Abstract
Aim
Patients with moderately to severely active ulcerative colitis have an increasing number of advanced therapy options including several biologics and Janus kinase inhibitors. Though data on efficacy and safety of these advanced therapies are available, less is known about the potential economic implications of their utilization in Japan. We evaluated the relative value of these advanced therapies in Japan using a locally developed cost per responder model.
Methods
A model was developed using relevant clinical endpoints and treatment costs to calculate cost per responder of all advanced therapies used for moderately to severely active ulcerative colitis treatment in Japan. Cost per responder was assessed in biologic-naïve and biologic-exposed populations, respectively. The model incorporated induction and maintenance therapy pathways as patients progressed through based on efficacy rates (clinical response, clinical remission and endoscopic improvement). Total costs for induction and maintenance included: drug acquisition, drug administration and serious adverse event management (as necessary) for responders, with additional rescue treatment cost only for non-responders.
Results
Upadacitinib showed lower cost per clinical response and cost per clinical remission across both biologic-naïve and biologic-exposed populations with only one exemption in cost per clinical remission in biologic-naïve population. In addition, upadacitinib demonstrated lower cost per endoscopic improvement in both populations. Janus kinase inhibitors outperformed with lower cost per responder than other mediations across all outcomes and patient populations with the exception of tofacitinib for clinical remission in biologic-exposed UC population.
Limitations
Comparative data used in this analysis have been derived from network meta-analysis, not from direct comparison.
Conclusions
The results of this cost per responder analysis suggest upadacitinib is a cost-effective option for the first- and second-line treatment of moderately to severely active ulcerative colitis in Japan.
Introduction
Ulcerative colitis (UC) is a type of inflammatory bowel disease that results in symptoms such as bloody diarrhea, abdominal pain and constipation. If not properly treated, UC can lead to morbidity, surgery, and a poor quality of life [Citation1]. The pooled prevalence of UC in Europe and North America ranges from 286 to 505 per 100,000 population and the global prevalence has continued to increase [Citation2]. However, in Japan, the prevalence UC per 100,000 population has rapidly surged from 5 in 2010 to 98 in 2019 [Citation3]. This increase has received attention from the Ministry of Health, Labor and Welfare as UC is a designated intractable disease which entitles patients to government support schemes [Citation4].
Treatment for UC is determined based on the severity of symptoms, and is generally classified as mild, moderate, and severe [Citation5]. Biologic agents (e.g. infliximab, adalimumab, and golimumab) have been approved for moderate-to-severe UC as advanced therapies in several countries, expanding the range of non-surgical treatment options available to patients [Citation6,Citation7]. However, more than 60% of patients still do not achieve remission when treated with biologics [Citation8], and patients who have failed to respond to first-line anti-tumor necrosis factor (TNF) agents are at increased risk for treatment failure to second-line biologics [Citation9]. New modes of treatment such as α4β7 integrin antagonist, anti-IL-12/23p40 antibodies or Janus kinase (JAK) inhibitors have been continuously added to advanced therapies as alternative options for UC. However, according to the guidelines of the Japanese Society of Gastroenterology, a curative medical therapy has not been established for UC [Citation10]. Further investigation is needed to understand the value of advanced therapies in UC in terms of providing a short-term relief for patients suffering from symptoms as well as a long-term benefit.
There are a number of potential efficacy outcomes which can be used to assess response to advanced therapies when treating UC. Systematic reviews and meta-analyses of UC treatments include trials with the following efficacy endpoints: proportion of patients receiving colectomy (1 to 3 years) after treatment [Citation11], mucosal healing [Citation12], clinical response, clinical remission [Citation13], and quality of life as key efficacy endpoints. The updated Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) statement from the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) ranked clinical response, clinical remission and endoscopic healing as the three most important targets for treatment of UC [Citation14]. Systematic comparison using each of these important endpoints is warranted to better understand the value of current treatments.
The high drug cost of advanced therapies in the treatment of UC has led to research on the cost per response for those therapies available on the market. The cost per responder (CPR) model is a precise analytical tool for evaluating the economic value of medical treatments based on achieving specific patient outcomes. Reducing complex clinical outcomes to a binary response metric allows for an efficient comparison of interventions based on cost-effectiveness by factoring associated costs into the economic model [Citation15,Citation16]. Evidence from the US for patients with moderate-to-severe UC reported significant variation in cost per responder, with $173,948 at 52 weeks for tofacitinib (orally; 10 mg) compared to $658,162 for ustekinumab (subcutaneous; 90 mg) [Citation17]. In a biological-naïve cohort from the US, infliximab emerged as the most cost-effective UC treatment at $99,171 per mucosal healing (MH), outperforming adalimumab ($316,378 per MH) and vedolizumab ($301,969 per MH), with its cost-effectiveness sensitive to non-drug administration costs [Citation18]. Considerable variation was also identified in Italy, with cost per patients in sustained response at 52-weeks ranging between €47,772 with vedolizumab 300 mg and €101,181 with adalimumab [Citation19]. In Japan, the level of evidence on cost per response for advanced therapies remains scarce, signaling the need for further research to understand the likely financial impact of using advanced therapies for treating UC.
The objective of this study was to evaluate the cost-effectiveness of the advanced therapies used in the treatment of moderately to severely active UC using a CPR model with Japanese healthcare system perspective. The cost-effectiveness of these options was explored in biologic-naïve (individuals who have not been previously treated with biologics) and biologic-exposed (individuals who have previously been treated with biologics) populations, respectively.
Methods
Population and response definitions
The population of interest for the model were Japanese adults with moderate-to-severe active UC. Japanese adults were considered to be ≥15 years old according to pharmaceutical labeling rules of Japan [Citation20]. Both biologic-naïve and biologic-exposed patients were considered under different scenarios. Response to treatment was measured as clinical response, clinical remission, and endoscopic improvement rates. All three endpoints were evaluated at the end of 52 weeks of treatment as part of a “treat-through” intent-to-treat (ITT) design. Patients remained on the treatment they were first prescribed at induction and maintenance phases as long as patients achieve at least a clinical response at the end of induction phase. Clinical response was defined as a decrease from baseline in full Mayo score ≥3 points and ≥30%, accompanied by a decrease in rectal bleeding score ≥1 or an absolute rectal bleeding score ≤1. The full Mayo score consists of four subscores, each scored from 0 to 3: stool frequency, rectal bleeding score, Mayo endoscopic score, and physician’s global assessment, for a total score range of 0 to 12 [Citation21]. Clinical remission was defined as a full Mayo score ≤2 with no subscore >1. Endoscopic improvement was defined as Mayo endoscopic score ≤1.
Model structure and analysis
A simple linear model was developed to allow patients to move from induction therapy to maintenance therapy or switch treatments when they failed to achieve a clinical response at the end of induction, over the course of 52 weeks (). Treatment regimen codes for the drugs of interest (infliximab, adalimumab, golimumab, ustekinumab, vedolizumab, tofacitinib, filgotinib and upadacitinib) as well as dosages of interest are described in . It was assumed that patients who respond to advanced therapies by the end of induction phase continue on the same treatment during maintenance phase. At the end of induction phase, patients were classified as a responder or non-responder as determined only by the clinical response endpoint. Patients that did not respond to any of the advanced therapies by the end of induction moved to a maintenance phase scenario but switched to an alternative therapy. It was assumed that among non-responders in the induction phase, patients switch from their induction treatment to one of two possible maintenance treatment scenarios: 5-biologics-basket (a weighted average of infliximab, vedolizumab, adalimumab, golimumab, and ustekinumab based on market share (Supplementary Table 1) or no treatment. These two scenarios were included separately in the model. Non-responders who moved to 5-biologics-basket started their new treatment with another induction dosing. Non-responders at the end of induction phase were assumed to retain their non-responder status until the end of maintenance phase in all scenarios. Any efficacy benefits from the bio-basket treatment were not considered due to data limitations.
Figure 1. Model schematic.
This schematic represents treatment with advanced therapies as 8-week induction and 44-week maintenance phases. Duration of induction and maintenance phases varies according to differing specifications for each advanced therapy in the model (). Patients classified as non-responders were switched to either of the alternative treatment scenarios (i) 5-biologics-basket (weighted average of infliximab, adalimumab, vedolizumab, ustekinumab, and golimumab) or (ii) no treatment. Non-responder frequency of visits was once per month. Response rate was stable until the end of the maintenance phase (formula applied: 1-clinical response rate). Patients in the responder population, continued the advanced therapies they received in the induction phase once per two months. Clinical response was reduced in the same proportion every month.
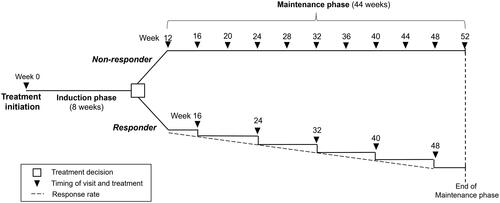
Table 1. Comparators in the model.
A proportion of responders at the end of induction were assumed to lose efficacy during maintenance and require rescue therapy (for patients who failed to maintain a prespecified response level during maintenance phase); the difference in clinical response between end of induction and at week 52 represents those who lost response status. It was assumed that loss of response took place at the midpoint to allow for a half-width adjustment of costs. Those patients losing a clinical response status during the maintenance phase were assumed to continue their respective advanced treatment until week 52 while receiving a course of rescue treatment. A treatment course refers to a single, continuous series of treatments over a few weeks, with costs averaged according to standard dosing schedules or clinical trial practices for ulcerative colitis. The model was created in Microsoft Excel (Microsoft 365, Version 2311).
Model inputs
In order to populate the CPR model for the Japanese setting, a targeted literature review was carried out to identify the appropriate parameter inputs. In particular, this review focused on extracting local cost data for treatments and management, including those related to safety.
The drug dosing schedule for each drug was obtained from the respective package insert and drug price was obtained from the Ministry of Health, Labour and Welfare medical fee schedule (Supplementary Tables 2 and 3) [Citation22]. The administration items and schedule, as well as rescue treatment items (Supplementary Tables 4–6), were obtained from input by clinical experts and recommendations from treatment guidelines [Citation23]. Only serious infections were considered as serious adverse events (SAE) in the model and rates were obtained, as described, from a network meta-analysis (NMA) in moderately to severely active UC (biologic-naïve or biologic-exposed patients). The efficacy rates and 95% credible intervals (CrIs) at the end of induction and at week 52 were derived from the outcome rates in the NMA, which evaluated clinical response, clinical remission, and endoscopic improvement for both biologic-naïve and biologic-exposed at induction and maintenance separately [Citation24] thus the same endpoints were used in the model (). The NMA included adults aged ≥16 years old. In order to accurately capture the overall treatment efficacy and translate it in an interpretable manner to decision-makers, treat-through ITT outcome rates were applied. According to specifications of the NMA and from the Markov Chain Monte Carlo samples, the estimated absolute efficacy rate of the respective induction clinical response was multiplied by the estimated absolute efficacy rate for the respective maintenance.
Table 2. Median efficacy rates with median 95% credible intervals.
Table 3. SAE rates.
Costs
The model was structured such that all patients on the same drug require the same drug cost and treatment administration cost during the induction phase. Drug costs and treatment administration costs of 5-aminosalicylic acid (5-ASA) and steroids, including steroid dose tapering as recommended in Japan [Citation23], were applied to all patients throughout the study period as concomitant therapies. Induction phase cost consisted of drug acquisition cost and administration cost of the advanced therapies, 5-ASA and steroid during the induction period of each respective therapy.
Treatment cost for an induction responder in maintenance phase consisted of drug acquisition cost and administration cost of the same advanced therapy, 5-ASA plus steroid as concomitant therapy, rescue treatment cost (one time for all patients who failed to maintain a prespecified response level during maintenance phase; Supplementary Table 6) and SAE management cost. Serious infection was considered in this SAE management category and patients with an SAE had SAE management cost and a corresponding break in advanced therapy. Diagnostic Procedure Classification (DPC) electronic score table for community acquired pneumonia requiring hospitalizations was referred to in order to determine the daily cost and duration of SAE management [Citation25]. The probability of SAE was dependent on the drug administered () [Citation24].
Cost for an induction non-responder consisted of drug acquisition cost, administration cost of a 5-biologics basket and 5-ASA plus steroid with rescue treatment cost, or drug acquisition cost, administration cost of 5-ASA plus steroid with rescue treatment cost.
CPR was calculated by total annual treatment cost (induction cost, maintenance cost for induction responder and maintenance cost for induction non-responder) divided by efficacy rate at week 52, which combines both efficacy rates at the end of induction phase and at the end of maintenance phase for the respective endpoint.
As a sensitivity analysis, patients who did not demonstrate a response to treatment at the end of induction moved to the maintenance arm with no treatment. Although this is an unrealistic scenario, the additional costs associated with initiating and maintaining a new advanced therapy have been removed, thereby not penalizing a failure with additional drug costs.
Costs were calculated from the perspective of the Japanese National Healthcare System as of April 2023 expressed as Japanese yen and US dollars ($) calculated with the exchange rate of $1 = 133 Japanese yen [Citation26].
Compliance with ethics guidelines
This study was based on published, publicly available data, did not involve primary data collection and did not analyze individual-level data. Therefore, this study complied with ethical guidelines and ethics approval was not required.
Results
Upadacitinib had the lowest median CPR with the smallest 95% CrI for almost all endpoints for biologic-naïve and biologic-exposed populations regardless of whether non-responders progressed to a 5-biologic-basket or no treatment. The only exception to this trend was for the biologic-naïve population for cost per clinical remission.
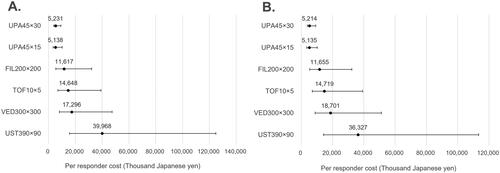
The CPR for clinical remission among biologic-naïve patients receiving either UPA45x30 or UPA45x15 and progressed to 5-biologic-basket was 6,972,000 yen (95%Crl: 4,298,000–17,972,000) ($52,421; 95%Crl: 32,316–135,128) and 7,021,000 yen (95%Crl: 3,900,000–20,074,000) ($52,789; 95%Crl 29,323–150,932), respectively (). The CPR for endoscopic improvement in the same population was 5,231,000 yen (95%Crl: 3,831,000–9,193,000) ($39,331; 95%Crl: 28,805–69,120) and 5,138,000 yen (95%Crl: 3,467,000–10,199,000) ($38,632; 95%Crl: 26,068–76,784), respectively (). For the biologic-exposed population on the same regimens (UPA45x30 and UPA45x15), the CPR for clinical remission was 5,527,000 yen (95%Crl: 3,838,000–10,847,000) ($41,556; 95%Crl: 25,414–81,556) and 5,106,000 yen (95%Crl: 3,363,000–10,751,000) ($38,391; 95%Crl: 25,286–80,835), respectively (). The CPR for endoscopic improvement in the same population was 5,214,000 yen (95%Crl: 3,819,000–9,163,000) ($39,203; 95%Crl: 28,714–68,895) and 5,135,000 yen (95%Crl: 3,464,000–10,193,000) ($38,609; 95%Crl: 26,045–76,639), respectively ().
Figure 2. Cost per responder for clinical remission (5-biologic-basket for non-responders). (A) Biologic-naïve cost per clinical remission; (B) biologic-exposed cost per clinical remission; in figure, dots represent the median cost per responder and the whiskers represent the 95% CrI.
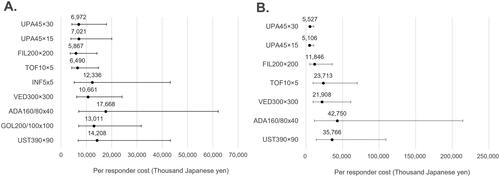
Figure 3. Cost per responder for endoscopic improvement (5-biologic-basket for non-responders). (A) Biologic-naïve cost per endoscopic improvement; (B) biologic-exposed cost per endoscopic improvement; in figure, dots represent the median cost per responder and the whiskers represent the 95% CrI.
The median CPR to achieve clinical remission with upadacitinib in a biologic-naïve population was roughly 7 million yen ($52,632) for both regimens (maintenance dose of 15 mg and 30 mg, once per day (QD)) (). The two other JAK inhibitors, filgotinib and tofacitinib, had lower values at approximately 6 million ($45,113) and 6.5 million yen ($48,872), respectively, the 95% CrI of all other biologics overlapped considerably. All other biologics had a median CPR of more than 10 million yen (>$75,188). In terms of endoscopic improvement in the same patient population (), upadcitinib (maintenance dose of 15 mg and 30 mg QD) had an even lower CPR of roughly 5.2 million yen ($39,098). This value was lower than the other JAK inhibitors which had CPRs greater than 11 million yen (>$82,707). Ustekinumab had the highest median value of nearly 40 million ($300,752). Similar to the results of clinical remission, 95% CrIs for all treatments overlapped to some degree for the endoscopic improvement endpoint.
To achieve clinical remission in a biologic-exposed population (), median CPR values for upadacitinib were the lowest at approximately 5 million yen ($37,594) while filgotinib and tofacitinib had median CPR value of 12 million yen ($90,226) and 24 million yen ($180,451), respectively. Adalimumab had the highest value at approximately 43 million yen ($323,308). For endoscopic improvement (), median CPR values for upadacitinib were again the lowest at 5 million yen ($37,594) while filgotinib had median CPR value of 12 million yen ($90,226) and tofacitinib 15 million yen ($112,782). Ustekinumab had the highest median value of 36 million yen ($270,677). The 95% CrIs overlapped for most of the advanced therapies but upadacitinib had the smallest intervals.
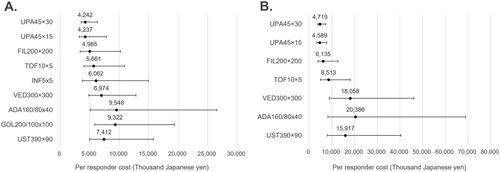
Results on clinical response are also presented in .
Figure 4. Cost per responder for clinical response (5-biologic-basket for non-responders). (A) Biologic-naïve cost per clinical response; (B) biologic-exposed cost per clinical response; in figure, dots represent the median cost per responder and the whiskers represent the 95% CrI.
For all initiated treatments, an alternative scenario where induction non-responders receives no treatment in the maintenance phase yielded lower CPR than the base case scenario (Supplementary Figures 1–3) as sensitivity analyses. This was true for all treatments with reductions ranging from approximately 22 to 48% except upadacitinib, in which there was only a 15 to 18% reduction. This result was possibly due to the higher clinical response rate for upadacitinib at the end of the induction phase. The sensitivity analyses showed similar results in ranking among advanced therapies and supported robustness of our base results.
Discussion
Upadacitinib was shown to be a cost-effective option based on the lowest cost to achieve clinical response, clinical remission and endoscopic improvement, not only in second line or later treatment (biologic-exposed patients) but also as a first line treatment (biologic-naïve). Previous studies have shown upadacitinib to be effective in biologic-exposed and biologic-naïve patients [Citation27,Citation28]. The current analysis suggests optimal use of upadacitinib, across endpoints, may be as a first line therapy for biologic-naïve patients with moderate-to-severe UC.
Few previous CPR models have included upadacitinib and were thus not able to make broad comparisons. In a study comparing the short-term costs of tumor necrosis factor inhibitors (infliximab, adalimumab, and golimumab) versus conventional therapy for moderately-to-severely active ulcerative colitis, golimumab 100 mg emerged as the most cost-effective option, yielding the lowest additional cost per year of sustained remission ($935) and response ($701) [Citation29]. In that study from Canada, adalimumab incurred the highest costs per additional remission ($7,430) and response ($2,361), and infliximab, despite its higher efficacy, did not offer the lowest cost per outcome. Another CPR model from the US supported infliximab ($147,379) as more cost effective for remission than adalimumab ($320,097) at 52 weeks [Citation30]. These findings were partially supported when assessed in the current study (biologic-naïve cohort) for clinical remission where golimumab and infliximab had similar median cost (12 million and 13 million yen ($90,226 and $97,744), respectively) and adalimumab was much larger cost at nearly 18 million yen ($135,338). When the clinical response endpoint was measured, costs for infliximab (nearly 6 million yen ($45,113)) were approximately 30% lower than either golimumab or adalimumab costs.
It is critical for pharmacoeconomic analyses to, as much as possible, accurately reflect the real-world treatment pathway in their structure. Clinical response, clinical remission and endoscopic improvement are important endpoints for UC evaluation as standards for assessing patients’ clinical condition and future healthcare utilization. Evidence indicates that endoscopic improvement like mucosal healing decreases the frequency of further surgery and hospitalization [Citation31]. To closely align with clinical practice, our analysis used these specific clinical endpoints, which are also recommended by the STRIDE-II Consensus [Citation14]. Moreover, our model structure is aligned with the patient pathway described in the 2020 Japanese clinical practice guidelines, which stipulate that the objective of pharmaceutical treatment is early induction of remission followed by maintenance of this outcome in the long-term to prevent relapse [Citation10]. Furthermore, our model considered SAE occurrence over the course of advanced therapies and possible treatment cessation due to SAE. Hence, the cost-benefit of upadacitinib examined in this study is closely aligned with real-world clinical practice, and shows treatment value for patients with moderate-to-severe UC regardless of previous biologic treatment.
There are several limitations that must be taken into account when interpreting the results of this analysis. First, the efficacy endpoints and SAE used in the model are based on a separate NMA of clinical trial data which does not always include data from Japan. Some heterogeneity in these definitions may exist between the regional clinical trials included in the NMA. In addition, clinical trial data used in the NMA may not entirely be reflective of routine clinical practice due to special circumstances such as reduced medical fees during the trial period. Nevertheless, given the limited comparative data between advanced therapies in clinical practice, the methodology of NMA would be useful to indirectly compare efficacy as well as safety data among advanced therapies in UC even from randomized controlled trials [Citation32]. Second, given UC is a lifelong disease, longer-term analysis may be beneficial to evaluate cost-effectiveness. However, extrapolation over the clinical trial period would be needed to analyze longer-term cost-effectiveness which may produce increased uncertainty in the results. Thus, we used data only from the actual trial period to minimize uncertainties from analysis. The absence of a consensus on the optimal method for measuring improvement in UC is underscored by a literature review that identified eight different endoscopic indices used in clinical trials [Citation33], alongside suggestions that assessments combining endoscopic and histologic outcomes may offer a more comprehensive reflection of disease improvement [Citation34,Citation35]. The model also penalizes patients those who fail to reach responder status at the end of induction phase. These patients then move to a new biologic therapy to re-initiate induction and maintenance therapy. However, this structure was confirmed to align with clinical practice based on opinions from clinical experts as well as the treatment guidelines in Japan [Citation23]. To address the limitations of the model, a “no treatment” alternative for patients who fail to achieve a response was used to remove subsequent treatment costs. This scenario, although unrealistic in the real world, helps to conservatively demonstrate the value of treatment. Finally, CPR models do not incorporate health utility data or extend over long-term time horizons. This omission limits our understanding of the long-term cost-effectiveness and quality-of-life impacts of UC therapies and necessitates further research. However, the results of this model were carefully reviewed and confirmed by clinical experts in Japan with an awareness of potential biases and the complexities inherent in sponsored research methodologies [Citation36].
Conclusions
We conclude that the results of this CPR analysis suggest upadacitinib is a promising treatment option for first and second line of advanced therapy in Japan as a cost-effective treatment for moderately to severely active UC.
Transparency
Author contributions
All authors contributed to the design of the study, model development, parameterization, and analysis or interpretation of the data. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (153.6 KB)Acknowledgements
We express our sincere gratitude to Alexis Masclet of Syneos Health for medical writing support for this article. In addition, the authors would like to thank Real World Evidence and HEOR Consulting teams at Syneos Health for technical support for this article.
Declaration of financial/other interests
MS has received grants or contracts from Mochida Pharmaceutical Co., Ltd, Zeria Pharma Co., Ltd, EA Pharma Co., Ltd, Kissei Pharmaceutical Co., Ltd and EPS Corporation; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Ltd, Takeda Pharmaceutical Co., Ltd, EA Pharma Co., Ltd, Gilead Sciences K.K. and Kissei Pharmaceutical Co., Ltd. IK and YO are employees of AbbVie GK and may hold AbbVie stocks/stock options. YSG is an employee of AbbVie Inc. and may hold AbbVie stock/stock options. NN was an employee of AbbVie GK at the time of conducting this study. XT and RM are employees of Syneos Health.
Data availability statement
The data supporting the findings of this study are available within the article and its online supplementary materials.
Additional information
Funding
References
- Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. doi: 10.1016/s0140-6736(12)60150-0
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0
- Yamazaki M, Chung H, Xu Y, et al. Trends in the prevalence and incidence of ulcerative colitis in Japan and the US. Int J Colorectal Dis. 2023;38(1):135. doi: 10.1007/s00384-023-04417-6.
- Japan Intractable Diseases Information Center. Ulcerative colitis (Designated Difficult Disease 97) 2022 [cited 2023 February 3]. Available from: https://www.nanbyou.or.jp/entry/62. (in Japanese)
- Berends SE, Strik AS, Löwenberg M, et al. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of ulcerative colitis. Clin Pharmacokinet. 2019;58(1):15–37. doi: 10.1007/s40262-018-0676-z.
- Park SC, Jeen YT. Current and emerging biologics for ulcerative colitis. Gut Liver. 2015;9(1):18–27. doi: 10.5009/gnl14226.
- Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12(2):113–122. doi: 10.25122/jml-2018-0075.
- Peyrin-Biroulet L, Lémann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33(8):870–879. doi: 10.1111/j.1365-2036.2011.04599.x.
- Hyun HK, Zhang HS, Yu J, et al. Comparative effectiveness of second-line biological therapies for ulcerative colitis and Crohn’s disease in patients with prior failure of anti-tumour necrosis factor treatment. BMC Gastroenterol. 2022;22(1):143. doi: 10.1186/s12876-022-02225-w.
- Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489–526. doi: 10.1007/s00535-021-01784-1.
- Jia X, Guo R, Hu Z, et al. Efficacy of infliximab, cyclosporine and tacrolimus on ulcerative colitis: a meta-analysis. Medicine (Baltimore). 2020;99(44):e22894. doi: 10.1097/md.0000000000022894.
- Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45(10):1291–1302. doi: 10.1111/apt.14030.
- Vickers AD, Ainsworth C, Mody R, et al. Systematic review with network meta-analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One. 2016;11(10):e0165435. doi: 10.1371/journal.pone.0165435.
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031.
- Meltzer DO, Basu A, Sculpher MJ, et al. Theoretical foundations of cost-effectiveness analysis in health and medicine. In: Neumann PJ, Ganiats TG, Russell LB, editors. Cost-effectiveness in health and medicine. New York (NY): Oxford University Press; 2016. p. 39–65.
- Desai U, Kirson NY, Guglielmo A, et al. Cost-per-remitter with esketamine nasal spray versus standard of care for treatment-resistant depression. J Comp Eff Res. 2021;10(5):393–407. doi: 10.2217/cer-2020-0276.
- Jairath V, Cohen RD, Loftus EV, et al. Evaluating cost per remission and cost of serious adverse events of advanced therapies for ulcerative colitis. BMC Gastroenterol. 2022;22(1):501. doi: 10.1186/s12876-022-02590-6.
- Yokomizo L, Limketkai B, Park KT. Cost-effectiveness of adalimumab, infliximab or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ Open Gastroenterol. 2016;3(1):e000093. doi: 10.1136/bmjgast-2016-000093.
- Ruggeri M, Rolli FR. Cost per response/remission in biologics available in Italy for the treatment of TNF-α inhibitors-naïve patients with ulcerative colitis. Glob Reg Health Technol Assess. 2019;6(1) doi: 10.33393/grhta.2019.456.
- Ministry of Health Labour and Welfare. Regarding the guidelines and points of caution for the labeling and documentation of pharmaceutical drugs for medical use 2017 [cited 2024 Feburuary 19]. Available from: https://www.pmda.go.jp/files/000218448.pdf (in Japanese)
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi: 10.1056/nejm198712243172603.
- Ministry of Health Labour and Welfare. Medical fee information service 2022 [cited 2022 September 3]. Available from: https://shinryohoshu.mhlw.go.jp/shinryohoshu/searchMenu/. (In Japanese)
- Ministry of Health Labour and Welfare Science Research Grants Subsidy Policy Research Project for Intractable Diseases. Ulcerative colitis and Crohn’s disease: diagnostic criteria and treatment guidelines 2021 [cited 2022 December 14]. Available from: http://www.ibdjapan.org/pdf/doc15.pdf. (In Japanese)
- Panaccione R, Collins EB, Melmed GY, et al. Efficacy and safety of advanced therapies for moderately to severely active ulcerative colitis at induction and maintenance: an indirect treatment comparison using Bayesian network meta-analysis. Crohns Colitis 360. 2023;5(2):otad009. doi: 10.1093/crocol/otad009.
- Ministry of Health Labour and Welfare. About the diagnostic procedure classification (DPC) electronic score table 2022 [cited 2022 April 5]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000198757_00003.html. (In Japanese)
- Bank of Japan. List of exchange rates for reporting ministerial ordinance [cited 2023 April 20]. Available from: https://www.boj.or.jp/about/services/tame/tame_rate/syorei/index.htm. (In Japanese)
- Loftus EV, Jr., Colombel JF, Takeuchi K, et al. Upadacitinib therapy reduces ulcerative colitis symptoms as early as day 1 of induction treatment. Clin Gastroenterol Hepatol. 2022;21(9):2347–2358.e6. doi: 10.1016/j.cgh.2022.11.029.
- Ghosh S, Sanchez Gonzalez Y, Zhou W, et al. Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis. 2021;15(12):2022–2030. doi: 10.1093/ecco-jcc/jjab099.
- Toor K, Druyts E, Jansen JP, et al. Cost per remission and cost per response with infliximab, adalimumab, and golimumab for the treatment of moderately-to-severely active ulcerative colitis. J Med Econ. 2015;18(6):437–446. doi: 10.3111/13696998.2015.1012513.
- Lofland JH, Mallow P, Rizzo J. Cost-per-remission analysis of infliximab compared to adalimumab among adults with moderate-to-severe ulcerative colitis. J Med Econ. 2013;16(4):461–467. doi: 10.3111/13696998.2013.775134.
- Peyrin-Biroulet L, Ferrante M, Magro F, et al. Results from the 2nd scientific workshop of the ECCO. I: impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis. 2011;5(5):477–483. doi: 10.1016/j.crohns.2011.06.009.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. National Institute for Health and Care Excellence (NICE), London; 2014.
- Vuitton L, Peyrin-Biroulet L, Colombel JF, et al. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017;45(6):801–813. doi: 10.1111/apt.13948.
- Ma C, Sedano R, Almradi A, et al. An international consensus to standardize integration of histopathology in ulcerative colitis clinical trials. Gastroenterology. 2021;160(7):2291–2302. doi: 10.1053/j.gastro.2021.02.035.
- Li K, Marano C, Zhang H, et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology. 2020;159(6):2052–2064. doi: 10.1053/j.gastro.2020.08.037.
- Friedberg M, Saffran B, Stinson TJ, et al. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. JAMA. 1999;282(15):1453–1457. doi: 10.1001/jama.282.15.1453.

