the primary objective of obtaining adherence data for macitentan compared with ambrisentan and bosentan in a real-world Australian setting by using the Pharmaceuticals Benefits Scheme (PBS) 10% (PBS10) sample dataset.
Methods
Study design
This retrospective, observational study used sample data from the PBS10 dataset between January 2006 and October 2020. The primary objective was to compare treatment adherence in Australian patients with PAH who were treated with the ERAs macitentan, ambrisentan, and bosentan. The study was conducted in accordance with the International Conference on Harmonisation Harmonized Tripartite Guideline: Guideline for Good Clinical Practice with applicable local and regional regulations and with the Declaration of Helsinki’s ethical principles. Due to the study’s retrospective design and use of de-identified data, ethics approval and informed consent were not required. Access to the PBS10 sample dataset did require approval from the External Request Evaluation Committee.
Data sources
The PBS10 sample dataset is a national record of pharmaceutical claims consisting of a systematic random sample of 10% of the Australian population that is eligible for Medicare and all their reimbursed dispensations. The Australian government makes this 10% sample available through data custodians for research purposes, and longitudinal data are available for this study from January 2006 to October 2020. This dataset includes all PBS-reimbursed prescriptions for all patients included in the data, and unique patients can be nationally tracked over time, even after switching prescriber or pharmacy. The Australian Government reimburses community pharmacies and private hospitals for PBS-listed medicines, subsidizing approximately 75% of prescribed medicine use in Australia. Patient data were extracted based on prescriptions for PAH-specific medications, which were identified by the associated anatomical therapeutic classification code.
Patients
Patients with PAH were identified from the PBS10 sample dataset using the following inclusion criteria: (1) received ≥1 PAH-specific drug (Supplementary Table 1), (2) aged ≥18 years at the time of prescription for any PAH drug, (3) a period of ≥12 months from the initiation of treatment for PAH to the end of the PBS10 sample data (October 2020), and (4) a period of ≥6 months from the start of the PBS10 sample dataset (January 2006) to their first PAH drug prescription.
Outcomes
The primary outcome for this study was adherence to ERA treatment. The secondary outcomes were time to treatment change (add-on or switch) and overall survival. The effect of concomitant dispensing of inhaler therapy for chronic obstructive pulmonary disease (COPD) or asthma was also assessed. The exposure of interest was treatment with macitentan, ambrisentan, or bosentan. Patients were assigned to cohorts according to their index ERA, which was defined as the first prescribed ERA during the patient selection period. Subsequent changes in ERA prescription did not result in changes in the patient’s cohort and such treatment episodes were not investigated in the study. The index date was defined as the date of first prescription of the index ERA for each patient who had not received any ERA treatment for PAH within the previous 6 months or more.
Treatment adherence was defined as a patient receiving ≥80% of ERA doses over 12 months. Sensitivity analyses were also performed using thresholds of 70% and 60%. Treatment adherence for each patient was calculated using the proportion of days covered method, and if days’ supply data was not provided, an assumption was made that each prescription lasted 30 days.
Time to treatment change was calculated as the time from commencement of the index ERA to the first switch or add-on, calculated in consecutive days. Overall survival was defined as the time from commencement until the time of death from any cause measured in consecutive days. Since only the year of death is provided in the PBS10 sample dataset, the date of death was classified as the date of the patient’s final purchase on the PBS of that year or January 1 if there were no PBS purchases in the year of the patient’s death. Thus, in the absence of a specific date of death, the analysis used the earliest date the patient could have died.
Statistical analyses
Baseline demographic characteristics are summarized descriptively, using means and standard deviations (SDs) or medians (ranges) for continuous variables and frequency distributions and percentages for categorical variables.
Patients were censored at the last date of data extraction. The Kaplan-Meier method was used to plot the time to treatment change and overall survival curves. Differences in time to treatment change and overall survival were assessed with a Cox proportional hazards model, while differences in treatment adherence were evaluated with a multiple logistics regression model. For both models, the propensity score was used as the only covariate, which is calculated based on gender, age, comorbidity at baseline (hypertension, diabetes, renal disease, arterial disease), line therapy (first-line vs second-line or higher), and coronary heart disease.
For the outcomes of time to treatment change and overall survival, the same analysis was performed, but the analysis model included a variable for inhaler therapy versus no inhaler therapy instead of index ERA.
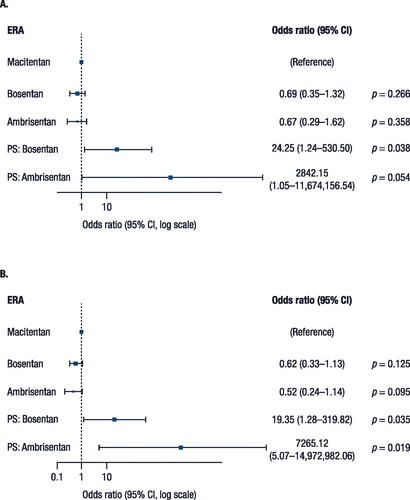
Propensity score adjustment increases the comparability of the observed baseline characteristics in patients treated with macitentan, ambrisentan, or bosentan. The propensity score is the conditional probability of receiving treatment [Citation19], which was estimated by multinomial logistic regression on the three ERA treatment groups. During the final modelling stage, two propensity scores, corresponding to non–reference-level ERAs, were included as covariates in the logistic and Cox regression models. Propensity scores for patients were calculated based on fitting the model to the data for patients who received an ERA at any time during the observation period. Compared to matching method, regression adjustment method has the advantage of preserving the sample size as all patients were used in the model.
The following covariates were used to estimate each patient’s propensity score: gender, age at index date (<50, 50–59, 60–69, 70–79, or ≥80 years), line of therapy (first, second, or higher), prior arterial disease, prior hypertension, prior renal disease, and prior diabetes. Propensity score adjustments were also made to increase comparability of observed baseline characteristics between patients who received or did not receive COPD/asthma inhaler therapy. For this binary outcome, the propensity score was estimated by binary logistic regression using the same independent variables as the primary outcome. During the final modelling stage, propensity scores from the binary logistic regression were included as a covariate.
Results were considered statistically significant for p < 0.05. All statistical analyses were performed using R (R Foundation) [Citation20].
Results
Patient disposition and characteristics
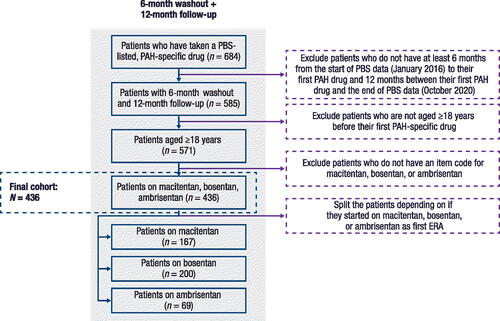
A total of 684 patients who had taken a PAH-specific drug were identified in the PBS10 sample dataset. After applying all eligibility criteria, the final cohort was comprised of 436 patients who had taken macitentan (n = 167), ambrisentan (n = 69), or bosentan (n = 200) as their index ERA ().
Figure 1. Patient disposition.
ERA, endothelin receptor antagonist; PAH, pulmonary arterial hypertension; PBS, Pharmaceuticals Benefits Scheme.
Overall, 67.0% of the patients were women (). Mean (SD) age was 62.0 (16.2) years, and 61.0% of patients were ≥60 years of age. For most patients (93.3%), macitentan, ambrisentan, or bosentan was a first-line therapy. Hypertension was the most common comorbidity, affecting 78.0% of patients, followed by arterial disease (35.1%), diabetes (19.7%), and comorbid renal disease (3.0%). A total of 15.6% of patients received inhaler therapy for COPD/asthma.
Table 1. Baseline demographic and clinical characteristics.
Primary outcome
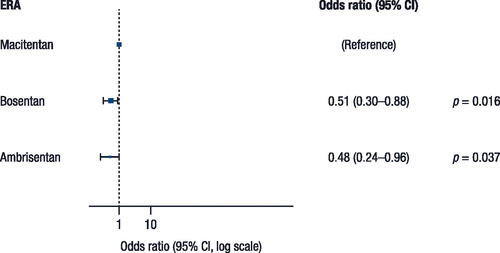
Treatment adherence was statistically significantly greater in patients who received macitentan (65.3%) versus ambrisentan (56.5%) and bosentan (58.0%). The odds ratio (OR) was 0.51 (95% confidence interval [CI]: 0.30–0.88; p = 0.016) for bosentan versus macitentan and 0.48 (95% CI: 0.24–0.96; p = 0.037) for ambrisentan versus macitentan, representing 49% and 52% lower odds of being adherent with bosentan and ambrisentan versus macitentan, respectively (). Overall, 68.3% of patients were classified as adherent to treatment when the threshold was lowered to 70% (70.1%, 68.0%, and 65.2% with macitentan, bosentan, and ambrisentan, respectively); 71.8% of patients were adherent when the threshold was 60% (71.9%, 72.0%, and 71.0%, respectively).
Sensitivity analyses revealed that statistical significances at the 80% threshold disappeared once the cutoffs were lowered to 60% and 70%, although the direction and magnitude of parameters remained consistent with the main result ().
Secondary outcomes
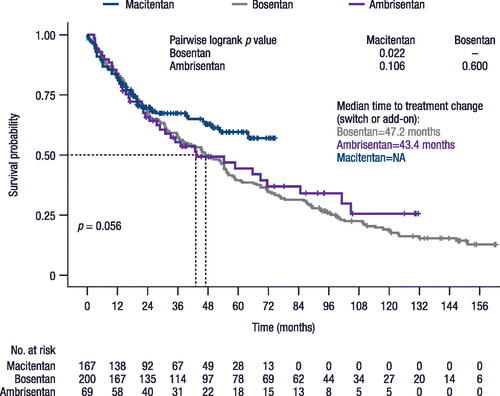
The median time to treatment change was 47.2 months and 43.4 months in patients with an index ERA of bosentan and ambrisentan, respectively (). This value could not be calculated for macitentan because insufficient duration of data were available to calculate median time. According to the Cox proportional hazards model, however, time to treatment change was statistically significantly longer with macitentan than bosentan. The hazard ratio (HR) was 1.74 (95% CI: 1.27–2.39; p = 0.001) for bosentan versus macitentan and 1.28 (95% CI: 0.85–1.92; p = 0.239) for ambrisentan versus macitentan. The proportional hazards assumption was not met for this outcome (Supplemental Figure 1).
Figure 4. Time to treatment change (switch or add-on).
Note, time before switching may be impacted by the varying approval dates of the treatments (i.e. 2004 for bosentan, 2009 for ambrisentan, and 2014 for macitentan). NA, not achieved.
No statistically significant differences in overall survival were identified among the three ERAs, with median values of 93.3 months for bosentan and 83.9 months for ambrisentan (p = 0.53). Median values were not available for macitentan. The HR was 1.36 (95% CI: 0.94–1.96; p = 0.103) for bosentan versus macitentan and 1.26 (95% CI: 0.80–1.97; p = 0.321) for ambrisentan versus macitentan.
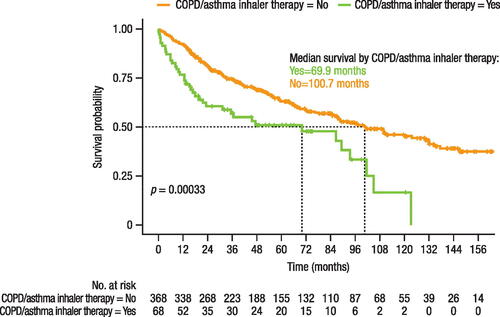
Patients who received COPD/asthma inhaler therapy had a statistically significantly shorter time (31.1 months) to treatment change than those who did not receive inhaler therapy (54.6 months; p = 0.0098; ). The HR for time to treatment change was 1.44 (95% CI: 1.03–2.00; p = 0.033) for patients taking inhaler therapy. Overall survival time was also statistically significantly shorter in patients receiving inhaler therapy (69.9 months) than those without inhaler therapy (100.7 months; p = 0.0003; ). The HR for overall survival was 1.66 (95% CI: 1.15–2.38; p = 0.006) for patients with versus without inhaler therapy. The proportional hazards assumption was met for overall survival in this population (Supplemental Figure 2), but not for time to treatment change (Supplemental Figure 3).
Figure 5. Effect of inhaler therapy for COPD/asthma use on time to treatment change.
COPD, chronic obstructive pulmonary disease.
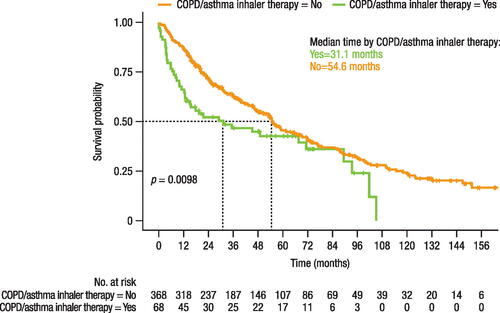
Figure 6. Effect of inhaler therapy for COPD/asthma use on overall survival.
COPD, chronic obstructive pulmonary disease.
No statistically significant differences were found when evaluating the effect of inhaler therapy on treatment adherence. For treatment adherence, the OR in patients taking inhaler therapy versus not taking inhaler therapy was 1.71 (95% CI: 0.84–3.81; p = 0.160).
Discussion
In this study, we generated real-world evidence comparing the three ERAs macitentan, ambrisentan, and bosentan in Australian patients with PAH using data from the Australian PBS10 sample dataset. For the primary outcome, overall treatment adherence appeared to be suboptimal and there were statistically significant differences among the three ERAs assessed. These findings are important for a lethal disease like PAH where treatment adherence may potentially impact the efficacy of therapies.
Treatment adherence, which can be influenced by patient age, dosing regimen, the presence of comorbidities, and the use of a specialist, is an important variable in the management of PAH [Citation21,Citation22]. Research on the adherence of patients with PAH to prescribed medications is scarce, but data from other chronic conditions show that nonadherence is associated with higher costs and other negative outcomes, such as higher hospitalization rates [Citation12,Citation21]. Prior studies in PAH showed that overall treatment adherence rates ranged widely, from 47% [Citation17] to 96% in patients treated in a specialty clinic [Citation16]. Adherence also varies between different PAH-specific drug classes, with higher rates observed with ERAs compared to PDE-5 inhibitors [Citation12,Citation13]. In our study, treatment adherence was highest in patients who received macitentan (65.3%) versus ambrisentan (56.5%) and bosentan (58.0%). Another study of several PAH-specific medications similarly found higher adherence in patients taking macitentan (97.0%) than ambrisentan (75.1%) and bosentan (67.4%) [Citation23]. Another study found adherence to be highest with ambrisentan (87% [number of days covered] and 82% [manual assessment method]), followed closely by macitentan (82% and 77%) and bosentan (71% and 68%) [Citation22]. It is possible that once-daily dosing of ambrisentan and macitentan may explain, in part, the superior adherence to the twice-daily dosing of bosentan. Our study underscores the fact that promoting adherence is an important aspect of PAH, which has been largely ignored by most physicians. Not only are PAH medications potentially lifesaving, but they also incur a great cost to the health care system. We postulate that improving PAH drug adherence/persistence could lead to better long-term outcomes by reducing hospitalization, improving exercise capacity, improving health-related quality of life, and improving productivity. We anticipate that these pharmacoeconomical aspects will be investigated in future studies. Also of high importance, more studies are urgently needed to identify factors that are associated with poor adherence in PAH. Potential strategies to improve adherence may include patient education, simplification of regimen (e.g. combination pills), electronic pill reminders, and evaluation of adherence at clinic visits. Together, potential improvements in adherence may be beneficial to clinical outcomes for patients with PAH.
The median time to treatment change could not be calculated for macitentan due to a lack of sufficient duration; however, according to the Cox proportional hazards model, patients taking macitentan remained on treatment significantly longer than in those taking bosentan and similar to those taking ambrisentan. Although our study could not answer the specific reasons for this finding, it is known that bosentan was associated with 4–5% risk of abnormal liver function tests [Citation6]. We hypothesize that this is perhaps one reason that bosentan was associated with the least time to treatment change.
Concurrent dispensing of inhaler therapy for COPD/asthma had a statistically significant impact on both time to treatment change and overall survival. The median time to treatment change was 31.1 months in patients receiving inhaler therapy versus 54.6 months in without inhaler therapy, while the median overall survival time was 69.9 months and 100.7 months in these patients, respectively. This outcome was of interest because the accurate diagnosis of PAH is often difficult and pulmonary comorbidity is common in PAH as more elderly patients are diagnosed with this condition [Citation24,Citation25]. Thus, many patients given a diagnosis of PAH may in fact have pulmonary hypertension due to lung disease (Group 3) or PAH with a lung disease phenotype [Citation9,Citation26,Citation27]. In either case, pulmonary hypertension due to lung disease or PAH with lung phenotypes are known to respond poorly to PAH medications and have a poorer survival compared to classical PAH [Citation28].
The results indicate that the proportional hazard assumption is met for the third outcome (i.e. overall survival), which is of particular clinical significance. However, this assumption is violated for the first two outcomes, which are considered secondary in our study. In light of these findings, the HR for the first two outcomes should be interpreted with caution as the HR is unlikely to remain constant throughout the study period, but the impact is considered moderate due to the moderate observed crossover in the Kaplan-Meier curves between groups ( and ). While the violation of the proportional hazard assumption may influence the interpretation, it is important to emphasize again that the HR of the third outcome, overall survival, remains consistent with the assumption.
Our findings should be interpreted carefully with the consideration of certain limitations. Analyses for this retrospective study were limited to the available data in the PBS10 sample dataset, some patient-level data were likely missing. In addition, clinical details regarding the severity of each patient’s PAH diagnosis were not available in the PBS10 sample dataset; therefore, surrogates, such as line of therapy and comorbidities, were used for data analysis. Furthermore, there may be discrepancies in the time of death as the analysis used the earliest possible date the patient could have died if a date was not available in the PBS10 sample. For the outcome of treatment adherence, since the number of days’ supply was not available, it was assumed that each prescription lasted for 30 days and that patients took all the medications prescribed.
Conclusions
Our findings from comparing adherence among ERAs demonstrate that real-world adherence of PAH medications is suboptimal, despite these medications being potentially life-saving treatment. These findings may be beneficial for researchers and health care providers who care for patients with PAH to inform treatment decisions and potentially improve clinical outcomes. Additional research is warranted to confirm our findings in a larger and more inclusive dataset and to clarify the potential reason for the lack of adherence in some patients—including the predictors of adherence of ERAs and other PAH therapies.
Transparency
Author contributions
All authors contributed to the study conception and design and interpretation of the data. Data analysis was performed by SL, MHK, and JC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
IRB information
The study was conducted in accordance with the International Conference on Harmonisation’s Good Clinical Practice Guidelines, the Declaration of Helsinki, and local and regional regulations. Because of its retrospective design and use of de-identified data, ethics approval, and informed consent were not required for this study. This study and publication of subsequent results were also approved by Services Australia (External Request Evaluation Committee Approval Number RMS3592). The Australian Government Department of Human Services External Request Evaluation Committee functions essentially as an ethics committee that reviews the purpose, anonymity, consent, secrecy, resource use, reputation, and other policies of research being conducted.
Supplemental Material
Download MS Word (295.8 KB)Acknowledgement
Medical writing support was provided by Alaina Mitsch, PhD, of Lumanity Communications Inc., and was funded by Janssen Asia Pacific Medical Affairs.
Declaration of funding
This study was funded by Janssen Asia Pacific Medical Affairs. The Sponsor was involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declaration of financial/other interests
EL and EK have received research support, honoraria, and speaking fees from Janssen, GlaxoSmithKline, and Pfizer. YM, DYY, JYT, and DBW are employees of Janssen Pharmaceuticals. MHK, JC, and SL are employees of Prospection, which received funding from Janssen to conduct the study analyses. PB was an employee of Janssen Pharmaceuticals at the time of the analysis.
Data availability statement
The de-identified participant data will not be shared.
References
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–1436. doi: 10.1056/NEJMra040291.
- Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the armadale echocardiography cohort. Heart. 2012;98(24):1805–1811. doi: 10.1136/heartjnl-2012-301992.
- The Pharmaceutical Benefits Scheme. Pulmonary arterial hypertension (PAH) medicines utilisation analysis. Sydney, Australia: The Pharmaceutical Benefits Scheme Drug Utilisation Sub-committee; 2015. [cited 2023 May 19]. Available from: http://www.pbs.gov.au/industry/listing/participants/public-release-docs/pulm-art-hypertension/pulmonary-arterial-hypertension-dusc-prd-2015-02-final.pdf.
- Anderson J, Lavender M, Lau E, et al. Pharmacological treatment of pulmonary arterial hypertension in Australia: current trends and challenges. Heart Lung Circ. 2020;29(10):1459–1468. doi: 10.1016/j.hlc.2020.01.017.
- Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. doi: 10.1056/NEJMoa1413687.
- Zhang YJ, Wang N, Gu ZC, et al. A network meta-analysis for safety of endothelin receptor antagonists in pulmonary arterial hypertension. Cardiovasc Diagn Ther. 2019;9(3):239–249. doi: 10.21037/cdt.2019.03.02.
- Gao XF, Zhang JJ, Jiang XM, et al. Targeted drugs for pulmonary arterial hypertension: a network meta-analysis of 32 randomized clinical trials. Patient Prefer Adherence. 2017;11:871–885. doi: 10.2147/PPA.S133288.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297.
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. doi: 10.1093/eurheartj/ehac237.
- Lin H, Wang M, Yu Y, et al. Efficacy and tolerability of pharmacological interventions for pulmonary arterial hypertension: a network meta-analysis. Pulm Pharmacol Ther. 2018;50:1–10. doi: 10.1016/j.pupt.2017.11.002.
- Wang S, Yu M, Zheng X, et al. A Bayesian network meta-analysis on the efficacy and safety of eighteen targeted drugs or drug combinations for pulmonary arterial hypertension. Drug Deliv. 2018;25(1):1898–1909. doi: 10.1080/10717544.2018.1523257.
- Frantz RP, Hill JW, Lickert CA, et al. Medication adherence, hospitalization, and healthcare resource utilization and costs in patients with pulmonary arterial hypertension treated with endothelin receptor antagonists or phosphodiesterase type-5 inhibitors. Pulm Circ. 2020;10(1):2045894019880086. doi: 10.1177/2045894019880086.
- Hull M, Pruett J, Koep E, et al. Treatment patterns and medication adherence and persistence among patients with pulmonary arterial hypertension in a real-world database representing a large US health plan. Paper presented at: 22nd Annual ISPOR International Meeting; 2017 May 20–24; Boston, MA.
- Grady D, Weiss M, Hernandez-Sanchez J, et al. Medication and patient factors associated with adherence to pulmonary hypertension targeted therapies. Pulm Circ. 2018;8(1):2045893217743616. doi: 10.1177/2045893217743616.
- Studer S, Hull M, Pruett J, et al. Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database. Pulm Circ. 2019;9(1):2045894018816294. doi: 10.1177/2045894018816294.
- Shah NB, Mitchell RE, Proctor ST, et al. High rates of medication adherence in patients with pulmonary arterial hypertension: an integrated specialty pharmacy approach. PLoS One. 2019;14(6):e0217798. doi: 10.1371/journal.pone.0217798.
- Waxman A, Chen SY, Boulanger L, et al. Factors associated with adherence to phosphodiesterase type 5 inhibitors for the treatment of pulmonary arterial hypertension. J Med Econ. 2013;16(2):298–306. doi: 10.3111/13696998.2012.756399.
- Jain S, Khera R, Girotra S, et al. Comparative effectiveness of pharmacologic interventions for pulmonary arterial hypertension: a systematic review and network meta-analysis. Chest. 2017;151(1):90–105. doi: 10.1016/j.chest.2016.08.1461.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786.
- Jansen AC, van Aalst-Cohen ES, Tanck MW, et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med. 2004;256(6):482–490. doi: 10.1111/j.1365-2796.2004.01405.x.
- Mandras SA, Gilkin RJ, Jr, Pruett JA, et al. Pulmonary arterial hypertension: progress and challenges in the modern treatment era. Am J Manag Care. 2014;20(9):S191–S199.
- Kjellström B, Sandqvist A, Hjalmarsson C, et al. Adherence to disease-specific drug treatment among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. ERJ Open Res. 2020;6(4):00299-2020. doi: 10.1183/23120541.00299-2020.
- Leo S, Liu Y, Brown-Gentry B, et al. Utilization and adherence rates to pulmonary arterial hypertension medications. Paper presented at: AMCP Managed Care & Specialty Pharmacy Annual Meeting; 2017 March 27–30; Denver, CO.
- Tatius B, Wasityastuti W, Astarini FD, et al. Significance of BMPR2 mutations in pulmonary arterial hypertension. Respir Investig. 2021;59(4):397–407. doi: 10.1016/j.resinv.2021.03.011.
- Hoeper MM, Pausch C, Grünig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant. 2020;39(12):1435–1444. doi: 10.1016/j.healun.2020.09.011.
- Hayes D. Jr. Idiopathic pulmonary arterial hypertension misdiagnosed as asthma. J Asthma. 2007;44(1):19–22. doi: 10.1080/02770900601125243.
- Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med. 2013;173(10):887–893. doi: 10.1001/jamainternmed.2013.319.
- Hoeper MM, Dwivedi K, Pausch C, et al. Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med. 2022;10(10):937–948. doi: 10.1016/S2213-2600(22)00097-2.