Abstract
Aims
Non‐cystic fibrosis bronchiectasis (NCFB) is a chronic progressive respiratory disorder occurring at a rate ranging from 4.2 to 278.1 cases per 100,000 persons, depending on age, in the United States. For many patients with NCFB, the presence of Pseudomonas aeruginosa (PA) makes treatment more complicated and typically has worse outcomes. Management of NCFB can be challenging, warranting a better understanding of the burden of illness for NCFB, treatments applied, healthcare resources used, and subsequent treatment costs. Comparing patients diagnosed with exacerbated NCFB, with or without PA on antibiotic utilization, treatments, and healthcare resources utilization and costs was the purpose of this study.
Materials and methods
This was a retrospective cohort study of commercial claims from IQVIA’s PharMetrics Plus database (January 1,2006–December 31, 2020). Study patients with a diagnosis of NCFB were stratified into two groups based on the presence or absence of PA, then followed to identify demographic characteristics, comorbid conditions, antibiotic treatment regimen prescribed, healthcare resources utilized, and costs of care.
Results
The results showed that patients with exacerbated NCFB who were PA+ had significantly more oral antibiotic fills per patient per year, more inpatient admissions with a longer length of stay, and more outpatient encounters than those who were PA−. For costs, PA+ patients also had significantly greater total healthcare costs per patient when compared to those who were PA−.
Conclusion
Exacerbated NCFB with PA+ was associated with increased antibiotic usage, greater resource utilization, and increased costs. The major contributor to the cost differences was the use of inpatient services. Treatment strategies aimed at reducing the need for inpatient treatment could lessen the disparities observed in patients with NCFB.
Introduction
Bronchiectasis is a chronic progressive respiratory disorder characterized by irreversibly and abnormally dilated airways, persistent cough, excessive sputum production, and recurrent pulmonary infectionsCitation1. Non-cystic fibrosis bronchiectasis (NCFB) is now recognized as the third most common respiratory disorder after asthma and chronic obstructive pulmonary diseaseCitation2–4. Among persons aged 18–34, NCFB occurs at an incidence of 4.2 cases per 100,000 persons. By the age of 75, however, the incidence rate increases to 271.8 cases per 100,000 patients. It is estimated between 237,464 and 431,585 adults in the United States (US) have NCFBCitation3,Citation5. Current literature has shown that patients with NCFB have an increased risk for all-cause mortality, further emphasizing the need for improved strategies of careCitation6,Citation7.
Bacterial infections are a commonly occurring manifestation of NCFB that drive airway inflammation and disease progressionCitation8. Pseudomonas aeruginosa (PA) is one of the most common and significant bacterium associated with chronic infection in NCFB and its presence can lead to greater lung function impairment, more frequent disease exacerbations, poorer health-related quality-of-life (HRQoL), and a greater risk of hospitalization and mortalityCitation8,Citation9. Exacerbations in NCFB are defined as acute deteriorations in a patient’s established baseline status of bronchiectasis, resulting in a change in symptoms and treatment strategy, including an addition or change in antibiotic therapy.
NCFB management aims to promote airway clearance, prevent exacerbations and improve HRQoLCitation10–12. Airway clearance techniques, such as chest physical therapy and pulmonary rehabilitation, are commonly used. While there are currently no approved medications with an indication for NCFB, antibiotic use is common in addition to inhaled mucolytic therapies to provide symptomatic relief and reduce the number of exacerbationsCitation11,Citation13,Citation14. In the case of infection, treatment typically involves empiric oral antibiotics (e.g. fluoroquinolones) selected based on local antibiogram data and patient sputum cultures. Though challenges remain in managing PA infection in severe cases of NCFB, more intensive medication regimens, including inhaled (e.g. colistin/tobramycin/gentamicin) and intravenous (IV) antibiotics (e.g. beta-lactam plus aminoglycoside) can be utilizedCitation15.
Maintenance therapy for NCFB can include long-term inhaled antibiotics with the selection of an agent guided by bacterial cultures, PA colonization status, and annual exacerbation rateCitation16. For the reduction of exacerbation frequency, patients without PA may receive chronic oral macrolide therapy, and those with bronchiectasis who are positive for PA can receive inhaled antibiotics such as nebulized colistin as first-line treatmentCitation17. Sputum cultures are also critical for guiding further antibiotic selection if initial treatment fails. However, no curative treatments are currently approved for NCFB, PA colonization, or exacerbations associated with the diseaseCitation18.
Additional evidence is required to increase awareness of NCFB and, to date, most studies investigating NCFB have utilized populations with relatively small sample sizes. While previous studies have investigated the burden of NCFB illness and assessed antibiotic prescribing patterns, the associated economic burden in the US remains largely understudiedCitation19. A recent claims analysis of patients diagnosed with NCFB demonstrated per patient costs associated with the treatment of NCFB to be $36,213 for patients who were PA− and $67,764 for patients who were PA+Citation20,Citation21. This study uses a large real-world sample to understand patterns of delivery of care, antibiotic use, HRU, and the economic burden incurred by patients with NCFB. Specifically, we characterize antibiotic prescribing patterns, HRU outcomes and costs in patients diagnosed with NCFB and experiencing exacerbations; both with and without PA colonization.
Methods
This is a retrospective cohort study conducted with commercial claims data from the IQVIA PharMetrics Plus database. The database contains de-identified adjudicated claims for approximately 150 million patients, with an annual capture of nearly 40 million enrolled in US commercial health plans. Because the study used de-identified PharMetrics claims, no IRB approval was required.
Patient identification
The study reviewed claims data from January 1, 2006 to December 31, 2020. The index date was the date of first claim with a qualifying NCFB diagnosis. This could occur during an imaging CT scan/bronchoscopy visit, a regular outpatient visit, or a hospitalization. Once patients were identified and selected based on inclusion and exclusion criteria, they were evaluated during the 6-month period prior to and 12 months following the index date. Due to the nature of claims data, loss to follow-up could occur due to health plan disenrollment, mortality, or end of the study identification period.
Patients were selected using convenience sampling within the study window. Inclusion criteria required patients to have the following features, at least one International Classification of Diseases diagnosis code claim for bronchiectasis ([ICD-9-CM]: 494.XX; [ICD-10-CM]: J47.XX), be aged between 18 and 65 years, and be continuously enrolled with a medical and pharmacy benefit for 12 months from the index date. The study’s specific definition to identify patients with NCFB was the presence of at least one inpatient claim for bronchiectasis; or two outpatient claims for bronchiectasis within a 1-year period but at least 7 days apart; or one outpatient claim for bronchiectasis in combination with a bronchoscopy or CT at least 7 days from the diagnosis, but within 6 months. Exclusion criteria included any evidence of cystic fibrosis using ICD codes (ICD-10-CM: E84. XX; ICD-9-CM: 277.XX). Patients were defined as having exacerbations if they had two or more claims for an acute NCFB exacerbation (ICD-9: 494.1; ICD-10: J47.1); or had one inpatient hospitalization for bronchiectasis; or received an IV antibiotic treatment; or had pneumonia (J15.8, J15.9) during their observation period.
Once identified with exacerbated NCFB, patients were stratified into one of two groups based on PA infection status: patients with evidence of PA infection (PA+) and patients with NCFB and exacerbations but no evidence of PA infection (PA−). PA infection status was evaluated by the presence of at least one non-ancillary claim for a PA infection (ICD-9: 041.7; ICD-10: B96-5) or at least one non-ancillary claim for pneumonia due to a PA infection (ICD-9: 482.1; ICD-10: J15.1), either occurring 6 months prior to or following the index date.
Outcomes
All outcome variables were evaluated in the 1-year period following the index date. Demographic and clinical characteristics, pharmacy utilization, healthcare resource utilization (HRU), and costs were obtained from standardized variables on claims within databases. Patient demographic data included age, gender, and insurance coverage. Clinical characteristics were evaluated using diagnosis and other clinical codes to determine the Charlson Comorbidity Index (CCI), an estimate of the 1-year risk of mortality based on pharmacy claimsCitation22,Citation23. A CCI of 0 indicates no risk of death while a higher CCI is associated with an increased risk of death and a poorer health status. All cause antibiotic treatments and prescriptions, route of administration (inhaled, oral, and IV) and HRU were measured using National Drug Codes (NDC) and Healthcare Common Procedure Coding System (HCPS) codes.
For HRU, inpatient hospitalization, ER visits, and outpatient visits by location were all identified by claims. Patients admitted to the hospital as a function of an emergency room (ER) visits are billed by providers as part of the hospitalization and are therefore not reliably distinguishable from the hospitalization. ER visits, then, are only those in which a patient was not admitted to the hospital. Estimates of cost were based on insurance payments to providers but excluded patient cost-sharing responsibilities. All costs were adjusted to 2022 US dollars using the Medical Care Component of the Consumer Price Index.
Statistical analyses
Univariate analyses included means and standard deviation for continuous variables with counts and percentages for discrete variables. HRU and costs were reported as mean per patient per year (PPPY) and included patients with zero encounters. Observations with missing values were omitted from analyses.
Bivariate analyses were used to compare differences in baseline characteristics, treatment patterns, HRU, and costs between the cohorts using Chi-square tests for categorical variables and Student’s t-test for continuous variables. P-values < 0.05 were considered statistically significant. All statistical analyses were conducted using SAS 9.3 and R 2022.02.
Results
The Pharmetrics commercial database identified a total of 36,285 patients diagnosed with NCFB who met the study’s inclusion criteria. Identified patients were largely female (63.3%), aged 53.7 (±9.85) years, and primarily covered by a PPO benefit plan.
Following stratification, 1,180 (3.3%) were identified as having exacerbated NCFB and PA (PA+) while 18,074 (49.8%) patients had exacerbations, but no PA (PA−). The remaining patients were those who had NCFB but no exacerbations or PA (not shown). The demographic data for the two groups under consideration are presented in . As shown for both groups, most patients were female (55.4% and 61.0%, respectively) with a mean age of 53.2 (± 10.7) and 53.3 (± 10.1), respectively. Patients diagnosed with PA+ had a significantly higher mean CCI than patients who were PA−, 2.86 ± 2.17 and 2.44 ± 2.08, respectively, p < 0.05.
Table 1. Demographic characteristics of commercially insured patients with NCFB and exacerbations.
Antibiotic treatment patterns
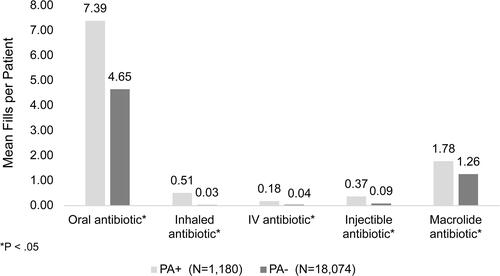
Among the overall NCFB population, 81.9% of patients (n = 29,721) received at least one prescription for any antibiotic during the study period. For oral antibiotic medications, which represented 79.5% of all antibiotics prescribed, there was a mean rate of 4.01 (±4.93) prescription fills PPPY. Among identified patients, those with PA+ had significantly more oral antibiotic fills (mean = 7.39 ± 6.92) PPPY than those who were PA− (4.65 ± 5.22), p < 0.05. This difference was also present for other routes of antibiotic administration (inhaled, IV, and injectable) ().
Healthcare resource utilization
Overall, commercially insured NCFB patients had a mean of 0.73 (± 1.49) inpatient (IP) admissions PPPY, with an average length of stay (ALOS) at 6.52 (± 8.34) days per admission (). Mean ICU admissions PPPY were 0.21 (± 0.66), with an ALOS of 9.19 (± 13.26) days per ICU admission.
Figure 2. Inpatient encounters PPPY for patients with NCFB and exacerbations.
Abbreviations: IV, intravenous; PA, Pseudomonas aeruginosa; IP, inpatient.
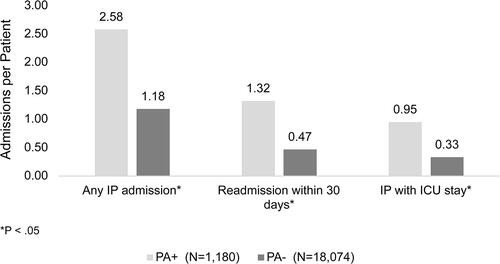
Patients who were PA+ had significantly higher rates of HRU when compared to patients who were PA−. For inpatient HRU, those who were PA+ had a mean of 2.58 (±3.00) admissions PPPY with a LOS of 9.94 (±11.06) days per admission. This can be compared to patients who were PA− with a mean of 1.18 (±1.70) inpatient admissions PPPY (p < 0.05) and an ALOS of 6.50 (± 8.42, p < 0.05). Patients who were PA+ also had a significantly higher PPPY rate of 30-day readmissions compared to patients who were PA− (1.32 ± 2.51 vs. 0.47 ± 1.30, p < 0.05). Similarly, for ICU admissions, the PPPY rate for patients who were PA+ was 0.95 (± 1.62) admissions compared to 0.33 (± 0.76) admissions for patients who were PA−, p < 0.05.
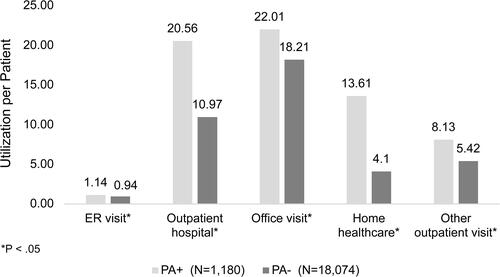
NCFB patients averaged outpatient HRU of 40.09 (± 37.9) total outpatient encounters PPPY. Like inpatient HRU, patients who were PA+ had significantly greater total outpatient encounters PPPY when compared to patients who were PA− (75.94 ± 57.33 vs. 45.87 ± 41.44, p < 0.05). PA+ patients demonstrated significantly greater outpatient HRU for many individual measures of resource utilization including ER visits, outpatient hospital visits, office visits, and home healthcare ().
Costs
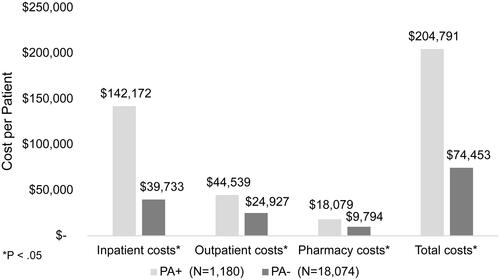
The mean total all-cause direct medical costs PPPY for patients diagnosed with NCFB was $55,800 (± $132,126). Of this total, $25,787 (± $105,264) were for inpatient services, $21,297 (± $48,573) for outpatient services, and $8,535 (± $24,997) for prescription pharmacy services. The results varied widely between treatment groups based on PA status. Patients who were PA+ had significantly greater mean PPPY total costs when compared to patients who were PA− ($204,791 ± $334,906 vs. $74,453 ± $149,343, respectively, p < 0.05). Similarly, as shown in , PA+ patients demonstrated significantly greater costs associated with inpatient services, outpatient services, and pharmacy costs. The cost of inpatient services accounted for 69.4% of total costs in patients who were PA+.
Discussion
This study utilized commercial medical and pharmacy claims to gain detailed insight into the demographic characteristics, antibiotic prescribing practices, HRU, and costs in patients diagnosed with NCFB and experiencing exacerbations in the United States. The results showed that patients with exacerbated NCFB and P. aeruginosa (PA+) had a significantly higher burden of illness when compared to those who were PA−. Specifically, patients who were PA+ had more comorbidities, received significantly more antibiotic prescriptions, experienced higher inpatient and outpatient HRU, and incurred greater healthcare costs than patients who were PA−.
The costs related to treatment of NCFB and PA+ were particularly revealing. First, the largest drivers of higher healthcare costs observed in these patients were associated with inpatient care, which comprised approximately two-thirds of all costs in PA+ patients. When compared to inpatient costs for patients who were PA−, there was a difference of $102,439 PPPY. While inpatient costs were the most striking (69.4% of total costs), PA+ patients showed significantly higher levels of HRU and costs across all categories of healthcare services, resulting in an additional $130,338 per year on average than patients who were PA−.
For comparative purposes, a separate group of patients with NCFB and no exacerbations or PA (PA−E−) (n = 16,946) was also examined. Total annual PPPY costs for this group were found to be only $25,587, with inpatient costs of $3,283 making up 12.8% of the total. The difference in total cost, then, between PA+E+ and PA–E– was $179,203, with inpatient costs contributing the greatest proportion of difference.
One caveat to the results is that actual differences between PA+ and PA− patient populations identified could be greater given that the presence of PA is likely under-coded within the claims data. First, not all patients with exacerbations are tested for PA. Beyond testing, providers and facilities may not have coded NCFB patients as PA+. One reason for this may be that such coding does not actually affect reimbursement. In literature examining the effect of PA infections on patient outcomes, colonization rates ranged between 25.4% and 34.0% in NCFBCitation15,Citation24. In this study, we found that only 3.25% of patients in the NCFB population were PA+. Regardless, the fact that PA infection rates may be under-reported highlights the need for consensus guidelines to provide clear and specific recommendations for PA screening and treatment in patients with NCFB.
As cited earlier, direct medical costs associated with PA infection in NCFB cost anywhere from $30,000 to $70,000 when compared to patients who are PA−. In addition, the excess costs in PA+ NCFB are typically attributable to a five-fold increase in hospitalizations that make up nearly 50% of total costs of care in this populationCitation20,Citation21. Our findings, however, suggest that the true cost incurred by patients with NCFB who are also PA+ is substantially greater than those demonstrated in prior studies. Although this study only focused on direct medical costs, there is no reason to suspect that other costs, including indirect medical costs, would also be affected.
As with all retrospective claims analyses, this study has limitations including missing and miscoded claims, comorbidity assessments based on pharmacy utilization, and a lack of detail on inpatient claims. For inclusion in our patient population, we relied on diagnosis codes but did not confirm them with chart review or other patient level data. Another potential limitation is that, due to large sample sizes, many associations that are not clinically significant may be found to be statistically significant. Finally, for costs, claims provide access only to costs paid by the health plan, but exclude coinsurance, copays, out of pocket costs, or other indirect costs.
Conclusion
This retrospective study of claims data in patients diagnosed with exacerbated NCFB suggests that those who are PA+ have increased antibiotic usage, increased HRU across all inpatient and outpatient services, and increased costs compared to patients with exacerbated NCFB but who are PA−. The primary driver of costs and resource utilization in this study was increased inpatient care, which is consistent with prior studies on NCFB. Further research is warranted to inform strategies to minimize exacerbations in patients with NCFB, particularly those who are PA+, as they have more exacerbations, lower QoL, higher mortality, and incur higher resource utilization and costs. These findings also suggest a need for updated treatment guidelines that maximize treatment strategies in this population.
Transparency
Declaration of funding
The study was sponsored by Zambon S.p.A.
Declaration of financial/other relationships
PRECISIONheor received funding for the study. M.F. is a consultant for PRECISIONheor. M.E.M. and F.P. are employees of the Zambon Group.
Author contributions
M.F. takes full responsibility for the article, including the data and analysis. M.F. drafted and revised the manuscript. M.F., M.E.M., F.P., and S.D. conceptualized and designed the study. M.F. and PRECISIONheor were responsible for the analysis. All authors contributed to data interpretation and manuscript revision.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
| Abbreviations | ||
| CCI | = | Charlson Comorbidity Index |
| HRQoL | = | health-related quality-of-life |
| HRU | = | healthcare resource utilization |
| NCFB | = | non‐cystic fibrosis bronchiectasis |
| PA | = | Pseudomonas aeruginosa |
| PA+ | = | patients diagnosed with PA |
| PA− | = | patient without PA |
| PPPY | = | per patient per year |
Acknowledgements
Maia R. Emden, Naomi C. Sacks, Katie Everson, and Ronae McLin were employees at PRECISIONheor when the study was conceptualized and initiated. They provided input on the study design and initial results interpretation.
References
- Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392(10150):880–890. doi: 10.1016/S0140-6736(18)31767-7.
- Maselli DJ, Amalakuhan B, Keyt H, et al. Suspecting non-cystic fibrosis bronchiectasis: what the busy primary care clinician needs to know. Int J Clin Pract. 2017;71(2):e12924. doi: 10.1111/ijcp.12924.
- Noone JG, Blanchette C, Zacherle E, et al. Estimates of the prevalence of non-cystic fibrosis bronchiectasis in the US Presented at the American Thoracic Society International Conference, Washington DC, May 21, 2017.
- Martinez-Garcia MA, Polverino E, Aksamit T. Bronchiectasis and chronic airway disease: it is not just about asthma and COPD. Chest. 2018;154(4):737–739. doi: 10.1016/j.chest.2018.02.024.
- Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12(4):205–209. doi: 10.1097/01.cpm.0000171422.98696.
- Munteanu O, Chesov D, Rusu D, et al. Mortality related risk factors in patients with non-cystic fibrosis bronchiectasis. Eur Respir J. 2022;60(suppl 66):4014. doi: 10.1183/13993003.congress-2022.4014.
- Sin S, Yun SY, Kim JM, et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir Res. 2019;20(1):271. doi: 10.1186/s12931-019-1243-3.
- Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonisation on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12(11):1602–1611. doi: 10.1513/AnnalsATS.201506-333OC.
- Wang R, Ding S, Lei C, et al. The contribution of Pseudomonas aeruginosa infection to clinical outcomes in bronchiectasis: a prospective cohort study. Ann Med. 2021;53(1):459–469. doi: 10.1080/07853890.2021.1900594.
- Imam JS, Duarte AG. Non-CF bronchiectasis: orphan disease no longer. Respir Med. 2020;166:105940. doi: 10.1016/j.rmed.2020.105940.
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J. 2015;45(5):1446–1462. doi: 10.1183/09031936.00119114.
- Quittner AL, O’Donnell AE, Salathe MA, et al. Quality of life questionnaire-bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax. 2015;70(1):12–20. doi: 10.1136/thoraxjnl-2014-205918.
- Lee AL, Hill CJ, McDonald CF, et al. Pulmonary rehabilitation in individuals with non–cystic fibrosis bronchiectasis: a systematic review. Arch Phys Med Rehabil. 2017;98(4):774–782.e1. doi: 10.1016/j.apmr.2016.05.017.
- Dhand R. The rationale and evidence for use of inhaled antibiotics to control Pseudomonas aeruginosa infection in non-cystic fibrosis bronchiectasis. J Aerosol Med Pulm Drug Deliv. 2018;31(3):121–138. doi: 10.1089/jamp.2017.1415.
- Wilson R, Aksamit T, Aliberti S, et al. Challenges in managing Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respir Med. 2016;117:179–189. doi: 10.1016/j.rmed.2016.06.007.
- Laska IF, Crichton ML, Shoemark A, et al. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta-analysis. Lancet Respir Med. 2019;7(10):855–869. doi: 10.1016/S2213-2600(19)30185-7.
- Hill AT, Sullivan AL, Chalmers JD, et al. British thoracic society guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69. doi: 10.1136/thoraxjnl-2018-212463.
- Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers. 2018;4(1):45. doi: 10.1038/s41572-018-0042-3.
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis – known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. doi: 10.1186/s12890-019-0818-6.
- Blanchette C, Noone J, Stone G, et al. Healthcare use and costs among patients with non-cystic fibrosis bronchiectasis in the United States. In: am J Respir Crit Care Med. 2016;193:A2939.
- Blanchette CM, Noone JM, Stone G, et al. Healthcare cost and utilization before and after diagnosis of Pseudomonas aeruginosa among patients with non-cystic fibrosis bronchiectasis in the U.S. Med Sci (Basel). 2017;5(4):20. doi: 10.3390/medsci5040020.
- Quan H, Li B, Couris CM, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8.
- Choate R, Aksamit TR, Mannino D, et al. Pseudomonas aeruginosa associated with severity of non-cystic fibrosis bronchiectasis measured by the modified bronchiectasis severity score (BSI) and the FACED: the US bronchiectasis and NTM Research Registry (BRR) study. Respir Med. 2021;177:106285. doi: 10.1016/j.rmed.2020.106285.